1. Introduction
Over the last decade, a variety of solar energy utilization systems have been extensively used in actual applications [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. These systems are deemed to be an attractive approach for solving global energy shortage problems due to its renewable and sustainable nature. However, the harvesting of solar energy varies intensely during different times, weather, and seasons, which inhibits the further application of the technology [
20]. To further improve the system flexibility, thermal energy storage (TES) units are employed as an indispensable component in the system.
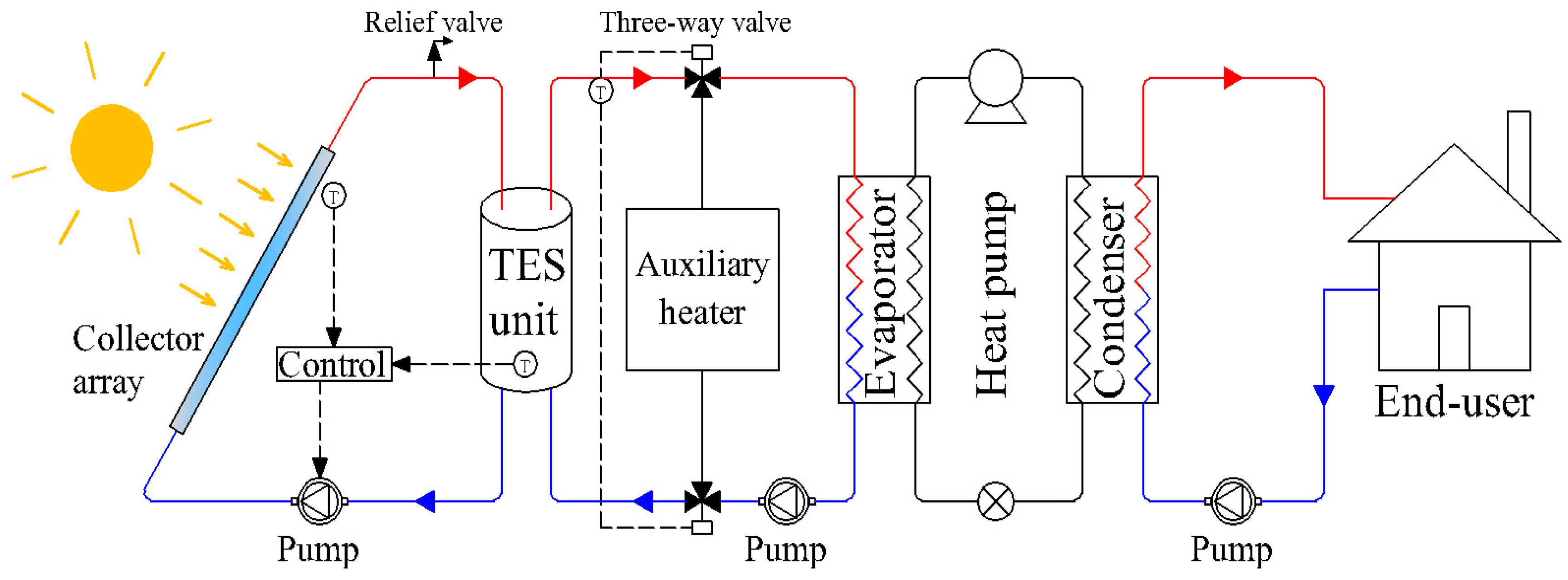
Figure 1 illustrates a solar assisted heat pump (SAHP) system using a TES unit, which consists of several components including a solar collector, TES unit and heat pump. During the daytime, the thermal energy gathered by the solar collector is stored in the TES unit, while during the evening or on cloudy days, when the solar radiation drops down to zero or is at an insufficient level, the previously stored thermal energy is released as a low-grade heat source to the evaporator. Therefore, a balance between the energy supply and demands is achieved, which greatly improves the system durability.
In general, TES systems can be divided into two types: sensible heat thermal energy storage (SHTES) systems and latent heat thermal energy storage (LHTES) systems [
21]. Compared to the widely used SHTES systems, LHTES systems provide a more promising TES solution [
22]. By using phase change materials (PCMs), these systems can provide better energy storage density with small thermal fluctuations.
Numerous experimental studies have been performed in this area [
23,
24,
25,
26,
27]. Dannemand et al. [
23] studied cylindrical PCM systems utilizing stable super-cooling sodium acetate trihydrate composites. They found that low heat exchange capacity was due to limited convection in the molten PCM. Yazici et al. [
24] investigated the effect of inner tube orientations on the melting behavior of paraffin. Their results demonstrated that the inner tube eccentricity was closely linked with the melting process. Murray and Groulx [
25,
26] studied the phase change process of dodecanoic acid under various charging and discharging conditions. They found that the natural convection played a significant role during the charging process, but became negligible during the discharging process. Gasia et al. [
27] studied the dynamic melting based on a cylindrical shell-and-tube system using water as PCM. The heat transfer coefficient of the system increased due to the forced convection of the liquid PCM.
Analytical approaches provide an alternative solution. Kalaiselvam et al. [
28] presented an analytical solution to locate interface locations over time. They found that the total solidification time depends on Stefan number and heat generation parameter, whereas the total melting time depended on equivalent thermal conductivity. Mosaffa et al. [
29] proposed an approximate model for a two-dimensional solidification process in a shell-and-tube finned thermal storage system. The PCM solid phase variation over time was monitored and compared with the cylindrical shell and rectangular storage arrangements. Xu et al. [
30] developed a mathematical model to analyze the overall exergy efficiency of combined charging-discharging processes using different kinds of PCMs and HTFs. Their results showed that the overall exergy efficiency can be improved by increasing the heat transfer units. Bechiri and Mansouri [
31,
32] developed an analytical solution for a shell-and-tube LHTES unit by applying an exponential integral function and a variables separation technique. Based on the analytical model, the effects of natural convection, HTF mass flow rate, outer tube radius, and pipe length on charging and discharging process were investigated.
Recently, numerical simulations using the computational fluid dynamics (CFD) approach have attracted considerable attention in this field. Compared to the experimental and analytical approaches, numerical simulations are more effective in dealing with influential factors, such as natural convection [
33,
34,
35,
36,
37,
38,
39], geometric configuration [
33,
34,
35,
36], dynamic melting process [
37] and non-dimensional number [
38], etc. Fan et al. [
33] studied a constrained melting process in a circumferentially finned spherical capsule, the influence of fin height on the TES performance was revealed. Li and Wu [
34] analyzed the TES performance with multiple geometric configurations and PCMs. Their results showed that the melting and solidification time of a TES unit with fins can be shortened by at least 14%. Xiao and Zhang [
35,
36] investigated the heat transfer in a tube-and-shell storage tank using paraffin/expanded graphite composite PCMs. They found that the type of PCM, HTF temperatures and flow rates dominate the TES performance. Tay et al. [
37] studied a dynamic melting in a tube-in-tank PCM storage system, which involves forced recirculation of melted PCM. The melting process was greatly accelerated compared to that based on natural convection. Archibold et al. [
38] investigated the effects of the Grashof and Stefan numbers on TES performance. It was found that increasing the Grashof number from 1.32 × 104 to 2.06 × 105 can strengthen the heat transfer. Also for a constant Grashof number (9.09 × 104), the PCM melted at a faster rate when the Stefan number increased from 0.077 to 0.097. Fornarelli et al. [
39] identified the natural convective flows within the molten PCM by temperature gradients and gravity, and the enhanced heat flux can reduce 30% of the charging time. Tao and Carey [
40] sequenced the PCM thermal properties affecting TES performance, which was in the order of melting temperature, thermal conductivity, specific heat, density and melting enthalpy, successively.
For the tube-in-tank LHTES system, thermal resistance dominates within the PCM. To accelerate the latent thermal energy storage process, one of the most effective methods is extending the heat transfer area [
37], such as using coil tubes. Compared to the serial coil tube, a helical coil tube is easier to install and has a greater heat transfer coefficient due to the secondary flow of fluid [
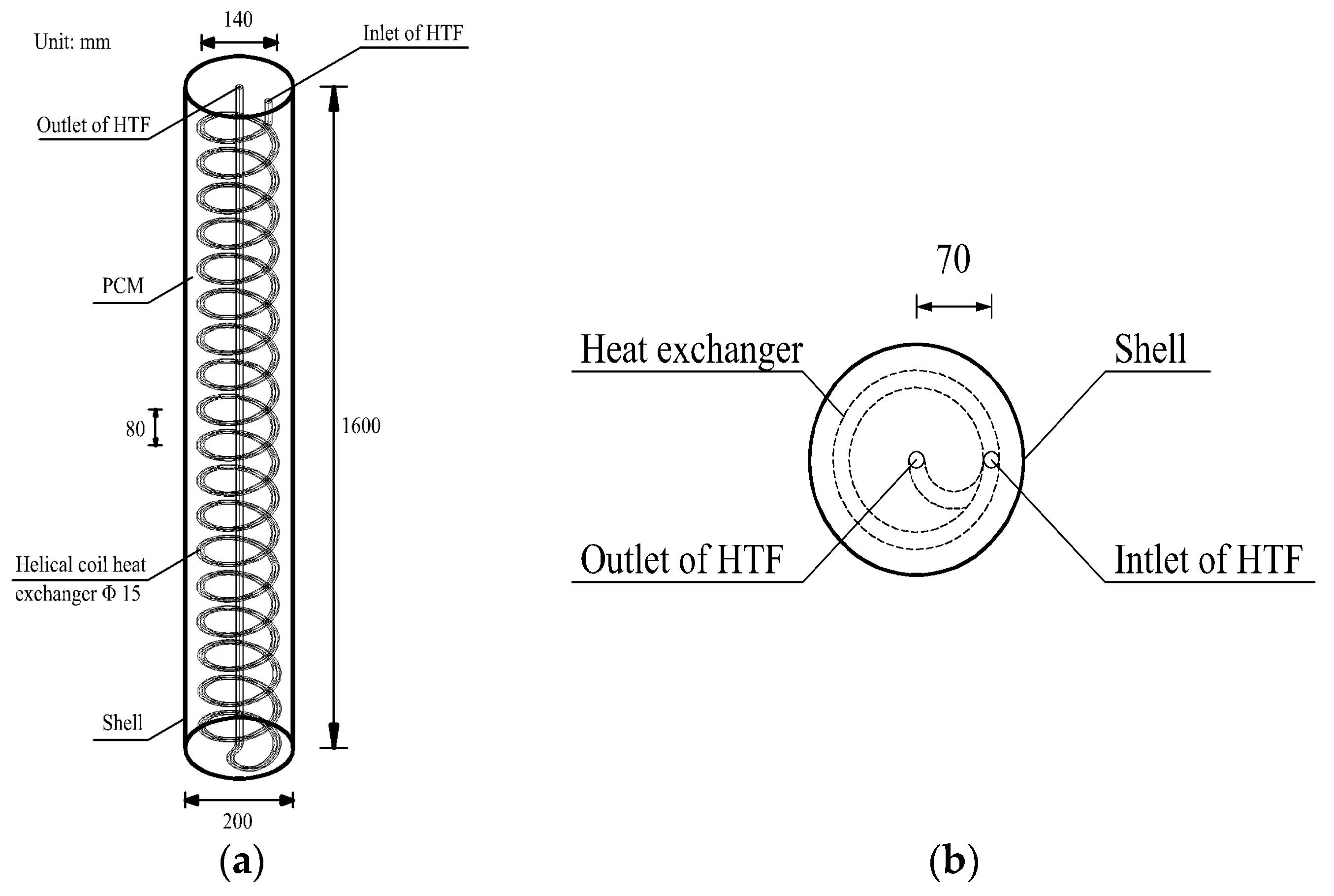
41]. Although numerous studies that have been conducted on LHTES systems, most of them were based on heat exchangers with straight tube. Studies on the enhancement of heat transfer performance in tube-in-tank LHTES systems by applying helical coil tubes are relatively scarce in the published literature. In order to get a rapid charging speed, an enhanced heat exchanger using a concentric helical coil tube was applied in a vertical cylindrical LHTES in this study. The dynamic PCM melting process was comprehensively studied through numerical modelling and experimental measurement. The dynamic melting process of the PCM including temperature and liquid fraction variations were analyzed in detail.
3. Numerical Modelling
For the HTF, the continuity, momentum and energy equations are shown below:
The HTF flow regime for all cases is turbulent flow, and the standard
k-ε model is applied. Turbulent kinetic energy transporting equation:
Turbulent dissipation rate transporting equation:
The turbulent viscosity:
where:
The values of constants in Equations (5)–(8) are listed in
Table 4 [
42].
For the PCM, the enthalpy-porosity method [
34,
35,
36,
37,
38,
39] was applied to model the dynamic melting process, modelling equations including the continuity, momentum, and energy equations are shown below:
Energy equation:
where
is the total enthalpy, which is the summation of the sensible enthalpy and latent enthalpy:
The sensible enthalpy
can be written as:
where
is the reference enthalpy,
is the reference temperature.
The latent enthalpy
can be written as:
where
is the latent heat,
is the liquid fraction and can be expressed as:
The source term
in Equation (10) is formulated as below:
where
is the mushy zone constant and varies between 10
4 and 10
7, according to previous literatures, 10
5 is applied [
43,
44,
45,
46]. The constant
is a small number to prevent division by zero.
For the HTF and PCM, the initial temperature equals to 30 °C:
The inlet temperature and velocity of the HTF complies with Equation (18) as:
A few assumptions were made to simplify the CFD model; the detailed model assumptions are:
The outer surface of the TES unit is adiabatic.
The thermal resistance of the heat exchanger tube is negligible.
The density of the PCM is subjected to the Boussinesq approximation [
43,
44,
45,
46,
47,
48].
The initial temperature of the TES unit is constant and uniform.
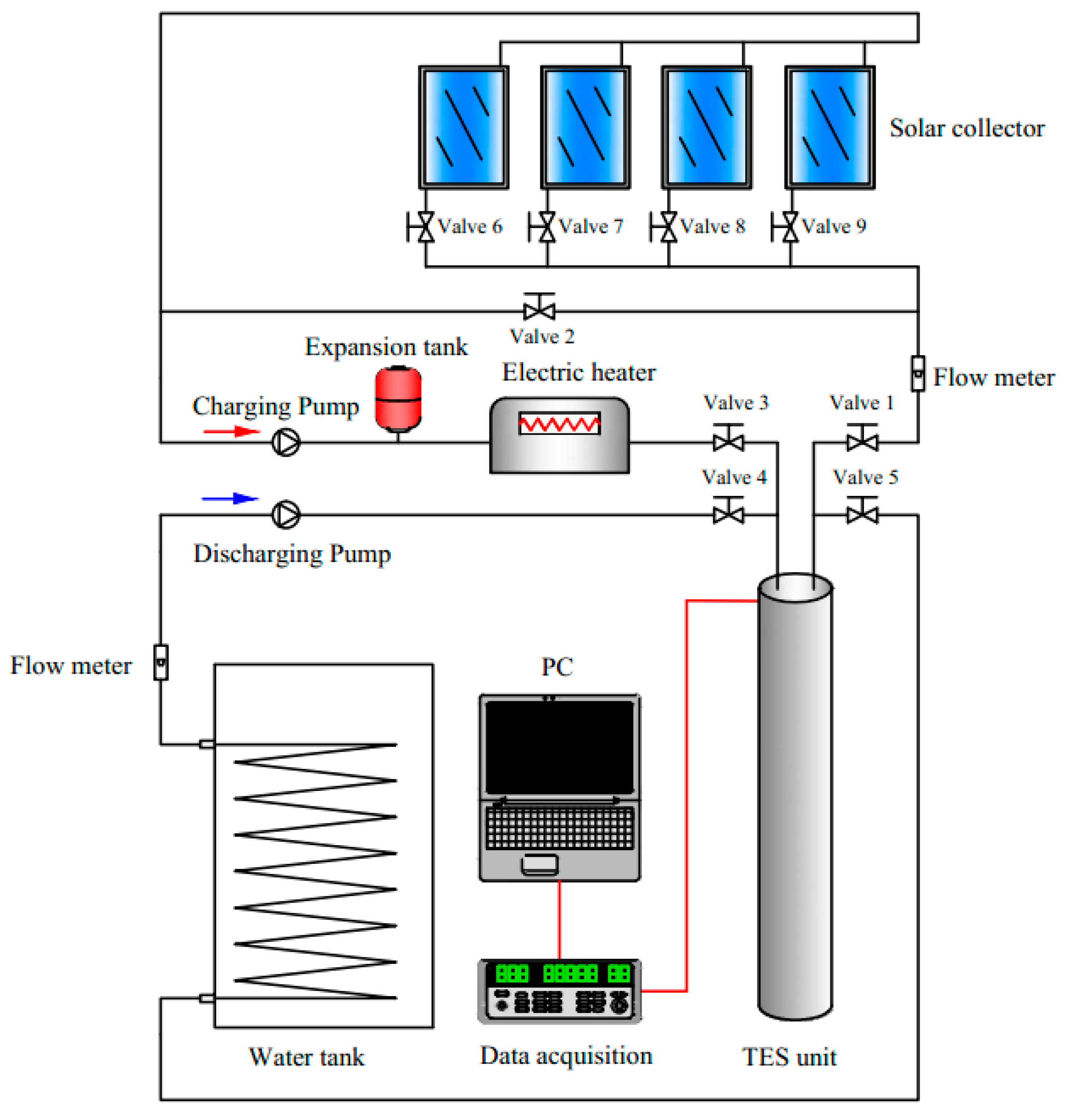
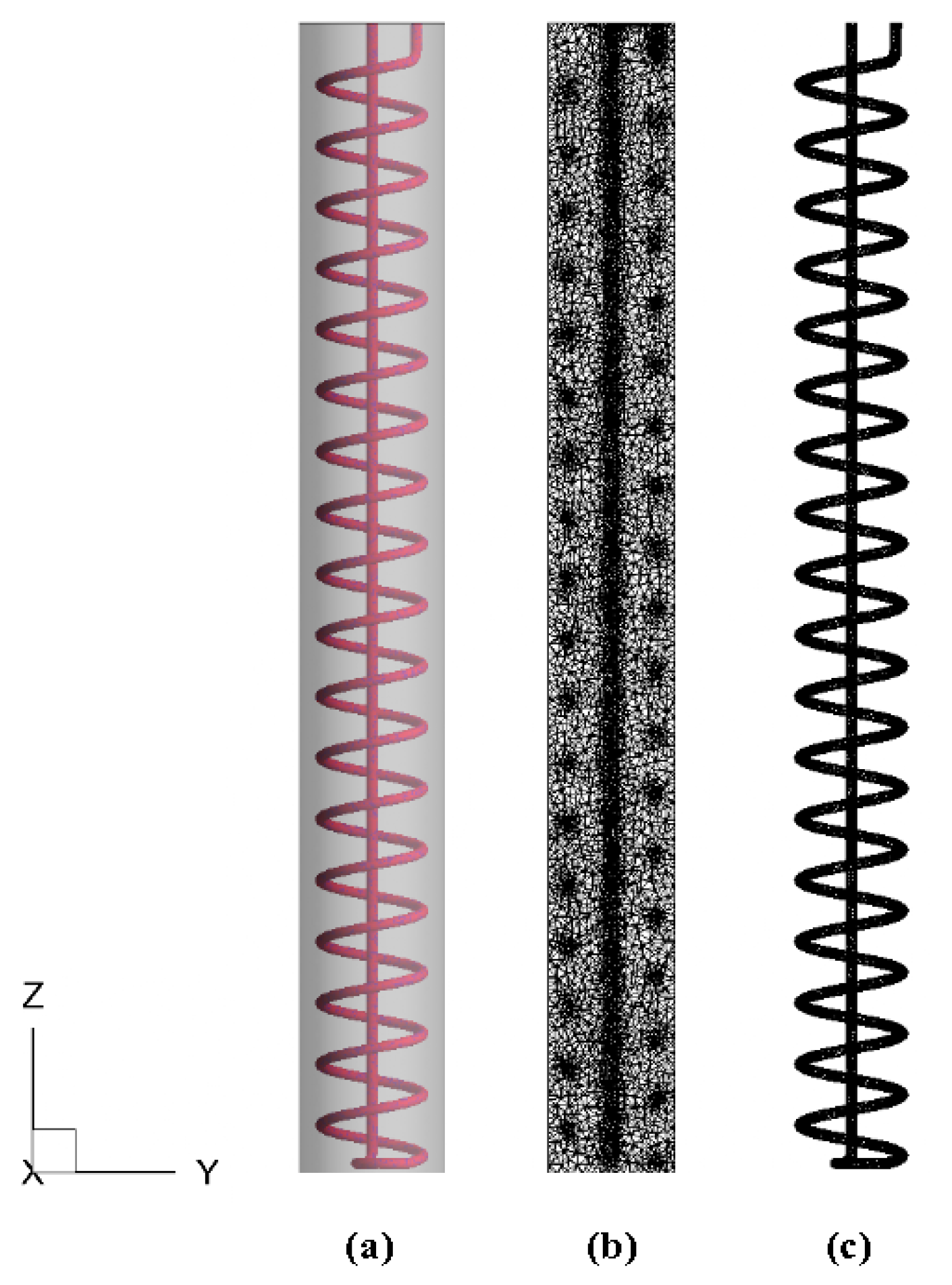
Figure 6 shows the 3D model and mesh results of the TES unit. Fluent 15.0 was used to mesh the TES unit and an unstructured mesh using tetrahedral cells was applied. All governing equations together with the boundary conditions over the whole domain were solved by the commercial CFD code Fluent 15.0. The Semi-Implicit Method for Pressure-Linked Equations (SIMPLE) algorithm was used for the pressure velocity coupling. The Pressure Staggering Option (PRESTO) scheme was applied for the pressure interpolation. The standard
model was adopted to describe the turbulent flow of HTF. The turbulent intensity and turbulent viscosity ratio for the HTF inlet were set as 5% and 10%, respectively. A second-order upwind scheme was adopted for the discretization of the momentum equation, energy equation, turbulent kinetic energy equation and dissipation rate equation. Solution convergence target was set as 10
−7 for the energy equation and 10
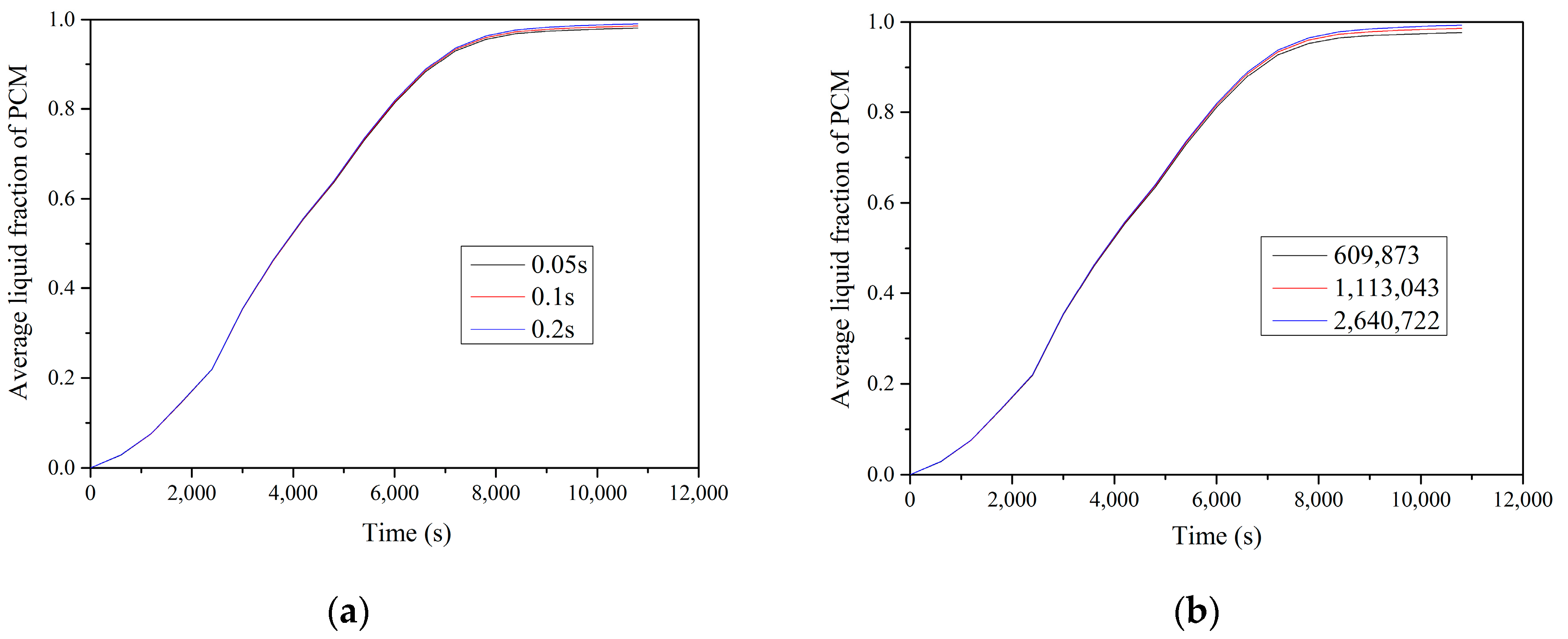
−3 for other variables. Independency tests had been conducted with three different meshes of 609,873, 1,113,043 and 2,640,722 grid elements and three time steps of 0.05 s, 0.1 s and 0.2 s to eliminate the influence from the grid number and time step on the average liquid fraction of PCM (
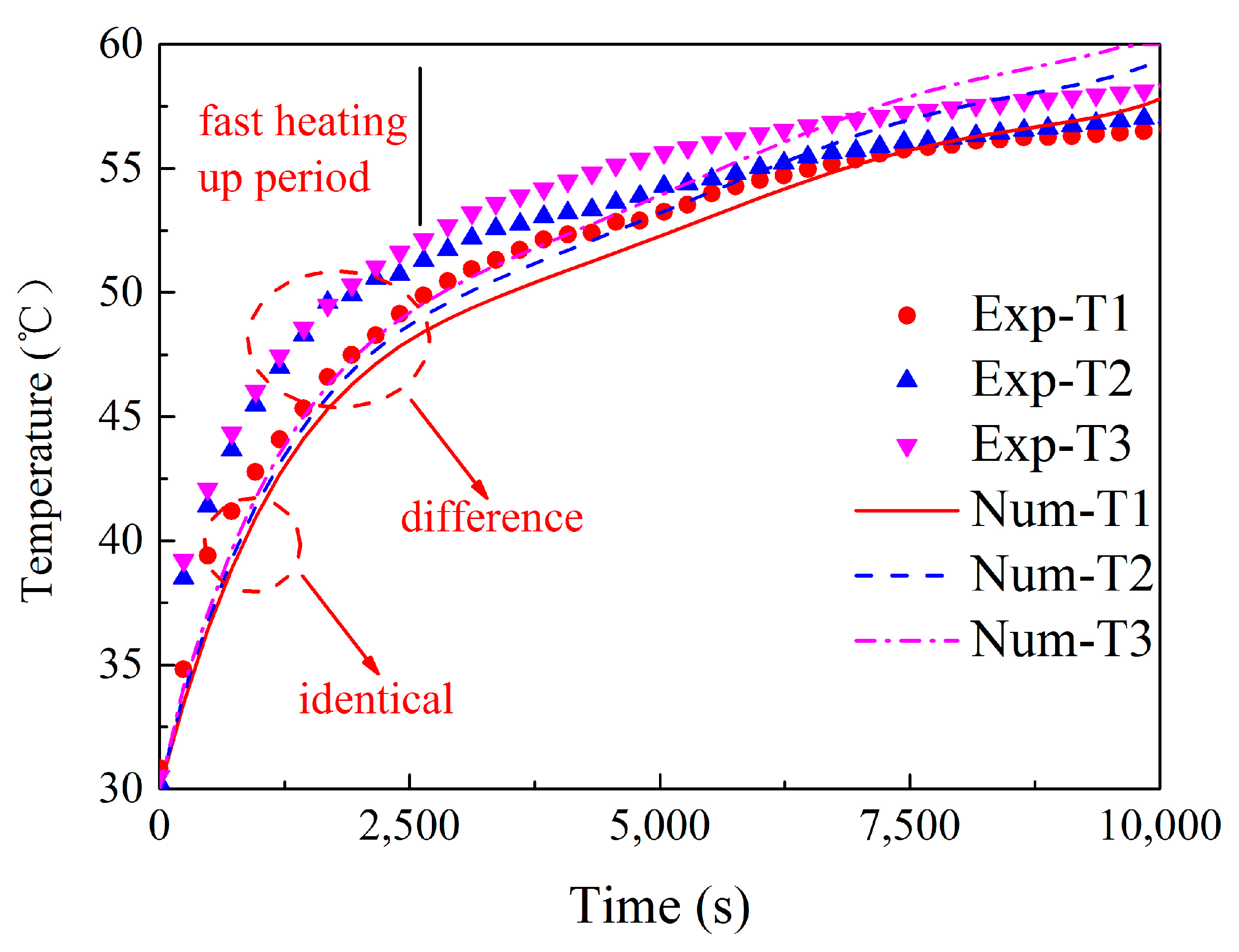
Figure 7). The numerical results did not show significant change as the number of grid elements increased to 2,640,722. In order to compromise the numerical accuracy and computational resource and time, 1,113,043 grid elements and 0.1 s were chosen in the present numerical calculation.
5. Conclusions
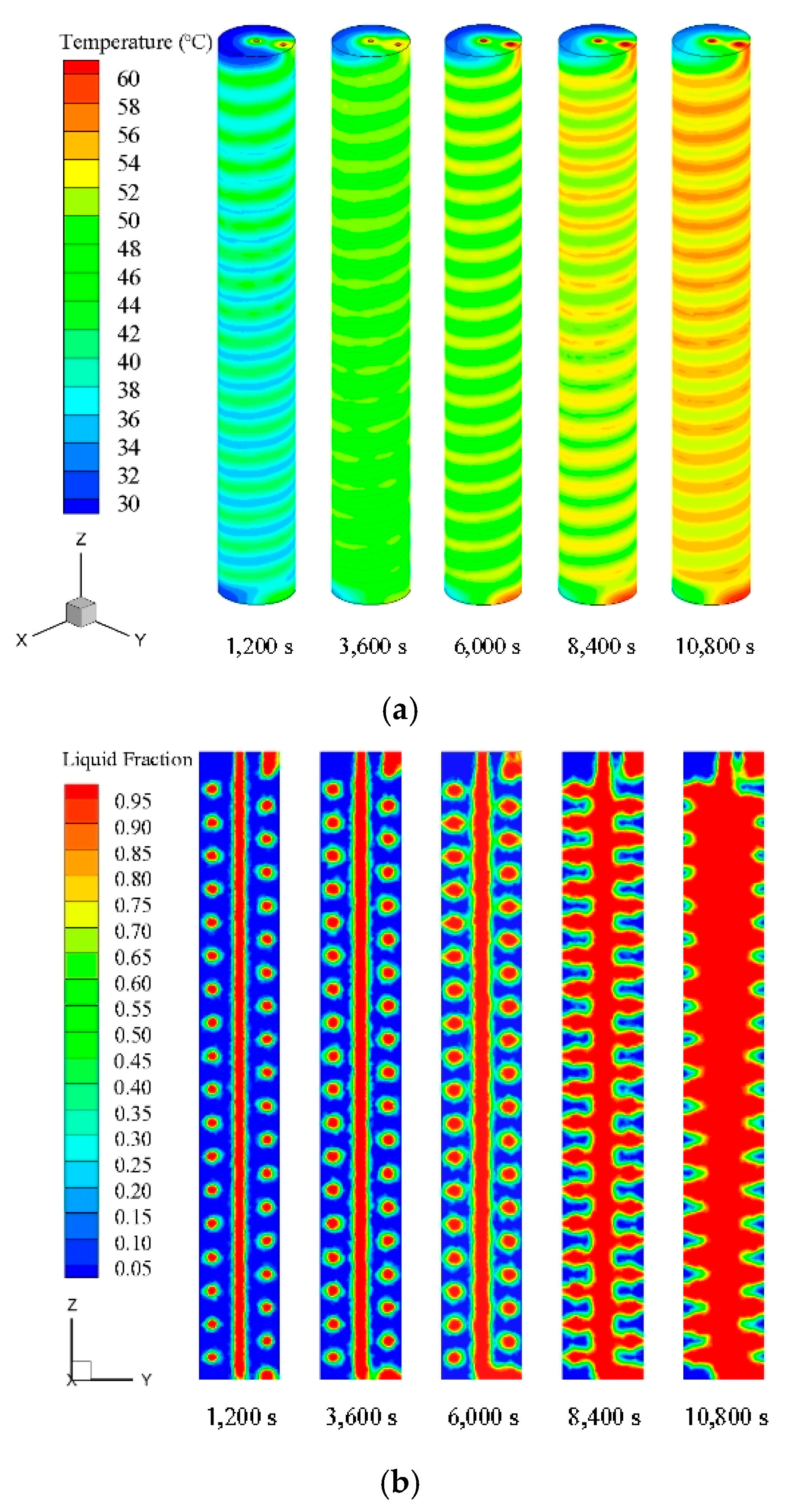
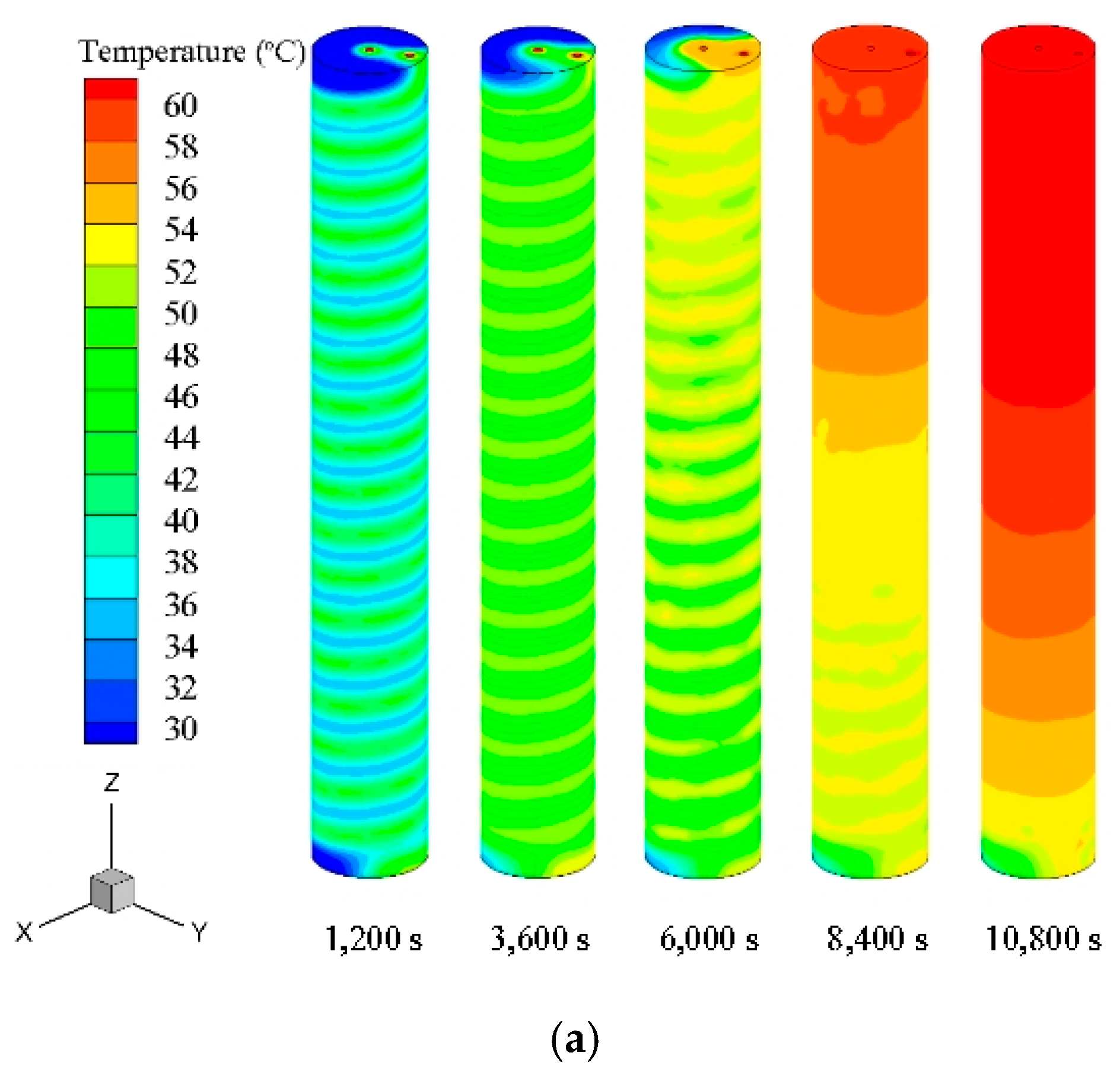
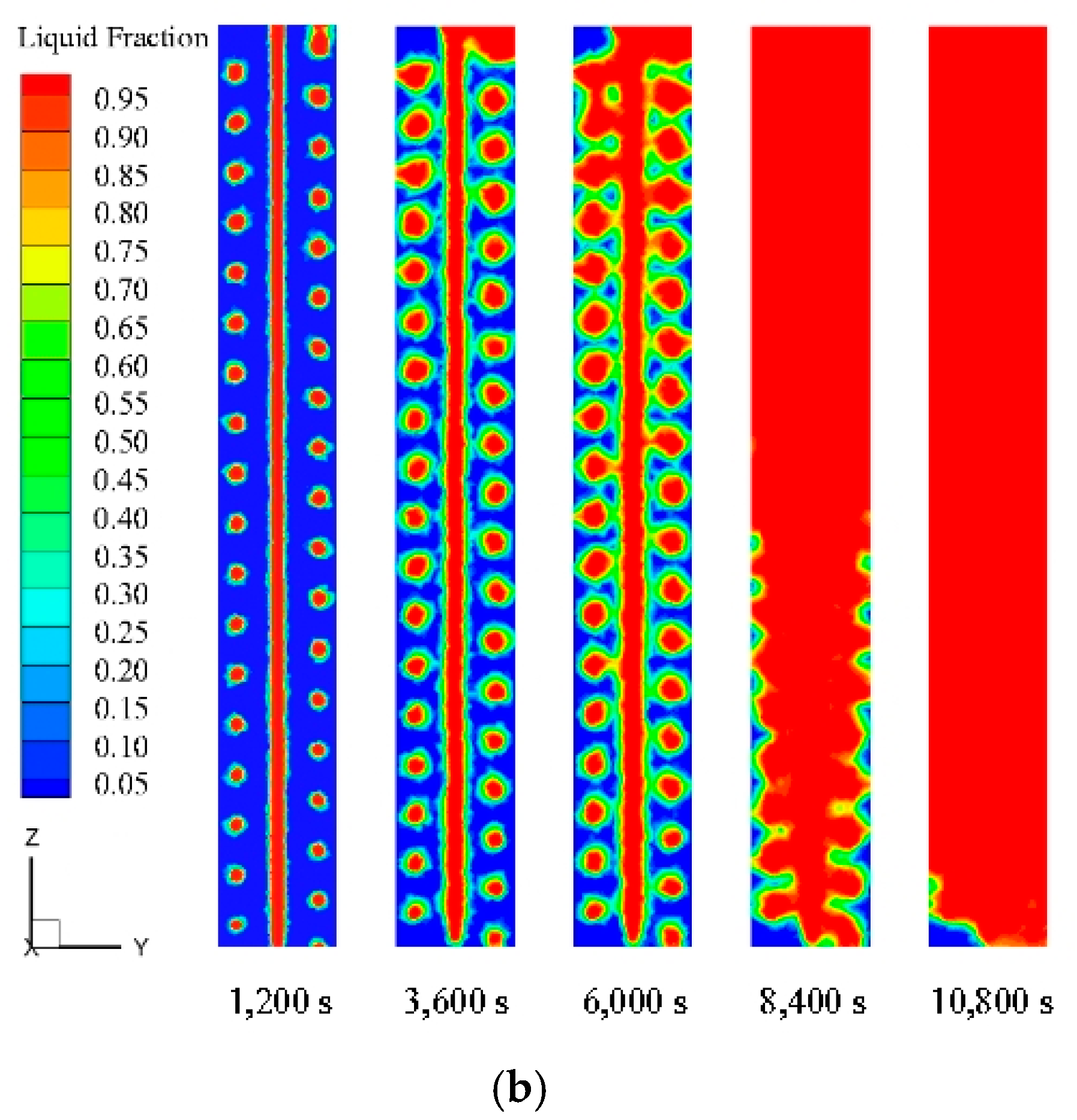
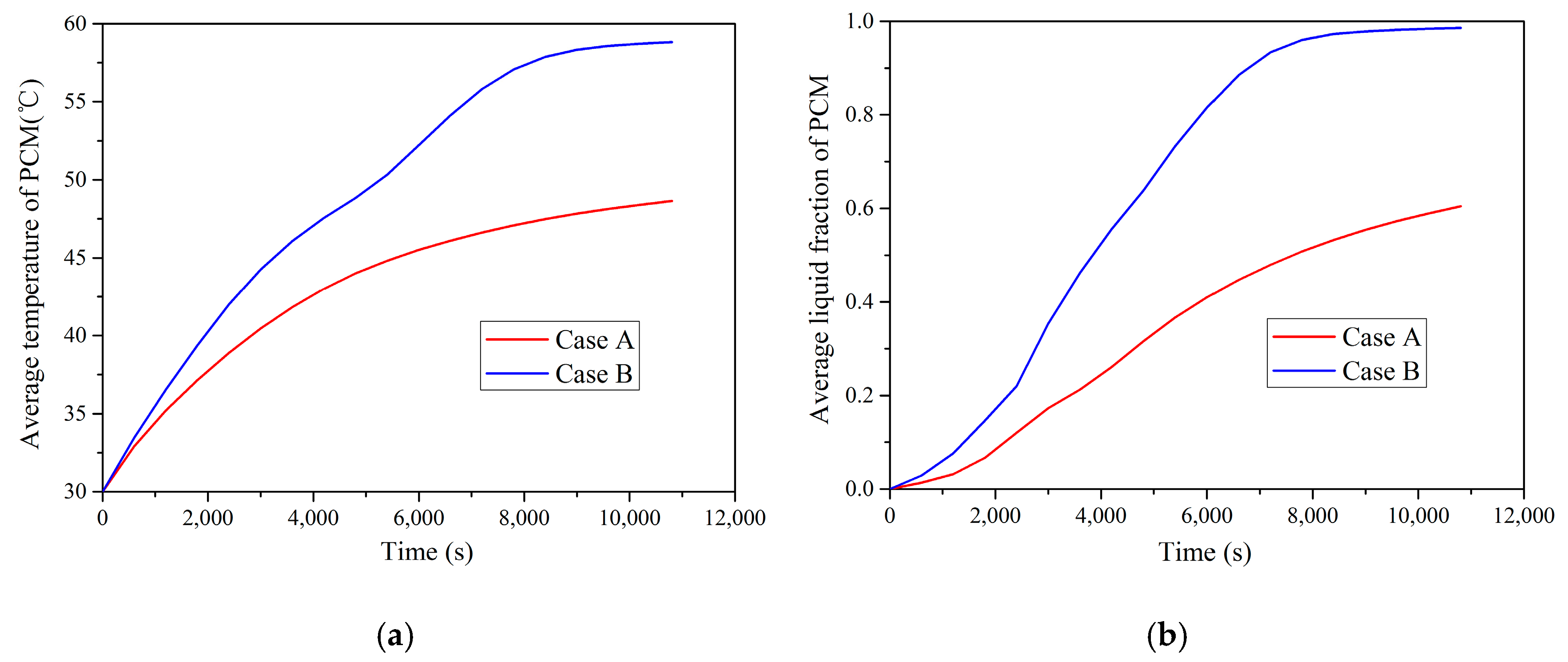
In this study, the melting process of PCM in a TES unit using a helical coil heat exchanger was investigated numerically and experimentally. Based on the numerical simulations, the PCM melting process was revealed and analyzed. The effect of natural convection on the charging process was studied by comparing two cases using different heat transfer mechanisms. The effects of HTF inlet parameters on the charging process were studied by seven cases using different temperatures and flow rate values. The following conclusions can be drawn:
The numerical simulation using a combined conduction and convection heat transfer mechanism showed faster and more complete melting rate than the case using only a conduction mechanism. Therefore, it is significant to consider the buoyancy effect for the dynamic melting process modelling of PCM.
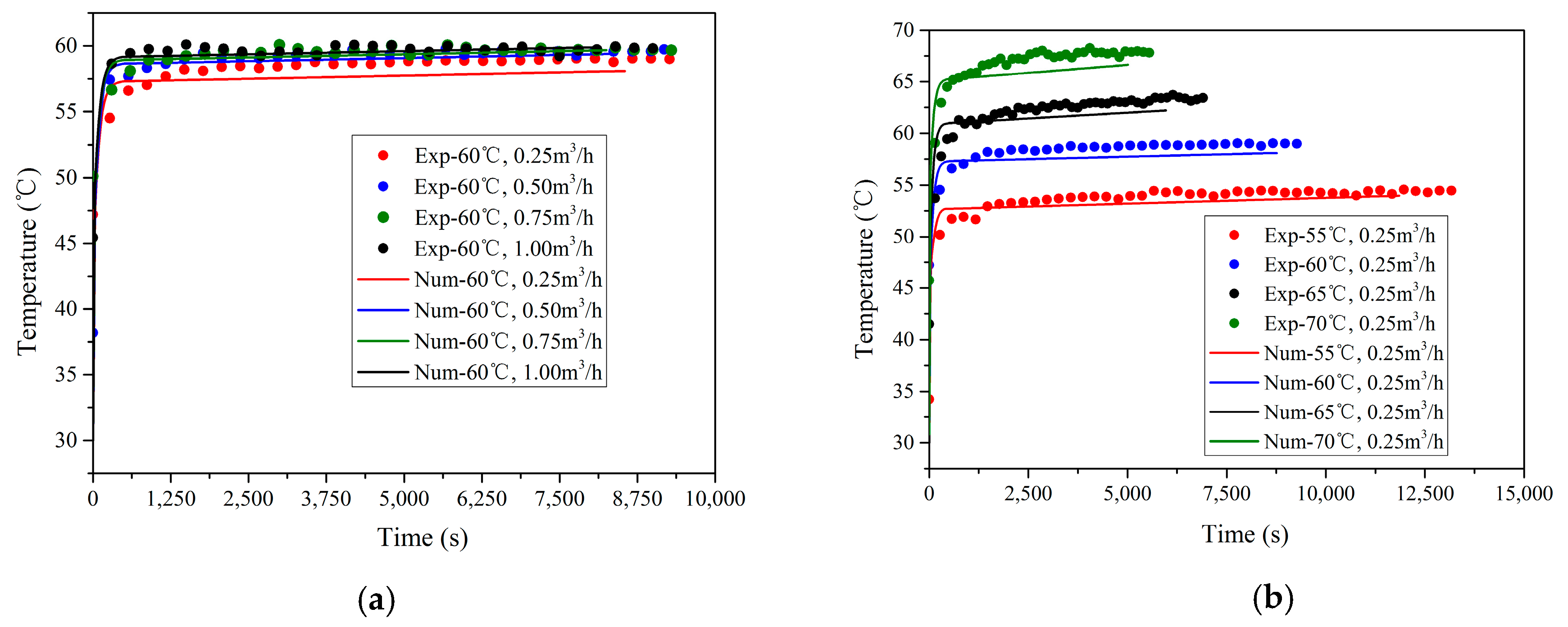
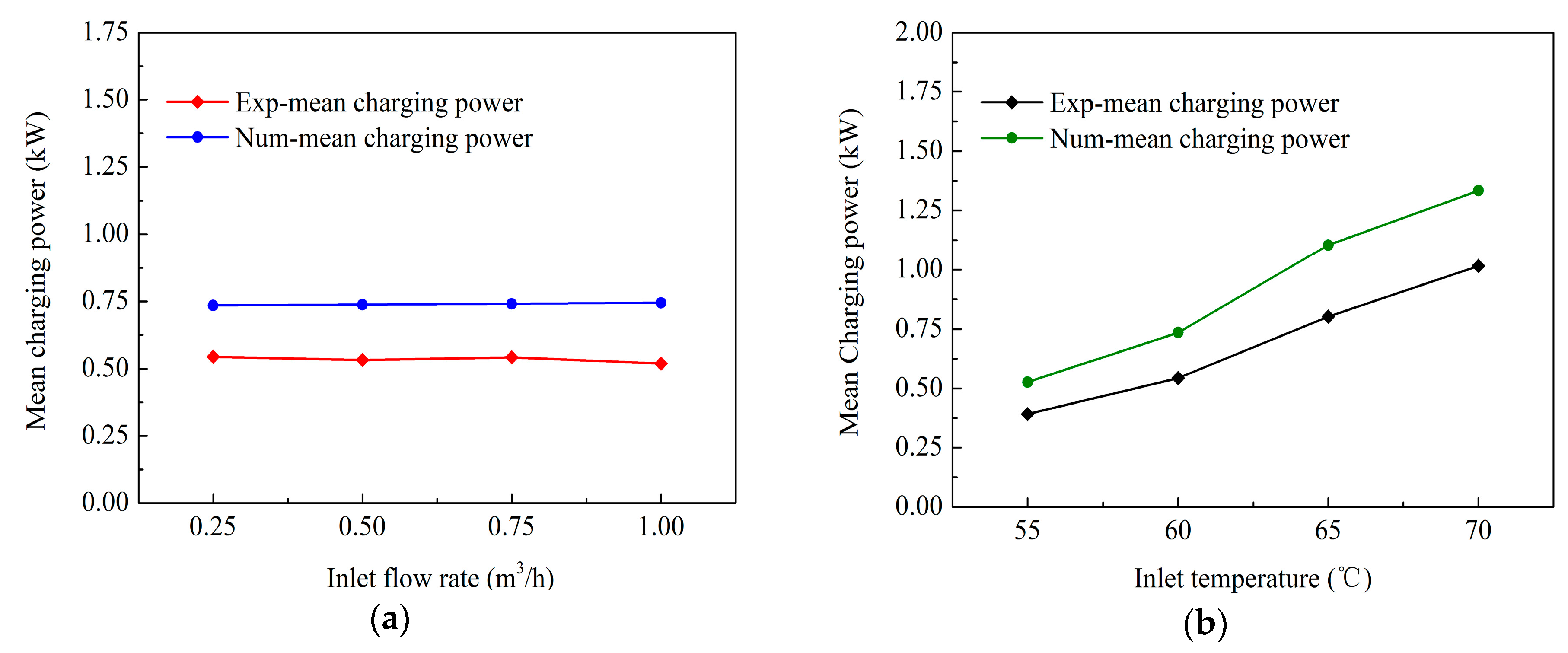
The HTF inlet temperature dominates the heat transfer performance of the TES unit, while the influence of the HTF flow rate is negligible. For a constant HTF inlet temperature, the mean charging power is independent of HTF flow rate variation. In contrast, for a constant flow rate, a 15 °C HTF temperature change from 55 °C to 70 °C increases the mean charging power by 2.5 times.
Based on the results obtained from the present study, the HTF inlet temperature range between 60 °C and 65 °C causes a rapid mean charging power increase with the highest gradient, which indicates that the TES unit is compatible with regular heat sources, including solar collectors and heat pump systems.
The present study focused on the PCM melting process, which is directly related to the heat source. However, for heat sources such as solar collectors, the HTF inlet boundary conditions usually change with time, inducing a variable inlet boundary condition profile. Therefore, further investigations should be carried out to test the system TES performance under variable inlet boundary conditions. Further more, solidification pocess and melting process are equally important in the TES performance. The solidification process of TES unit is a key focus for the heat utlization and should be examined in subsequent studies.