Effect of Pristine Palygorskite Powders on Explosion Characteristics of Methane-Air Premixed Gas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
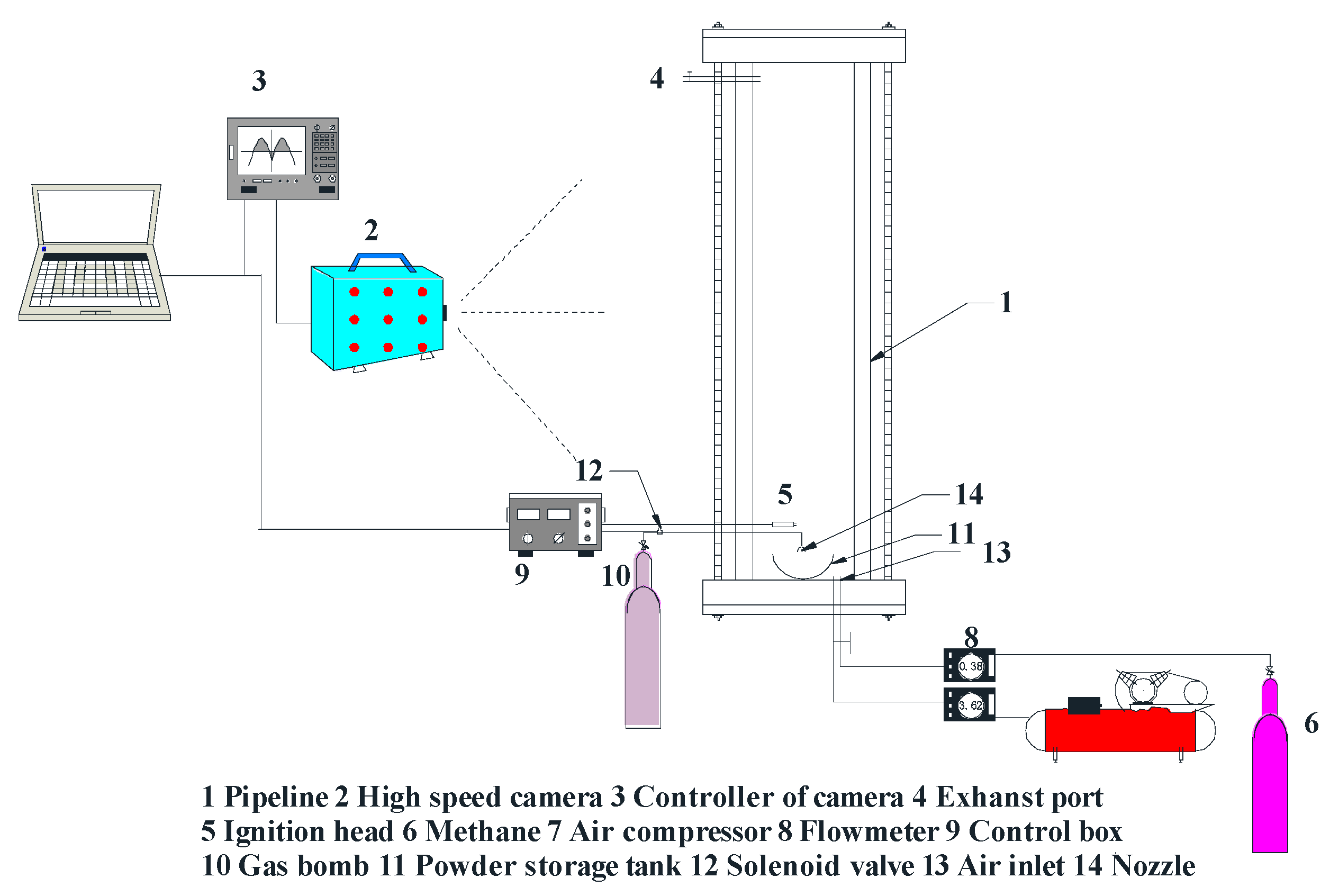
2.3. Explosion Experiment Device and test Process
3. Results and Discussion
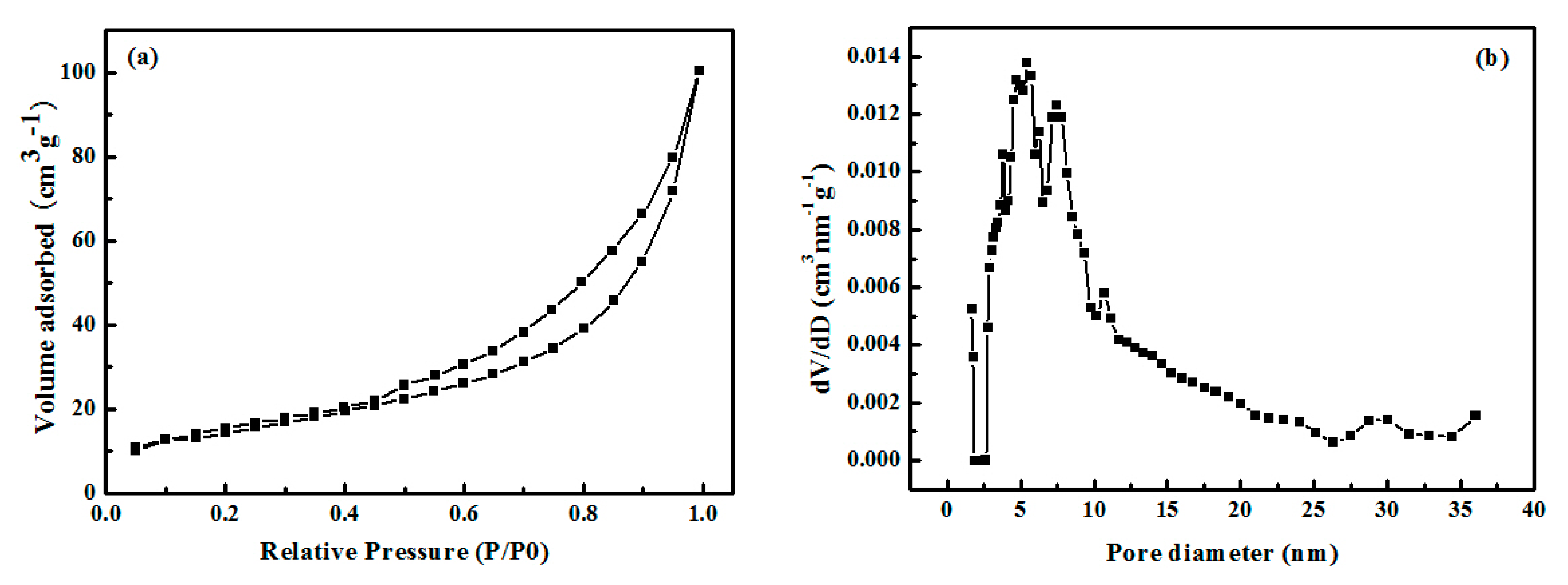
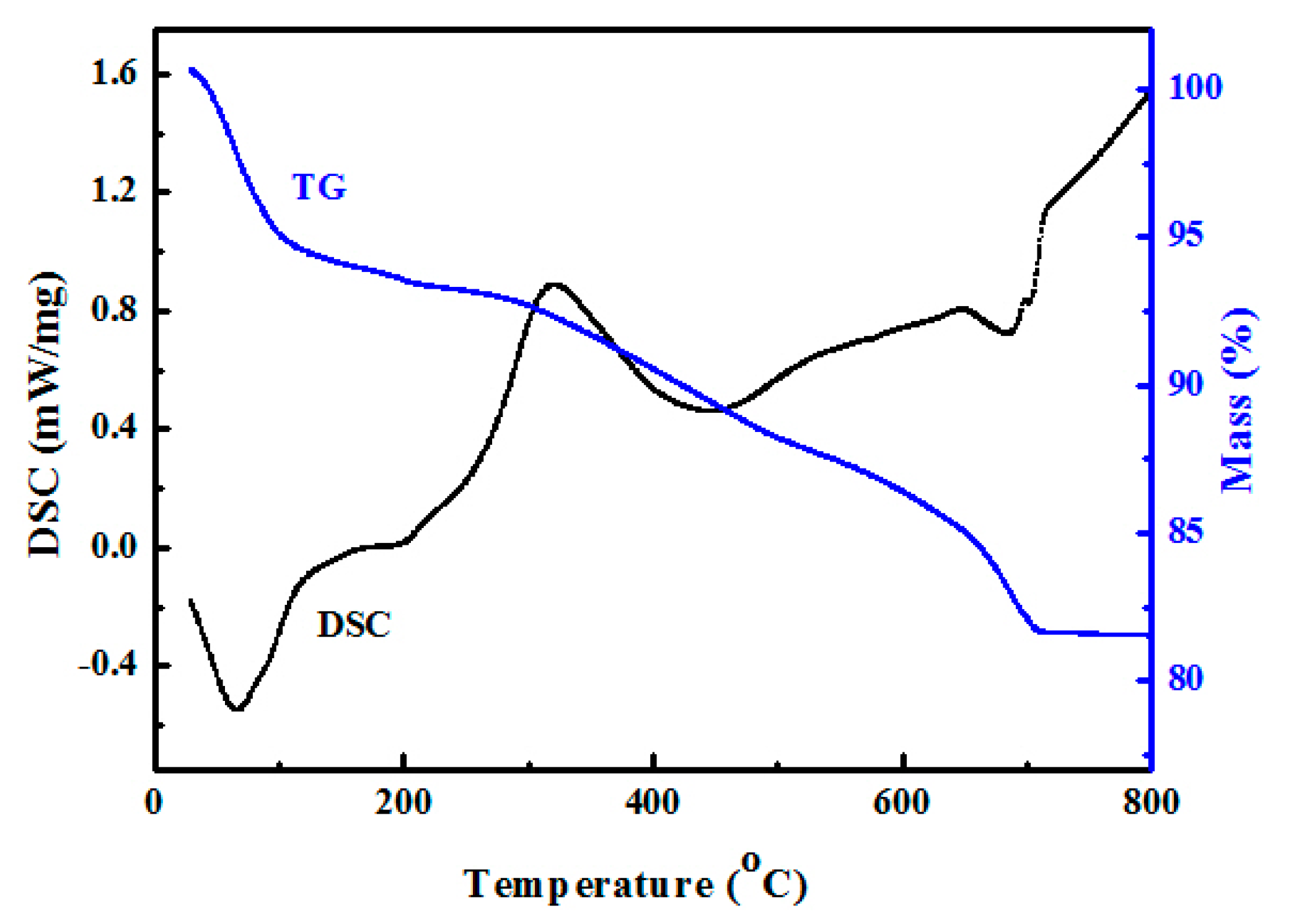
3.1. Characterization of Palygorskite Powders
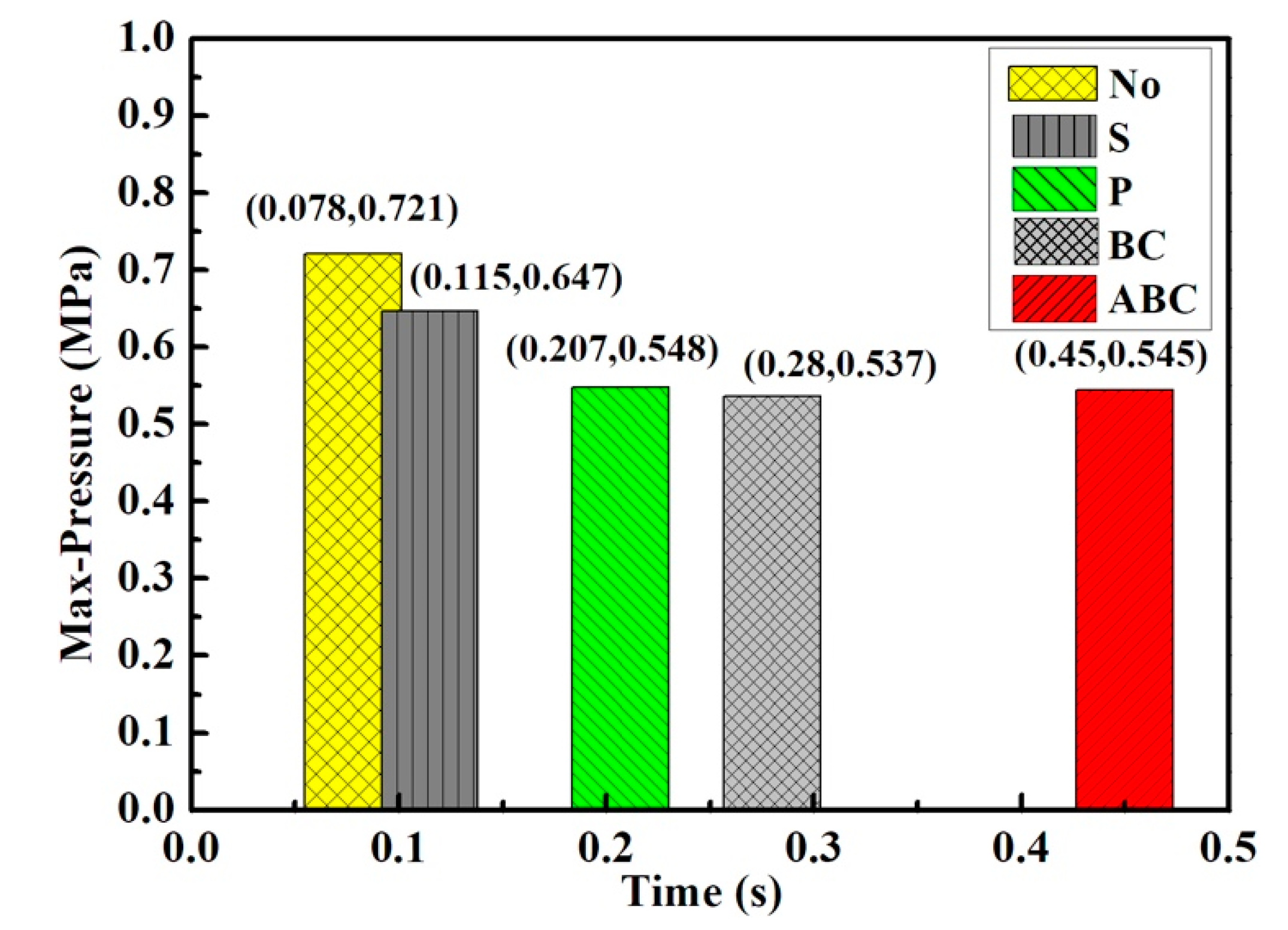
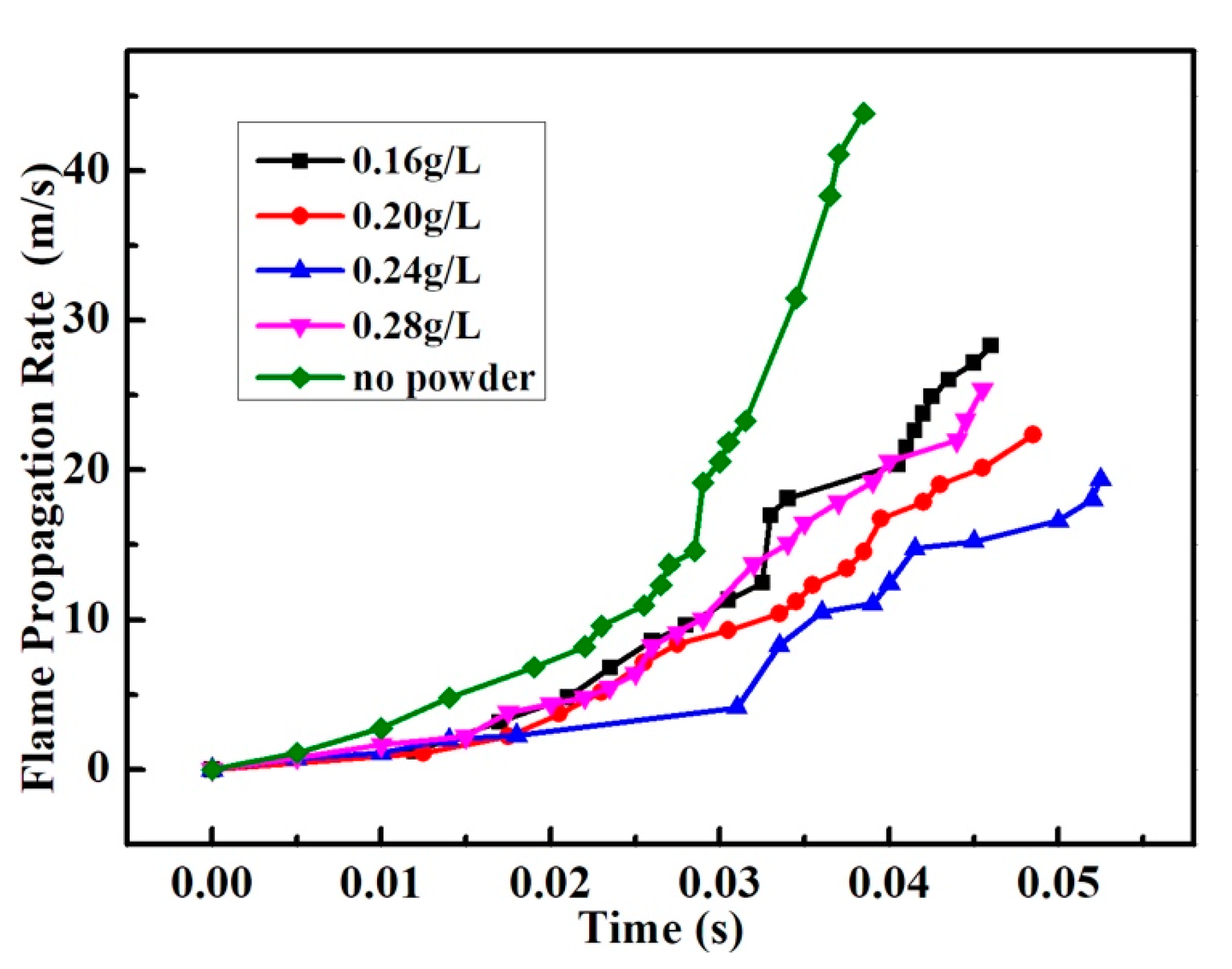
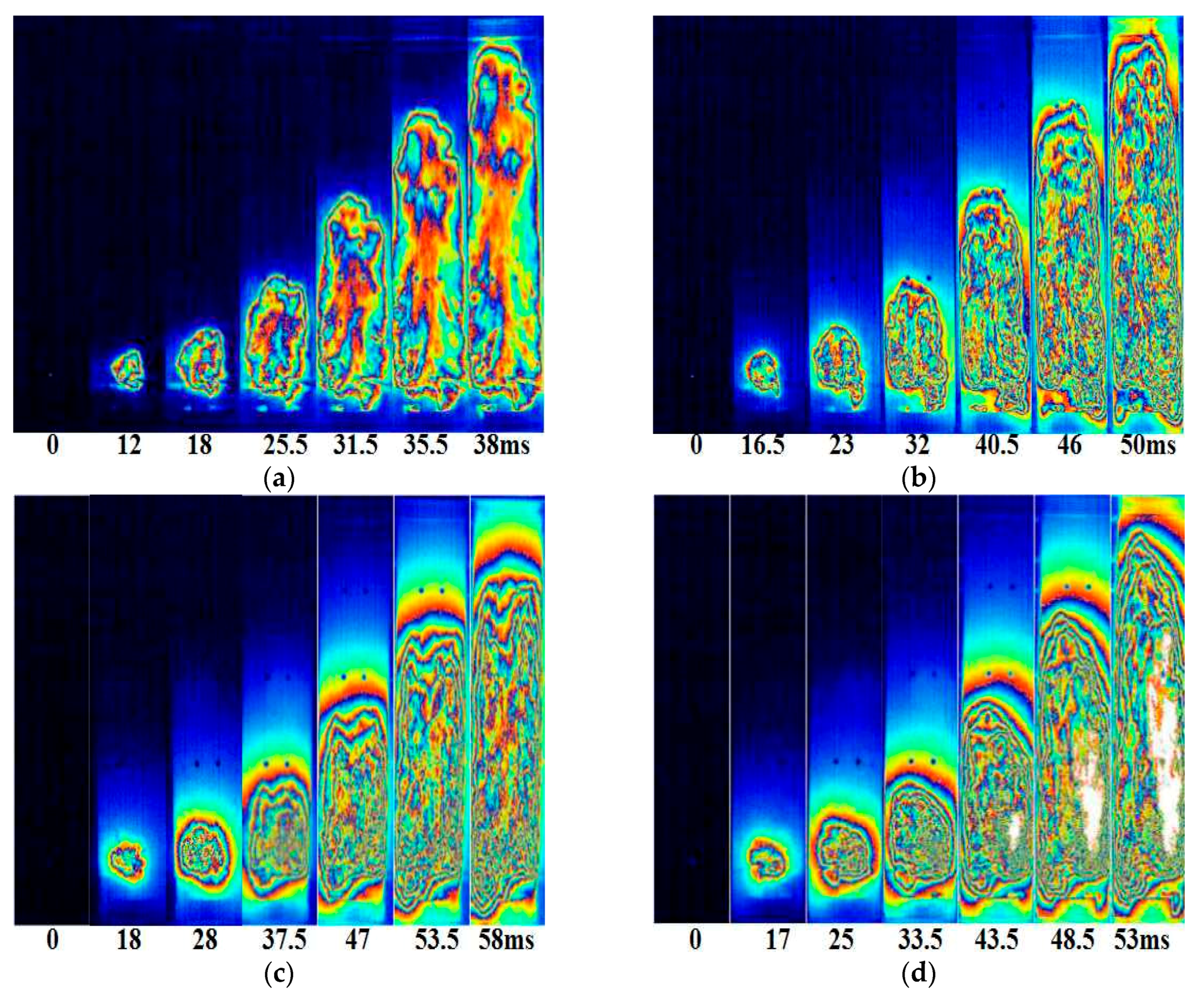
3.2. Suppression Properties of Palygorskite Powders
3.3. Suppression Mechanism of Palygorskite Powders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Z.H.; Fan, B.C.; Jiang, X.H. Suppression effects of powder suppressants on the explosions of oxyhydrogen gas. J. Loss Prev. Process Ind. 2006, 19, 648–655. [Google Scholar] [CrossRef]
- Krasnyansky, M. Prevention and suppression of explosions in gas-air and dust-air mixtures using powder aerosol-inhibitor. J. Loss Prev. Process Ind. 2006, 19, 729–735. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Shmakov, A.G.; Shvartsberg, V.M.; Chernov, A.A.; Yakimov, S.A.; Koutsenogii, K.P.; Makarov, V.I. Fire suppression by low-volatile chemically active fire suppressants using aerosol technology. Fire Saf. J. 2012, 51, 102–109. [Google Scholar] [CrossRef]
- Pan, R.K.; Xiao, Z.J.; Yu, M.G. The characteristics of methane combustion suppression by water mist and its engineering applications. Energies 2017, 10, 1566. [Google Scholar] [CrossRef]
- Wang, Z.R.; Ni, L.; Liu, X.; Jiang, J.C.; Wang, R. Effects of N2/CO2 on explosion characteristics of methane and air mixture. J. Loss Prev. Process Ind. 2014, 31, 10–15. [Google Scholar] [CrossRef]
- Almerinda, D.B.; Francesco, C.; Valeria, D.S.; Ernesto, S. Effect of Diluents on Rapid Phase Transition of Water Induced by Combustion. AIChE J. 2012, 58, 2810–2819. [Google Scholar]
- Liu, Q.M.; Hu, Y.L.; Bai, C.H.; Chen, M. Methane/coal dust/air explosions and their suppression by solid particle suppressing agents in a large-scale experimental tube. J. Loss Prev. Process Ind. 2013, 26, 310–316. [Google Scholar] [CrossRef]
- Luo, Z.M.; Wang, T.; Ren, J.Y.; Deng, J.; Shu, C.M.; Huang, A.Q.; Cheng, F.M.; Wen, Z.Y. Effects of ammonia on the explosion and flame propagation characteristics of methane-air mixtures. J. Loss Prev. Process Ind. 2017, 47, 120–128. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, H.M.; Chen, X.; Liu, X.Y.; Niu, Y.; Zhang, Y.; Yuan, B.H. Effect of dust explosion suppression by sodium bicarbonate with different granulometric distribution. J. Loss Prev. Process Ind. 2017, 49, 905–911. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, Y.; Meng, X.Q.; Zheng, L.G.; Gao, J.L. The suppression characteristics of NH4H2PO4/red mud composite powders on methane explosion. Appl. Sci. 2018, 8, 1433. [Google Scholar] [CrossRef]
- Wang, Q.H.; Wen, H.; Wang, Q.S.; Sun, J.H. Inhibiting effect of Al(OH)3 and Mg(OH)2 dust on the explosions of methane-air mixtures in closed vessel. Sci. China-Technol. Sci. 2012, 55, 1371–1375. [Google Scholar] [CrossRef]
- Zheng, L.G.; Li, G.; Wang, Y.L.; Zhu, X.C.; Pan, R.K.; Wang, Y. Effect of blockage ratios on the characteristics of methane/air explosion suppressed by BC powder. J. Hazard. Mater. 2018, 355, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, Y.S.; Yu, M.G.; Li, Y.; Cao, J.L.; Zheng, L.G.; Yi, H.W. Methane explosion suppression characteristics based on the NaHCO3/red-mud composite powders with core-shell structure. J. Hazard. Mater. 2017, 335, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, Y.S.; Cao, J.L.; Zheng, L.G.; Yi, H.W.; Yu, M.G. Suppression characteristics of KHCO3/red-mud composite powders with core-shell structure on methane explosion. J. China Coal. Soc. 2017, 42, 653–658. [Google Scholar]
- Koshiba, Y.; Takahashi, Y.; Ohtani, H. Flame suppression ability of metallocenes (nickelocene, cobaltcene, ferrocene, manganocene, and chromicene). Fire Saf. J. 2012, 51, 10–17. [Google Scholar] [CrossRef]
- Luo, Z.M.; Cheng, F.M.; Wang, T.; Deng, J.; Shu, C.M. Suppressive effects of silicon dioxide and diatomite powder aerosols on coal mine gas explosions in highlands. Aerosol. Air. Qual. Res. 2016, 16, 2119–2128. [Google Scholar] [CrossRef]
- Cheng, W.M.; Hu, X.M.; Xie, J.; Zhao, Y.Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Liang, S.; Lin, Y.J.; Du, Q.G.; Wei, Z.; Yang, Y.L. Preparation and rheology of polyamide-6/attapulgite nanocomposites and studies on their percolated structure. Polymer 2005, 46, 5758–5766. [Google Scholar]
- He, L.; Zhou, Y.K.; Yan, J.W.; Yang, L. Modification and Engineering Application of Palygorskite and Its Micro-Nano Materials; National Defense Industry Press: Beijing, China, 2012. [Google Scholar]
- Cao, J.L.; Shao, G.S.; Wang, Y.; Liu, Y.P.; Yuan, Z.Y. CuO catalysts supported on attapulgite clay for low-temperature CO oxidation. Catal. Commun. 2008, 9, 2555–2559. [Google Scholar] [CrossRef]
- Pan, M.; Lin, X.M.; Xie, J.J.; Huang, X.M. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. RSC Adv. 2017, 7, 4492–4500. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.Q.; Wang, W.B.; Zheng, Y.A.; Zheng, M.S.; Liu, X.Q. The Dissociation of Attapulgite and Its Nano-Functional Composites; Science Press: Beijing, China, 2014; pp. 62–95. [Google Scholar]
- Zheng, M.S.; Wang, A.Q.; Zhan, G.S. Study on Application of Attapulgite Caly; Chemical Industry Press: Beijing, China, 2007; pp. 2–3. [Google Scholar]
- Di Sarli, V.; Russo, P.; Sanchirico, R.; Di Benedetto, A. CFD Simulations of the Effect of Dust Diameter on the Dispersion in the 20 L Bomb. Chem. Eng. Trans. 2013, 31, 727–732. [Google Scholar]
- Ranganathan, S.; Rockwell, S.R.; Petrow, D.; Zalosh, R.; Rangwala, A.S. Radiative fraction of dust entrained turbulent premixed flames. J. Loss Prev. Process Ind. 2018, 51, 65–71. [Google Scholar] [CrossRef]
- Wen, H.; Cao, W.; Wang, K.K.; Cheng, F.M. Expermental study on ABC dry powder to repress gas explosion. J. Saf. Sci. Technol. 2010, 7, 9–12. [Google Scholar]
- Krasnyansky, M. Studies of fundamental physical–chemical mechanisms and processes of flame extinguishing by powder aerosols. Fire Mater. 2008, 32, 27–47. [Google Scholar] [CrossRef]
- Ni, X.M.; Zheng, Z.; Wang, X.S.; Zhang, S.G.; Zhao, M. Fabrication of hierarchical zeolite 4A microspheres with improved adsorption capacity to bromofluoropropene and their fire suppression performance. J. Alloys Compd. 2014, 592, 135–139. [Google Scholar] [CrossRef]
- Wang, C.H.; Auad, M.L.; Marcovich, N.E.; Nutt, S. Synthesis and characterization of organically modified attapulgite/polyurethane nanocomposites. J. Appl. Polym. Sci. 2008, 109, 2562–2570. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, L.L.; Ren, Q.; Li, J.C.; Ding, Y.H. Study on HDPE flame retarded with attapulgite compound intumescent flame retardanty. Plast. Technol. 2012, 40, 37–41. [Google Scholar]
- Ni, X.M.; Kuang, K.Q.; Yang, D.L.; Liao, G.X. Experimental study on the suppression of methane/air diffusion flame by iron-modified zeolite powders. China Saf. Sci. J. 2012, 22, 53–57. [Google Scholar]
- Liu, J.H.; Cong, B.H. Experimental evaluation of water mist with metal chloride additives for suppressing CH4/Air cup-burner flames. J. Thermal Sci. 2013, 22, 269–274. [Google Scholar] [CrossRef]
- Ni, X.M.; Wang, X.S.; Zhang, S.G.; Zhao, M. Experimental study on the performance of transition metal ions modified zeolite particles in suppressing methane/air coflowing flame on cup burner. J. Fire Sci. 2014, 32, 417–430. [Google Scholar] [CrossRef]




| Sample | Concentration (g·L−1) | Max-Pressure (MPa) | The Time of Pressure Peak (s) | The rate of Max-Pressure Rise (MPa·s−1) | The ratio of Max-Pressure Drop (%) |
|---|---|---|---|---|---|
| No powders | 0 | 0.721 | 0.078 | 37.471 | 0 |
| Palygorskite | 0.12 | 0.603 | 0.153 | 12.912 | 16.3 |
| Palygorskite | 0.14 | 0.594 | 0.169 | 12.243 | 17.6 |
| Palygorskite | 0.16 | 0.571 | 0.191 | 10.664 | 20.8 |
| Palygorskite | 0.18 | 0.545 | 0.200 | 7.425 | 25.1 |
| Palygorskite | 0.20 | 0.548 | 0.207 | 7.152 | 23.9 |
| Palygorskite | 0.22 | 0.598 | 0.153 | 13.743 | 17.1 |
| Palygorskite | 0.24 | 0.617 | 0.159 | 15.121 | 14.4 |
| Silica | 0.20 | 0.647 | 0.115 | 23.408 | 10.2 |
| BC | 0.10 | 0.537 | 0.28 | 5.08 | 28.3 |
| ABC | 0.10 | 0.545 | 0.45 | 2.99 | 25.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Zheng, L.; Yang, T.; Gao, J.; Li, Z. Effect of Pristine Palygorskite Powders on Explosion Characteristics of Methane-Air Premixed Gas. Energies 2018, 11, 2496. https://doi.org/10.3390/en11102496
Zhang Y, Wang Y, Zheng L, Yang T, Gao J, Li Z. Effect of Pristine Palygorskite Powders on Explosion Characteristics of Methane-Air Premixed Gas. Energies. 2018; 11(10):2496. https://doi.org/10.3390/en11102496
Chicago/Turabian StyleZhang, Yimin, Yan Wang, Ligang Zheng, Tao Yang, Jianliang Gao, and Zhenhua Li. 2018. "Effect of Pristine Palygorskite Powders on Explosion Characteristics of Methane-Air Premixed Gas" Energies 11, no. 10: 2496. https://doi.org/10.3390/en11102496





