Experimental Investigation on Microscopic Residual Oil Distribution During CO2 Huff-and-Puff Process in Tight Oil Reservoirs
Abstract
:1. Introduction
2. Experimental Setup
2.1. Materials
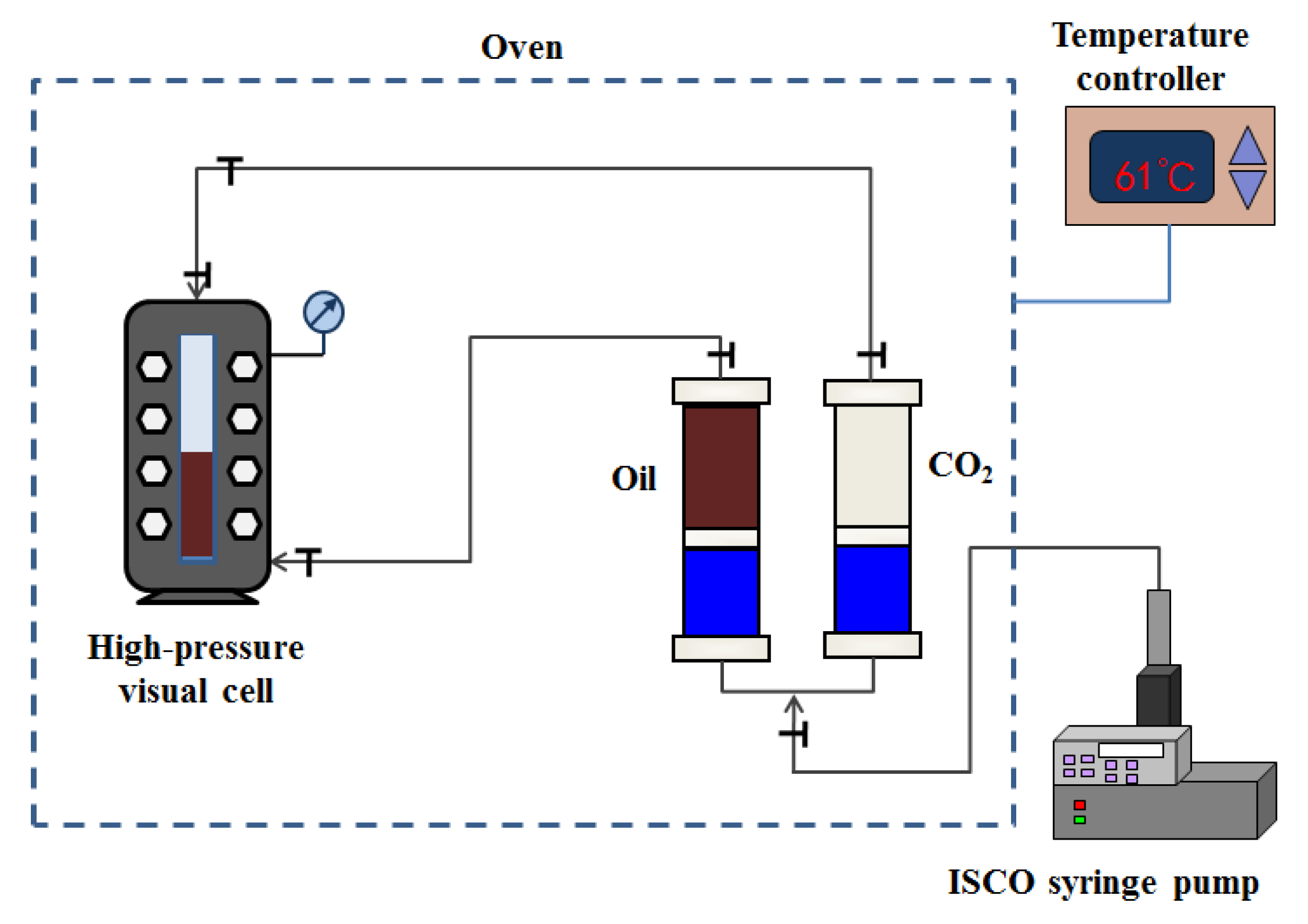
2.2. CO2 Solubility and Oil Swelling Factor Measurements
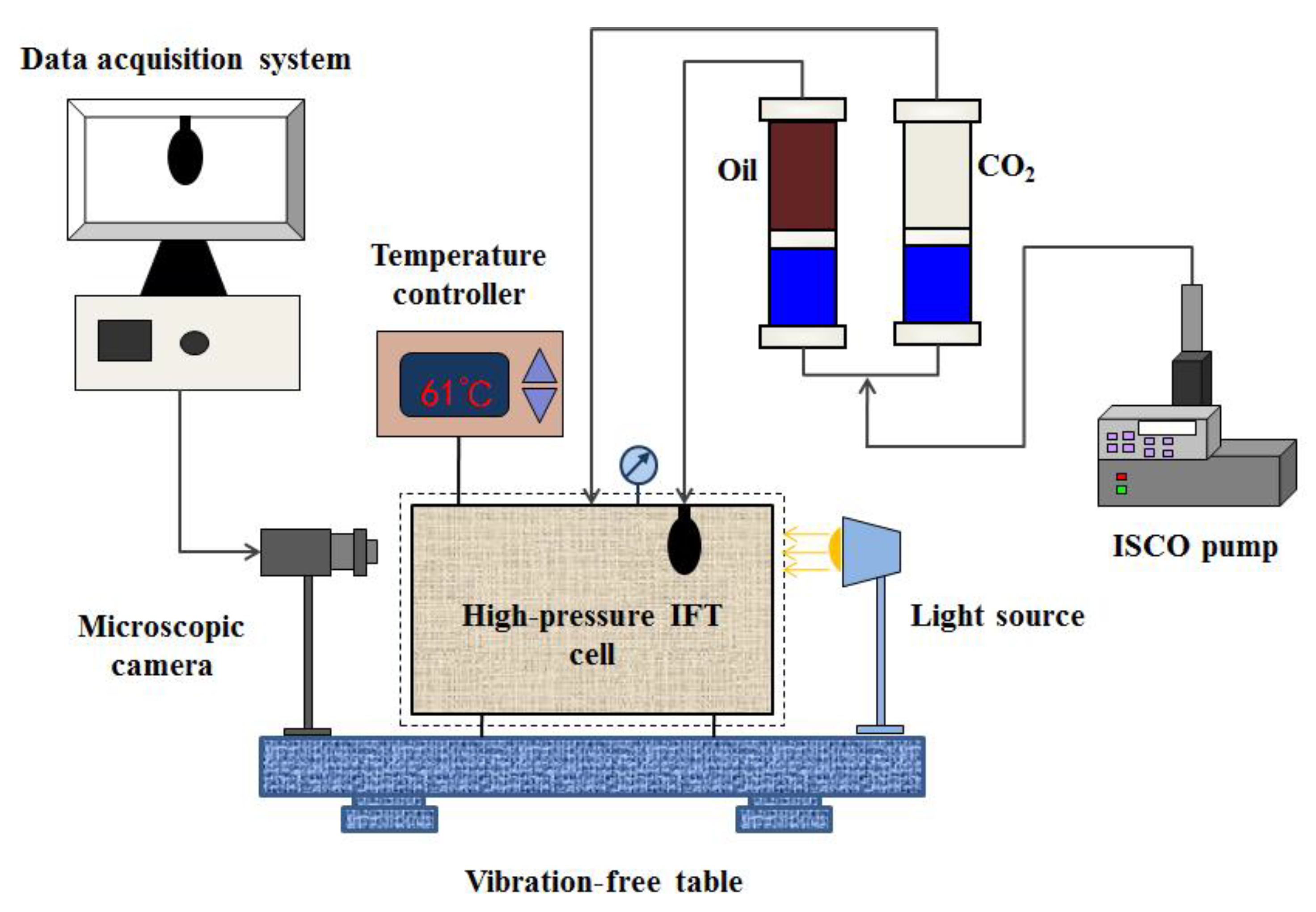
2.3. CO2-oil IFT Measurements
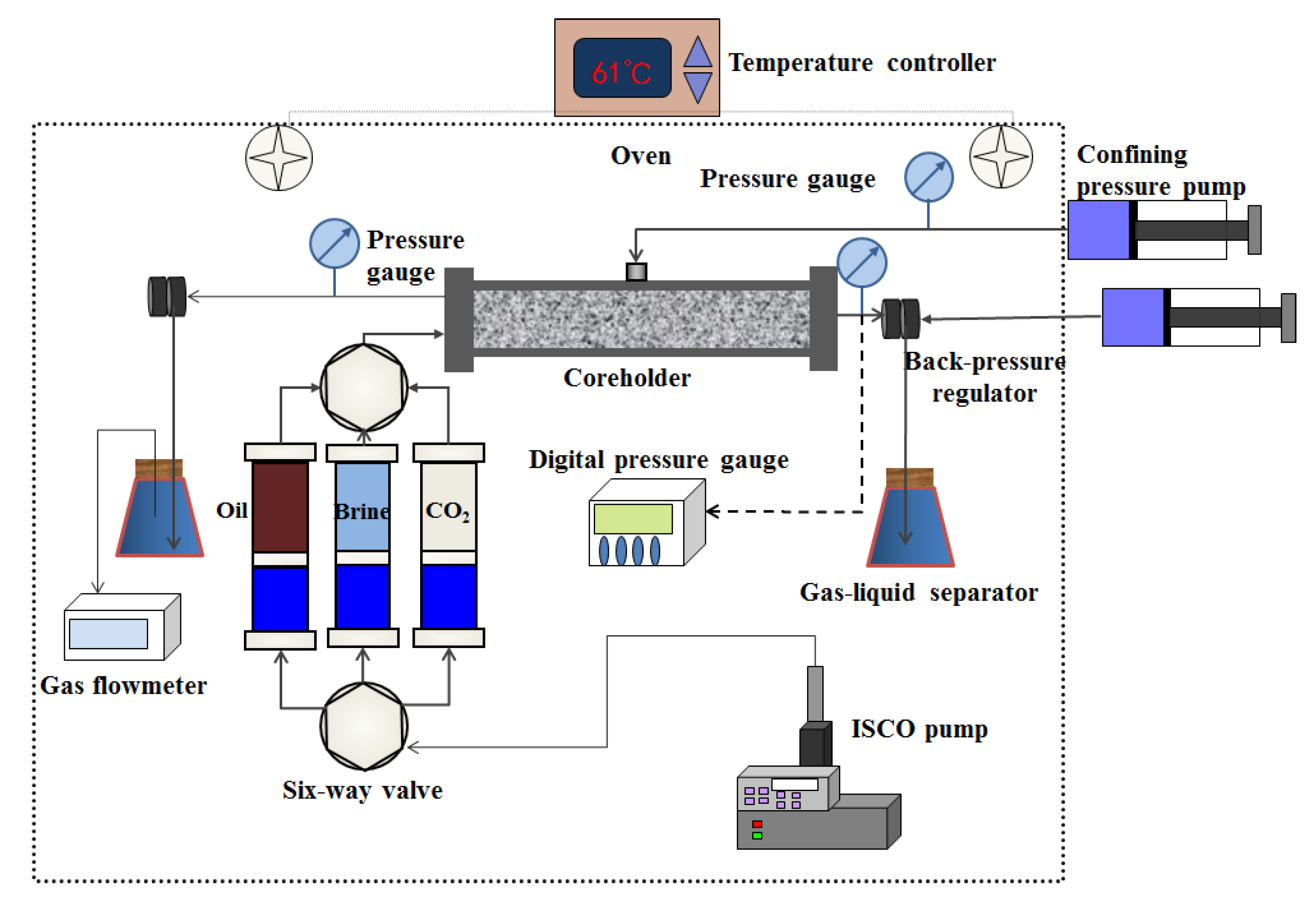
2.4. CO2 Huff-and-Puff Tests
- (1)
- Prior to each test, the core plugs were thoroughly cleaned using a Dean-Stark extractor (SXT-02, Shanghai Pingxuan Scientific Instrument Co., Ltd., Shanghai, China) for 20–30 days. After the core plugs were cleaned and dried at 100 °C. The gas permeability and porosity were measured with nitrogen (High-Pressure Gas Permeameter/Porosimeter, Temco, Tulsa, OK, USA).
- (2)
- The core plug was placed in a high-pressure coreholder (TY-4, Huada, Haian, China) and vacuumed for 24 hours. Then, the formation brine was injected at a flow rate of 0.2 cm3/min to saturate the core plug. After that, the NMR apparatus (Mini-NMR, Niumag, Suzhou, China) was used to measure the transverse relaxation time T2 of the core sample under the initial water-saturated condition. The magnetic intensity, gradient value control precision, and frequency range of the NMR apparatus were 0.5 T, 0.025 T/m, 0.01 MHz, and 1–30 MHz, respectively.
- (3)
- The core was displaced with MnCl2 solution (15,000 mg/L) of 5 PV. Then, the saturated core was scanned again by the NMR apparatus to ensure the hydrogen signal of the brine was eliminated.
- (4)
- After that, 3.0 PV of the crude oil was pumped through the core plugs at a constant rate of 0.1 cm3/min until no water was produced to achieve the connate water saturation (Swc) and the initial oil saturation (Soi) at a reservoir temperature of 61 °C. The T2 spectrum was measured again after the core had been saturated with crude oil.
- (5)
- In each test, the pressure of the CO2 in the high-pressure cylinder was increased to the prespecified injection pressure at the temperature of 61 °C. Then, the CO2 was injected into the oil saturated core at constant pressure to the desired pressure and the pump maintained the constant pressure condition for 30 min. After CO2 injection, CO2 was allowed to soak for tsoak = 6 h. Then, the oil was produced from the same end of the coreholder at atmospheric pressure. The huff-and-puff cycles continued until no considerable oil production was obtained. The injection and production pressure were continuously monitored and recorded during the entire test. A video camera was utilized to measure the cumulative produced oil volume.
- (6)
- After the CO2 huff-and-puff test, the T2 transverse relaxation time of the core samples was measured with the NMR apparatus.
3. Results and Discussion
3.1. Phase Behaviors of CO2-Oil System
3.1.1. CO2 Solubility and Oil Swelling Measurement
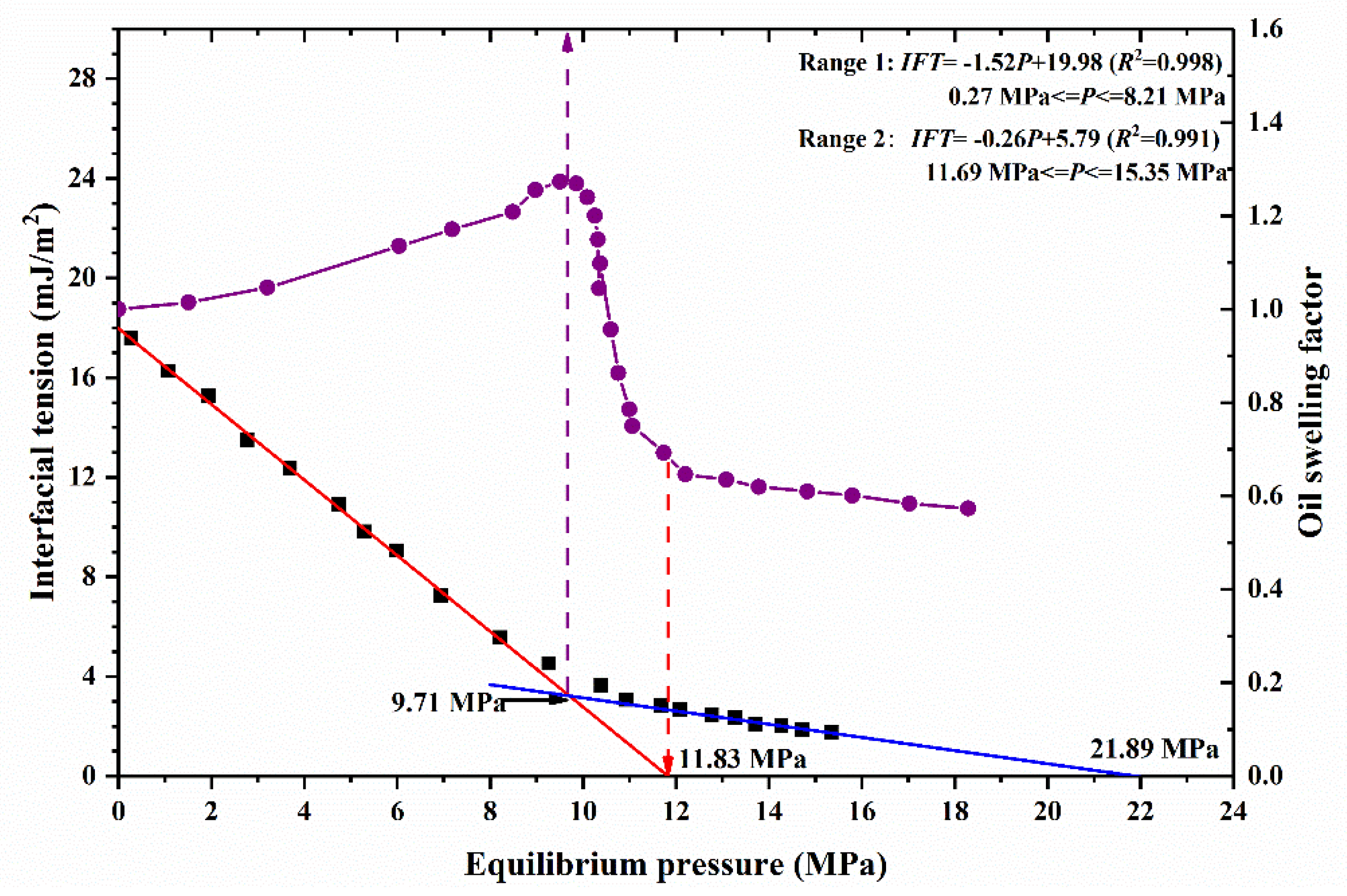
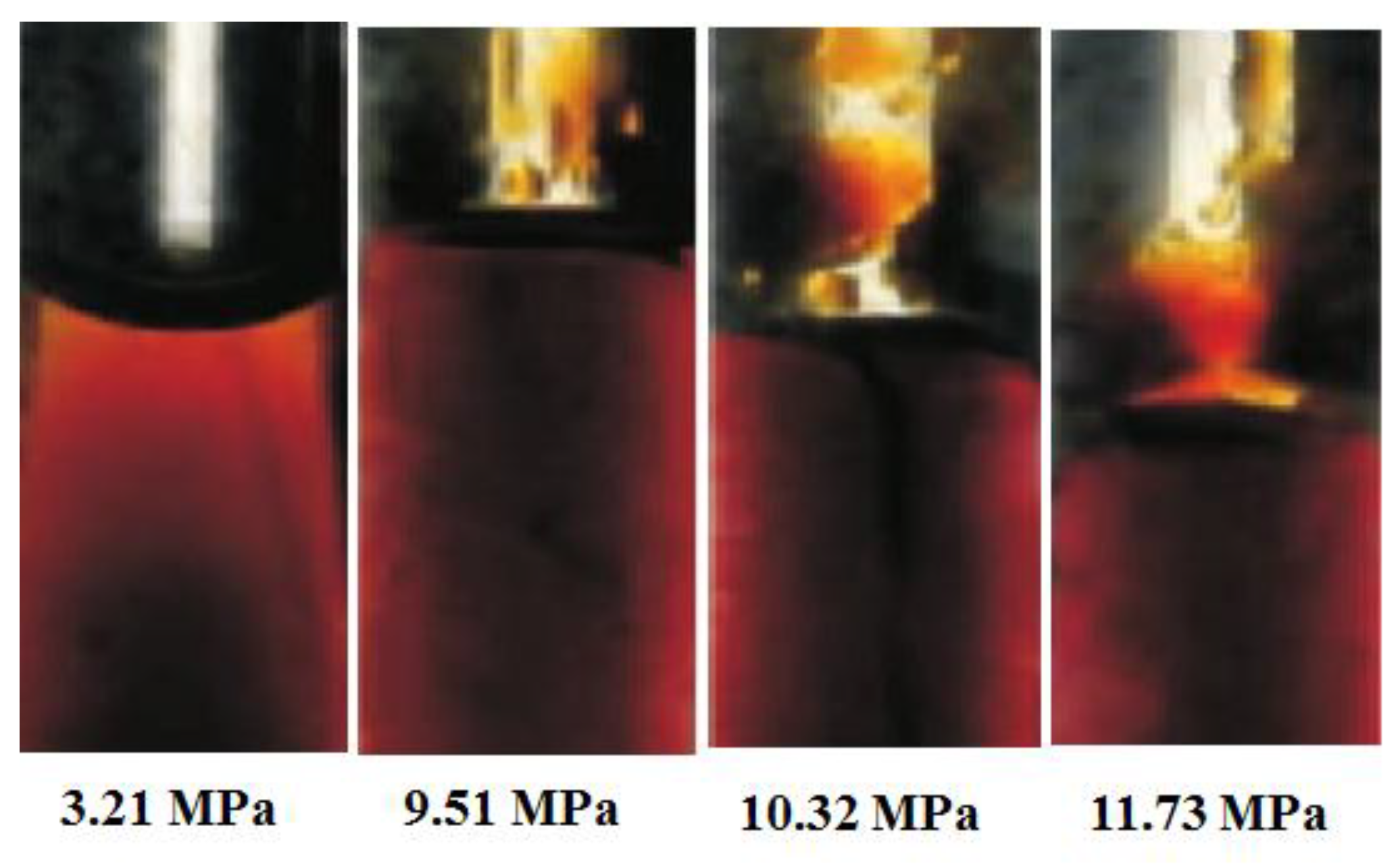
3.1.2. IFT Measurement
3.2. Microscopic Residual Oil Distribution During CO2 Huff-and-Puff Processes
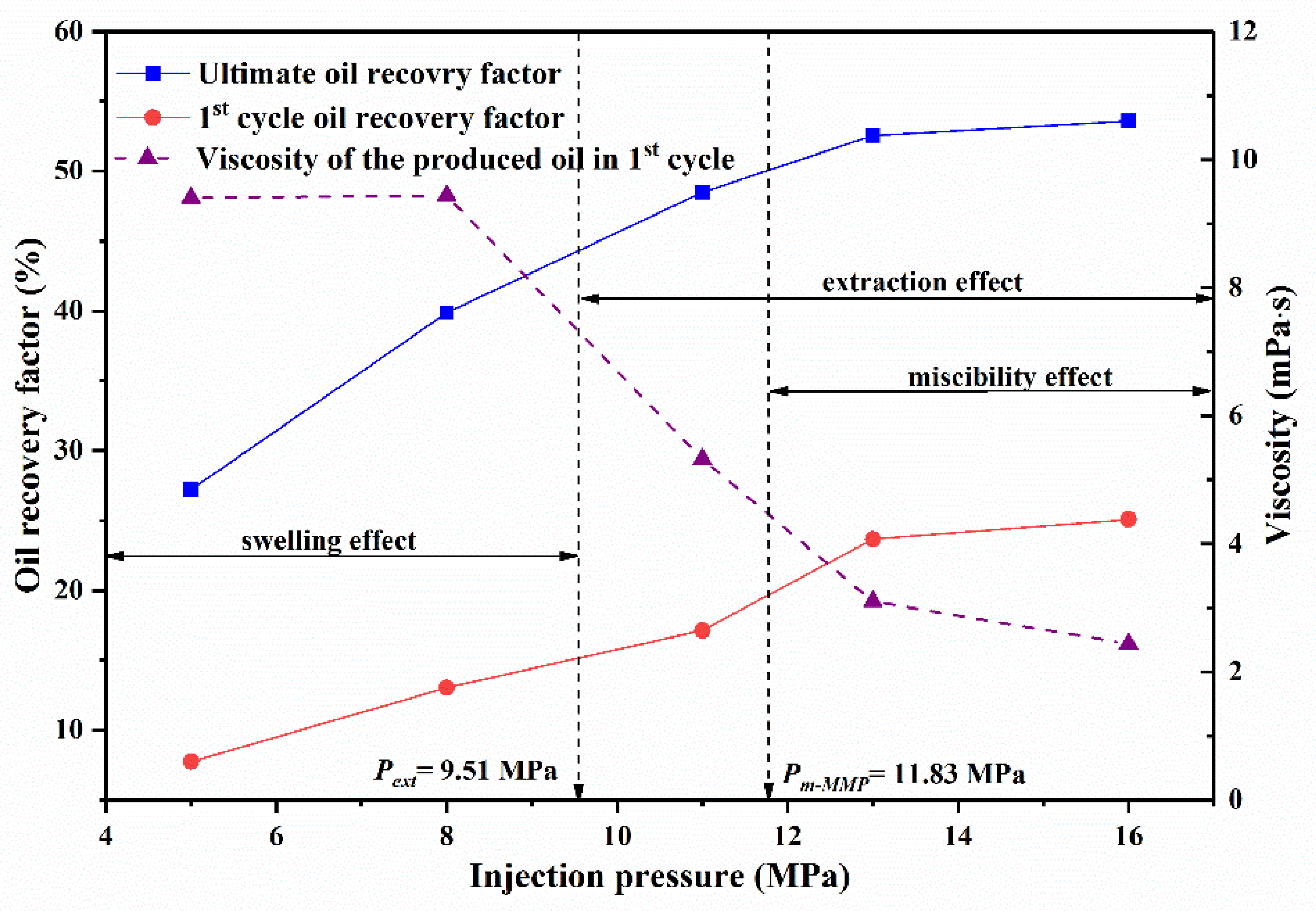
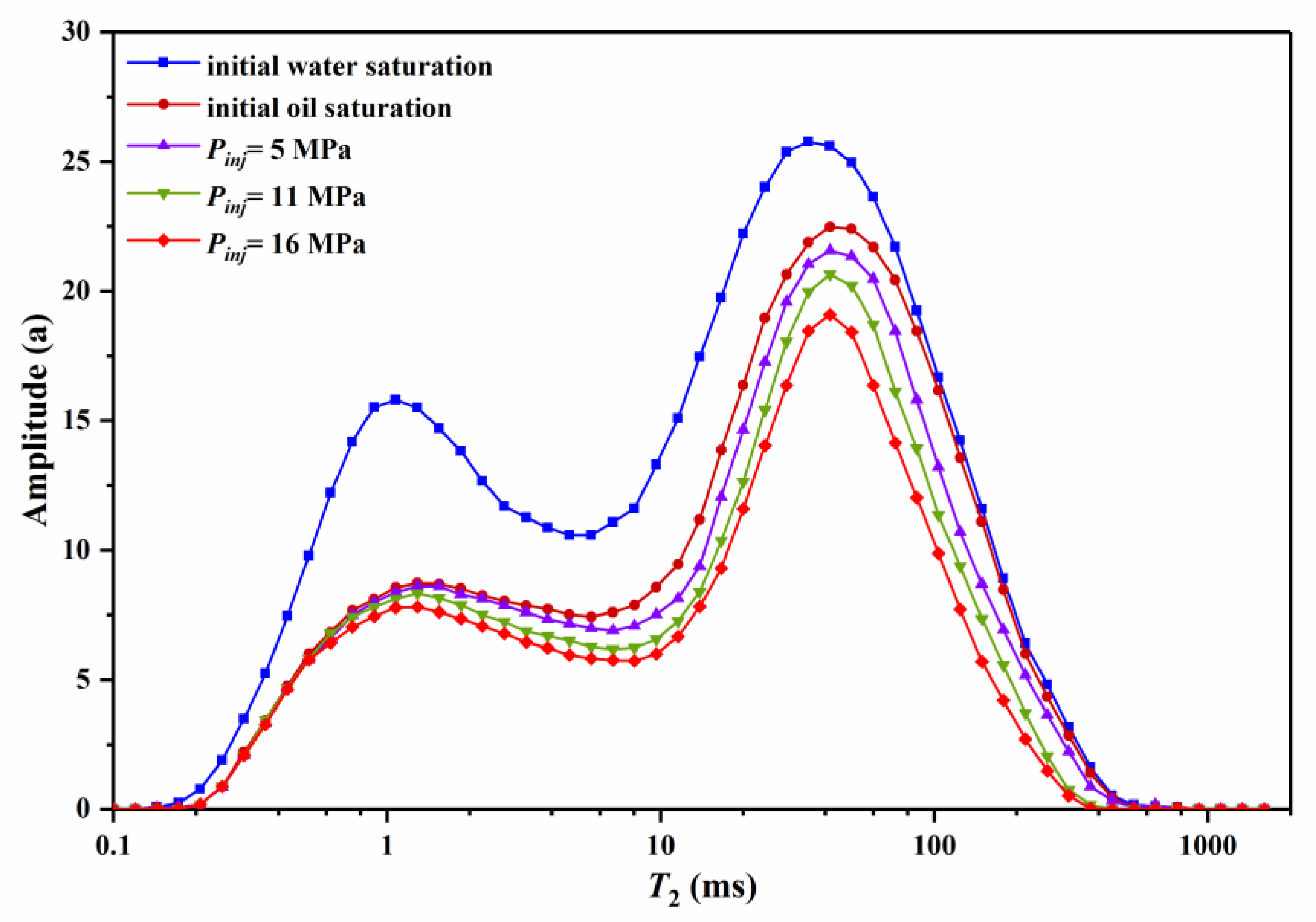
3.2.1. Effect of Injection Pressure on Residual Oil
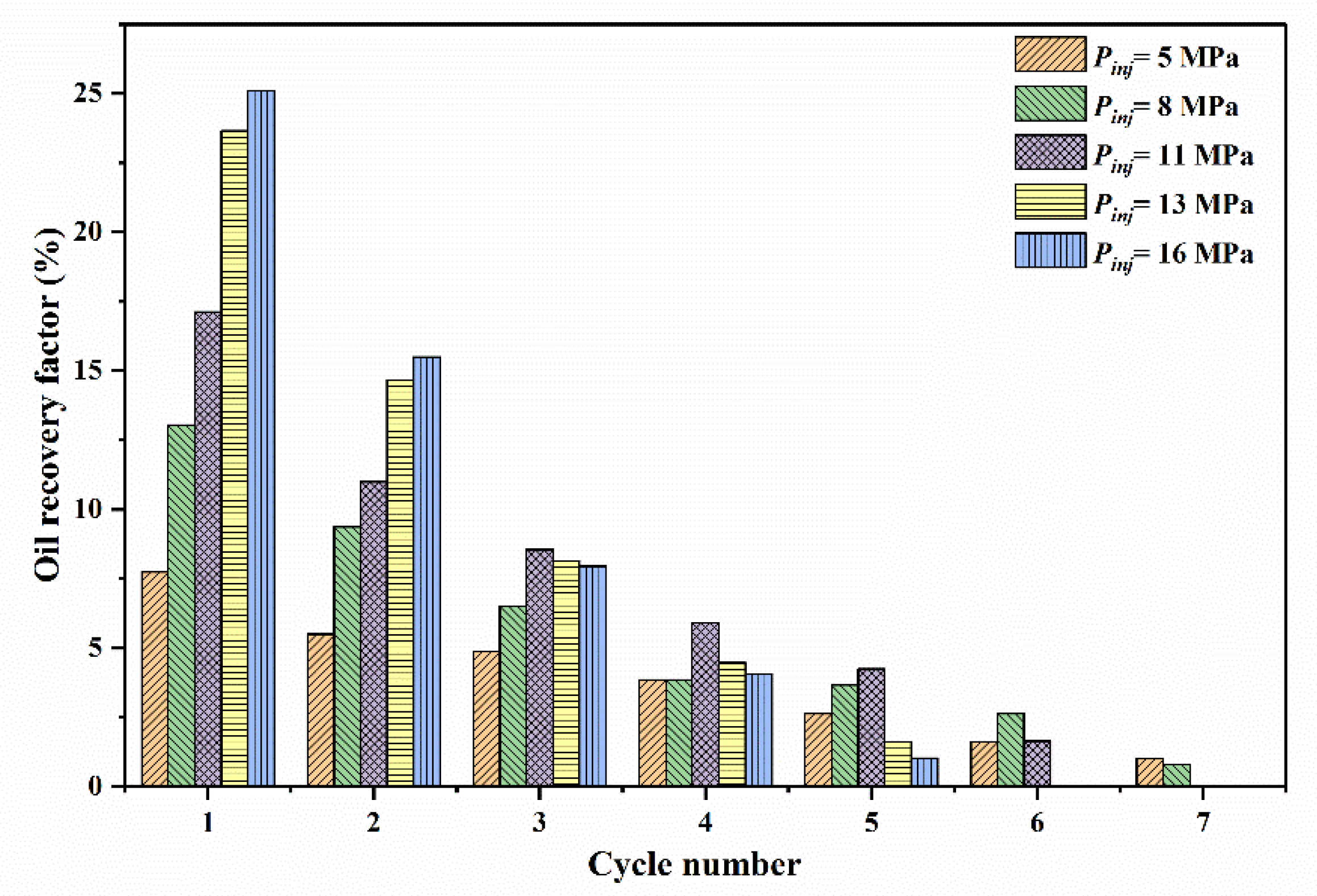
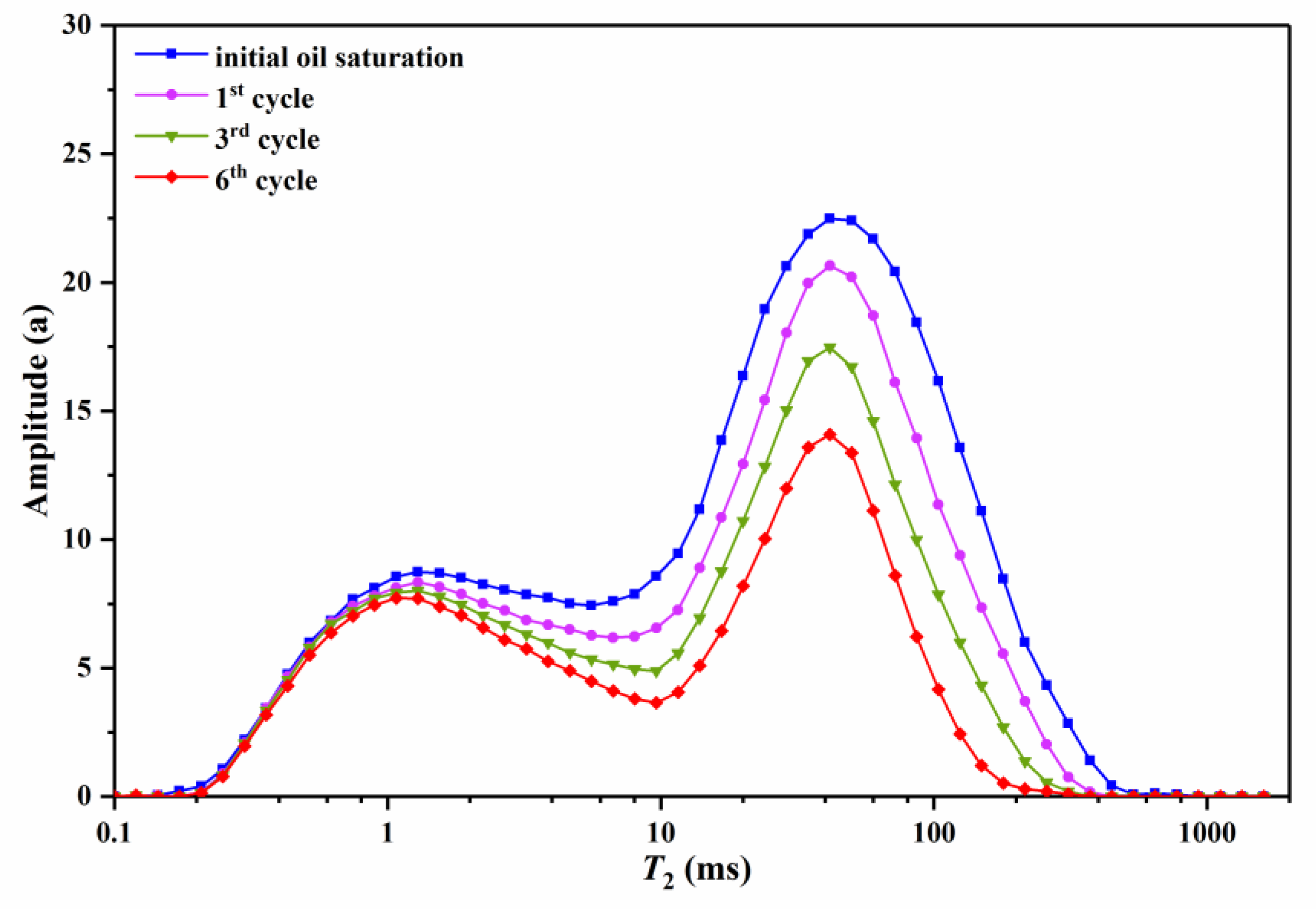
3.2.2. Effect of Cycle Number on Residual Oil
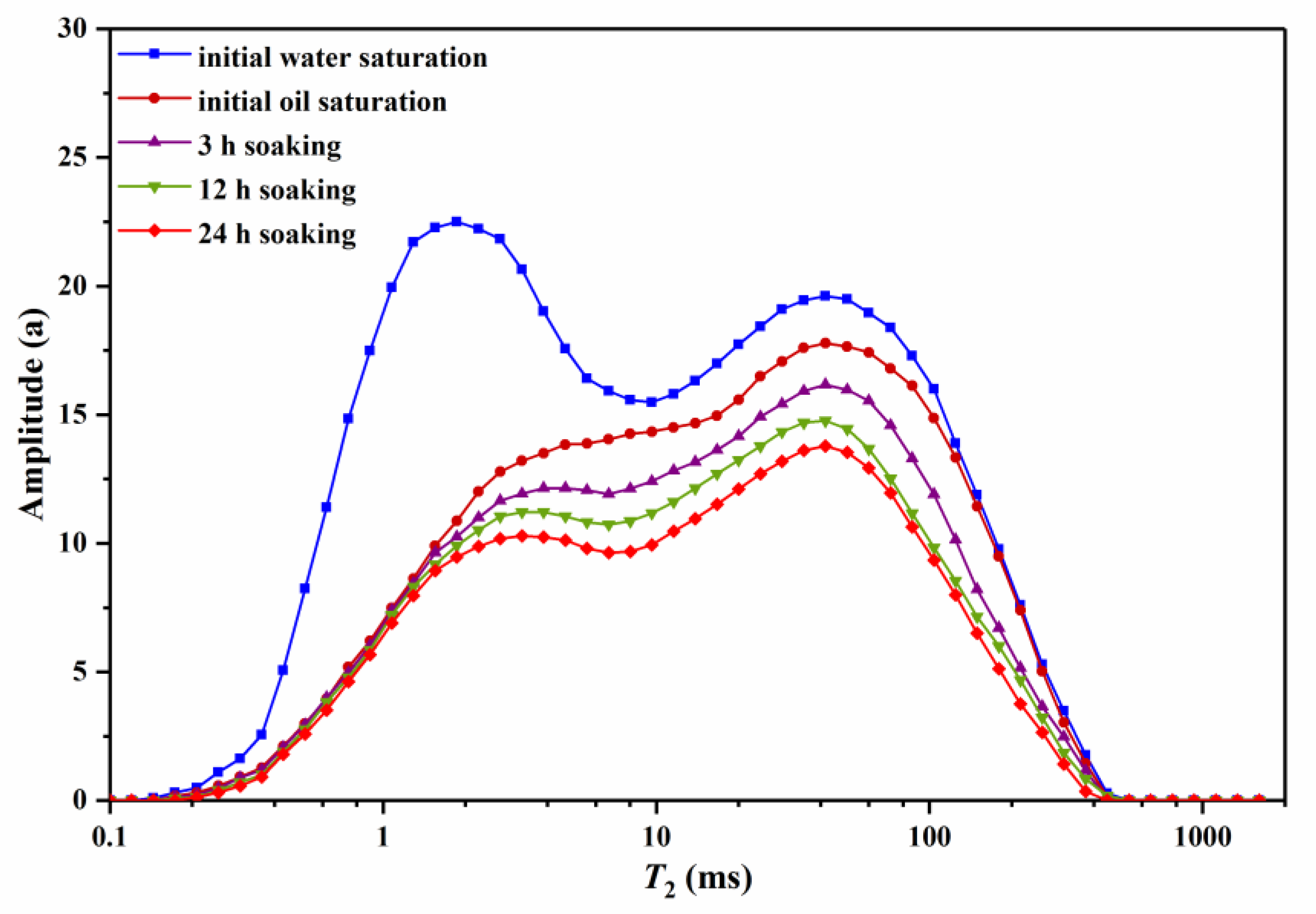
3.2.3. Effect of Soaking Time on Residual Oil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, Z.; Firoozabadi, A. Phase behavior and flow in shale nanopores from molecular simulations. Fluid Phase Equilibr. 2016, 430, 156–168. [Google Scholar] [CrossRef]
- Zhao, H.; Lai, Z.; Firoozabadi, A. Sorption hysteresis of light hydrocarbons and carbon dioxide in shale and kerogen. Sci. Rep. 2017, 7, 16209. [Google Scholar] [CrossRef] [PubMed]
- Dejam, M.; Hassanzadeh, H.; Chen, Z. Pre-Darcy Flow in Porous Media. Water Resour. 2017, 53, 8187–8210. [Google Scholar] [CrossRef]
- Dejam, M. Advective-diffusive-reactive solute transport due to non-Newtonian fluid flows in a fracture surrounded by a tight porous medium. Int. J. Heat Mass Transf. 2019, 128, 1307–1321. [Google Scholar] [CrossRef]
- Mashayekhizadeh, V.; Kord, S.; Dejam, M. EOR potential within Iran. Spec. Top. Rev. Porous Med. Int. J. 2014, 5. [Google Scholar] [CrossRef]
- Ren, B.; Ren, S.; Zhang, L.; Chen, G.; Zhang, H. Monitoring on CO2 migration in a tight oil reservoir during CCS-EOR in Jilin Oilfield China. Energy 2016, 98, 108–121. [Google Scholar] [CrossRef]
- Sheng, J.J. Critical review of field EOR projects in shale and tight reservoirs. J. Pet. Sci. Eng. 2017, 159, 654–665. [Google Scholar] [CrossRef]
- Sheng, J.J.; Chen, K. Evaluation of the EOR potential of gas and water injection in shale oil reservoirs. J. Unconv. Oil Gas Resour. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Zuloaga, P.; Yu, W.; Miao, J.; Sepehrnoori, K. Performance evaluation of CO2 Huff-n-Puff and continuous CO2 injection in tight oil reservoirs. Energy 2017, 134, 181–192. [Google Scholar] [CrossRef]
- Song, C.; Yang, D. Experimental and numerical evaluation of CO2 huff-n-puff processes in Bakken formation. Fuel 2017, 190, 145–162. [Google Scholar] [CrossRef]
- Todd, H.B.; Evans, J.G. Improved oil recovery IOR pilot projects in the Bakken formation. In Proceedings of the SPE Low Perm Symposium, Denver, CO, USA, 5–6 May 2016. [Google Scholar] [CrossRef]
- Rudyk, S.; Spirov, P.; Sogaard, E. Application of GC–MS chromatography for the analysis of the oil fractions extracted by supercritical CO2 at high pressure. Fuel 2013, 106, 139–146. [Google Scholar] [CrossRef]
- Ghedan, S. Global laboratory experience of CO2-EOR flooding. In Proceedings of the SPE/EAGE reservoir Characterization & Simulation Conference, Abu Dhabi, UAE, 19–21 October 2009. [Google Scholar] [CrossRef]
- Abedini, A.; Torabi, F. Parametric study of the cyclic CO2 injection process in light oil systems. Ind. Eng. Chem. Res. 2013, 52, 15211–15223. [Google Scholar] [CrossRef]
- Cao, M.; Gu, Y. Physicochemical characterization of produced oils and gases in immiscible and miscible CO2 flooding processes. Energy Fuels 2012, 27, 440–453. [Google Scholar] [CrossRef]
- Li, H.; Zheng, S.; Yang, D.T. Enhanced swelling effect and viscosity reduction of solvent (s)/CO2/heavy-oil systems. SPE J. 2013, 18, 695–707. [Google Scholar] [CrossRef]
- Habibi, A.; Yassin, M.R.; Dehghanpour, H.; Bryan, D. Experimental investigation of CO2-oil interactions in tight rocks: A Montney case study. Fuel 2017, 203, 853–867. [Google Scholar] [CrossRef]
- Abedini, A.; Torabi, F. Oil recovery performance of immiscible and miscible CO2 huff-and-puff processes. Energy Fuels 2014, 28, 774–784. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Gao, R.; Zeng, F.; Huang, C.; Tontiwachwuthikul, P.; Liang, Z. Enhanced light oil recovery from tight formations through CO2 huff ‘n’puff processes. Fuel 2015, 154, 35–44. [Google Scholar] [CrossRef]
- Pu, W.; Wei, B.; Jin, F.; Li, Y.; Jia, H.; Liu, P.; Tang, Z. Experimental investigation of CO2 huff-n-puff process for enhancing oil recovery in tight reservoirs. Chem. Eng. Res. Des. 2016, 111, 269–276. [Google Scholar] [CrossRef]
- McLendon, W.J.; Koronaios, P.; Enick, R.M.; Biesmans, G.; Salazar, L.; Miller, A.; Soong, Y.; McLendon, T.; Romanov, V.; Crandall, D. Assessment of CO2-soluble non-ionic surfactants for mobility reduction using mobility measurements and CT imaging. J. Pet. Sci. Eng. 2014, 119, 196–209. [Google Scholar] [CrossRef]
- Cui, M.; Wang, R.; Lv, C.; Tang, Y. Research on microscopic oil displacement mechanism of CO2 EOR in extra-high water cut reservoirs. J. Pet. Sci. Eng. 2017, 154, 315–321. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, W.; Wang, X.; Guo, S. 2-D Pore-scale experimental investigations of asphaltene deposition and heavy oil recovery by CO2 flooding. Energy Fuels 2018, 32, 3194–3201. [Google Scholar] [CrossRef]
- Tiwari, P.; Deo, M.; Lin, C.L.; Miller, J.D. Characterization of oil shale pore structure before and after pyrolysis by using X-ray micro CT. Fuel 2013, 107, 547–554. [Google Scholar] [CrossRef]
- Eslami, M.; Kadkhodaie-Ilkhchi, A.; Sharghi, Y.; Golsanami, N. Construction of synthetic capillary pressure curves from the joint use of NMR log data and conventional well logs. J. Pet. Sci. Eng. 2013, 111, 50–58. [Google Scholar] [CrossRef]
- Yang, P.; Guo, H.; Yang, D. Determination of residual oil distribution during waterflooding in tight oil formations with NMR relaxometry measurements. Energy Fuels 2013, 27, 5750–5756. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Z.; Ding, Y.; Luo, Y.; Qi, D.; Lin, W.; Zhao, X. Waterflooding Huff-n-puff in Tight Oil Cores Using Online Nuclear Magnetic Resonance. Energies 2018, 11, 1524. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Zhang, Z.; Niu, B.; Li, H. Impact of secondary and tertiary floods on microscopic residual oil distribution in medium-to-high permeability cores with NMR technique. Energy Fuels 2015, 29, 4721–4729. [Google Scholar] [CrossRef]
- Yang, C.; Gu, Y. Diffusion coefficients and oil swelling factors of carbon dioxide, methane, ethane, propane, and their mixtures in heavy oil. Fluid Phase Equilibr. 2006, 243, 64–73. [Google Scholar] [CrossRef]
- Siagian, U.W.R.; Grigg, R.B. The extraction of hydrocarbons from crude oil by high pressure CO2. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–22 April 1998. [Google Scholar] [CrossRef]
- Hoover, W.G. Molecular dynamics. In Molecular Dynamics; Springer: Berlin/Heidelberg, Germany, 1986; Volume 258. [Google Scholar]
- Rao, D.N. A new technique of vanishing interfacial tension for miscibility determination. Fluid Phase Equilibr. 1997, 139, 311–324. [Google Scholar] [CrossRef]
- Abedini, A.; Torabi, F. On the CO2 storage potential of cyclic CO2 injection process for enhanced oil recovery. Fuel. 2014, 124, 14–27. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Gao, H.; Zhao, J.; Li, H.A. Effect of asphaltene precipitation on CO2-flooding performance in low-permeability sandstones: A nuclear magnetic resonance study. RSC Adv. 2017, 7, 38367–38376. [Google Scholar] [CrossRef]
- Jamaloei, B.Y.; Kharrat, R.; Asghari, K. Pore-scale events in drainage process through porous media under high-and low-interfacial tension flow conditions. J. Pet. Sci. Eng. 2010, 75, 223–233. [Google Scholar] [CrossRef]
- Simpson, M.R. The CO2 huff’n’puff process in a bottomwater-drive reservoir. J. Pet. Technol. 1988, 40, 887–893. [Google Scholar] [CrossRef]





| No. | Length (cm) | Diameter (cm) | Permeability (mD) | Porosity (%) | Connate Water Saturation (%) |
|---|---|---|---|---|---|
| 1 | 6.652 | 2.506 | 0.81 | 13.34 | 24.49 |
| 2 | 6.138 | 2.506 | 0.36 | 12.20 | 26.01 |
| Experimental Condition | Cumulative ORFs in Different-Sized Pores (%) | ||||
|---|---|---|---|---|---|
| Micro (0.1–1 ms) | Small (1–10 ms) | Medium (10–100 ms) | Large (100–1000 ms) | Sum | |
| Soi | 57.45 | 64.45 | 82.25 | 94.58 | 75.51 |
| 1st cycle | 4.02 | 12.16 | 15.96 | 37.59 | 17.13 |
| 3rd cycle | 5.84 | 21.11 | 32.17 | 64.41 | 36.68 |
| 6th cycle | 9.79 | 29.29 | 48.23 | 86.18 | 48.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, K.; Yang, S.; Dou, H.; Wang, Q.; Wang, L.; Huang, Y. Experimental Investigation on Microscopic Residual Oil Distribution During CO2 Huff-and-Puff Process in Tight Oil Reservoirs. Energies 2018, 11, 2843. https://doi.org/10.3390/en11102843
Qian K, Yang S, Dou H, Wang Q, Wang L, Huang Y. Experimental Investigation on Microscopic Residual Oil Distribution During CO2 Huff-and-Puff Process in Tight Oil Reservoirs. Energies. 2018; 11(10):2843. https://doi.org/10.3390/en11102843
Chicago/Turabian StyleQian, Kun, Shenglai Yang, Hongen Dou, Qian Wang, Lu Wang, and Yu Huang. 2018. "Experimental Investigation on Microscopic Residual Oil Distribution During CO2 Huff-and-Puff Process in Tight Oil Reservoirs" Energies 11, no. 10: 2843. https://doi.org/10.3390/en11102843
APA StyleQian, K., Yang, S., Dou, H., Wang, Q., Wang, L., & Huang, Y. (2018). Experimental Investigation on Microscopic Residual Oil Distribution During CO2 Huff-and-Puff Process in Tight Oil Reservoirs. Energies, 11(10), 2843. https://doi.org/10.3390/en11102843




