Solid Fuel Production from Semi-Natural Grassland Biomass—Results from a Commercial-Scale IFBB Plant
Abstract
:1. Introduction
- (i)
- energetic and chemical fuel properties of the fuels produced using the commercial scale IFBB process, especially focusing on the reduction of detrimental mineral elements from silage to solid fuel.
- (ii)
- the combustion performance and emission characteristics of the fuels.
- (iii)
- the ash melting behavior of the silage and the fuels produced.
2. Materials and Methods
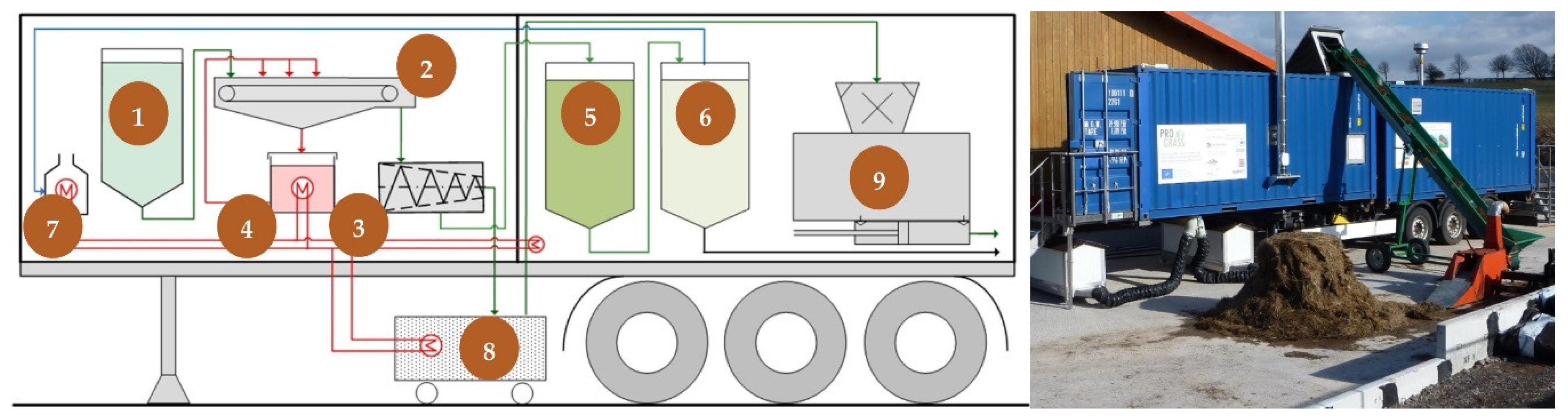
2.1. Commercial Scale Plant
2.2. Grassland Biomass
2.3. Chemical Analysis and Heating Value
2.4. Combustion and Ash Melting Test
2.5. Prototype Plant
2.6. Statistical Analysis
3. Results
3.1. Elemental Concentrations
3.2. Ash Melting
3.3. Combustion and Emission
3.4. Comparison between Commercial-Scale Plant and Prototype
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species Coverage (%) | Species Number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | DM (% FM) | FM (t·ha−1) | G | S/R | L | H | T | G | S/R | L | H | T | |
| AC | Agrostis capillaris, Holcus lanatus, Achillea millefolium | 28.50 | 20.33 | 109.50 | 2.67 | 14.33 | 107.17 | 233.67 | 11 | 2 | 6 | 32 | 51 |
| AE | Alopecurus pratensis, Arrhenatherum elatius, Holcus lanatus | 28.29 | 6.19 | 105.33 | 0.00 | 3.67 | 20.97 | 129.97 | 5 | 0 | 2 | 12 | 19 |
| AM | Agrostis x murbeckii, Holcus lanatus, Anthoxanthum odoratum, Galium spec. | - | - | 80.07 | 0.00 | 0.02 | 49.03 | 129.10 | 9 | 0 | 1 | 17 | 27 |
| AS | Agrostis stolonifera, Phragmites australis, Bolboschoenus maritimus | 51.33 | 16.93 | 68.07 | 21.53 | 0.07 | 18.20 | 107.87 | 4 | 9 | 1 | 8 | 22 |
| BS | Bromus erectus, Medicago falcata, Centaurea scabiosa | 24.85 | 11.00 | 22.87 | 1.87 | 51.40 | 40.07 | 116.20 | 11 | 2 | 12 | 26 | 51 |
| CD | Carex disticha, Phragmites australis, Holcus lanatus | 36.00 | 8.84 | 20.00 | 85.33 | 4.73 | 12.73 | 122.80 | 7 | 3 | 3 | 11 | 24 |
| DC | Deschampsia cespitosa, D. flexuosa, D. flexuosa, Carex leponina, Juncus acutiflorus | 49.00 | 5.23 | 75.80 | 79.87 | 37.00 | 26.80 | 219.47 | 6 | 6 | 2 | 8 | 22 |
| JE | Juncus effuses, Carex species, Alisma Plantago-aquatica | 32.50 | 16.23 | 3.23 | 75.33 | 0.00 | 35.93 | 114.50 | 3 | 5 | 0 | 9 | 17 |
| PA1 | Phalaris arundinacea, Urtica dioica | - | - | 84.33 | 0.67 | 0.00 | 24.00 | 105.00 | 3 | 2 | 0 | 3 | 8 |
| PA2 | Phalaris arundinacea, Carex acutiformis, Carex gracilis | 41.49 | 26.40 | 41.53 | 48.00 | 1.33 | 13.20 | 104.07 | 10 | 12 | 2 | 16 | 40 |
| PS | Phragmites australis, Phalaris arundinacea, Typha latifolia | 39.18 | 30.67 | 51.67 | 61.00 | 0.00 | 9.00 | 121.67 | 3 | 3 | 0 | 6 | 12 |
References
- BMBF. German Energy Transition. 2017. Available online: https://www.bmbf.de/en/german-energy-transition-2319.html (accessed on 5 November 2017).
- AGEE-Stat. Development of Renewable Energy Sources in Germany 2016. Available online: https://www.erneuerbare-energien.de/EE/Redaktion/DE/Downloads/development-of-renewable-energy-sources-in-germany-2016.pdf (accessed on 16 November 2017).
- Creutzig, F.; Ravindranath, N.H.; Berndes, G.; Bolwig, S.; Bright, R.; Cherubini, F.; Chum, H.; Corbera, E.; Delucchi, M.; Faaij, A.; et al. Bioenergy and climate change mitigation: An assessment. GCB Bioenergy 2015, 7, 916–944. [Google Scholar] [CrossRef] [Green Version]
- OECD. The Bioeconomy to 2030: Designing a Policy Agenda; OECD Publishing: Paris, France, 2009. [Google Scholar]
- Hauser, E.; Wern, B. The role of bioenergy in the German “Energiewende”—Whose demands can be satisfied by bioenergy? Energy Sustain. Soc. 2016, 6, 35. [Google Scholar] [CrossRef]
- BMU. National Biomass Action Plan for Germany: Biomass and Sustainable Energy Supply. Available online: http://www.bmel.de/SharedDocs/Downloads/EN/Publications/BiomassActionPlan.pdf (accessed on 3 October 2017).
- Pullin, A.S.; Báldi, A.; Can, O.E.; Dieterich, M.; Kati, V.; Livoreil, B.; Lövei, G.; Mihók, B.; Nevin, O.; Selva, N.; et al. Conservation focus on Europe: Major conservation policy issues that need to be informed by conservation science. Conserv. Biol. 2009, 23, 818–824. [Google Scholar] [CrossRef]
- Donnison, I.S.; Fraser, M.D. Diversification and use of bioenergy to maintain future grasslands. Food Energy Secur. 2016, 5, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Burrascano, S.; Chytrý, M.; Kuemmerle, T.; Giarrizzo, E.; Luyssaert, S.; Sabatini, F.M.; Blasi, C. Current European policies are unlikely to jointly foster carbon sequestration and protect biodiversity. Biol. Conserv. 2016, 201, 370–376. [Google Scholar] [CrossRef]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Amon, T.; Hobbs, P.J. Bioenergy from permanent grassland—A review: 2. Combustion. Bioresour. Technol. 2009, 100, 4945–4954. [Google Scholar] [CrossRef]
- Valin, H.; Peters, D.; van den Berg, M.; Frank, S.; Havlík, P.; Forsell, N.; Hamelinck, C. The Land Use Change Impact of Biofuels Consumed in the EU. Quantification of Area and Greenhouse Gas Impacts; ECOFYS Netherlands B.V.: Utrecht, The Netherlands, 2015; Available online: https://ec.europa.eu/energy/sites/ener/files/documents/Final%20Report_GLOBIOM_publication.pdf (accessed on 21 September 2017).
- Corton, J.; Donnison, I.S.; Patel, M.; Bühle, L.; Hodgson, E.; Wachendorf, M.; Bridgwater, A.; Allison, G.; Fraser, M.D. Expanding the biomass resource: Sustainable oil production via fast pyrolysis of low input high diversity biomass and the potential integration of thermochemical and biological conversion routes. Appl. Energy 2016, 177, 852–862. [Google Scholar] [CrossRef]
- Heinsoo, K.; Melts, I.; Sammul, M.; Holm, B. The potential of Estonian semi-natural grasslands for bioenergy production. Agric. Ecosyst. Environ. 2010, 137, 86–92. [Google Scholar] [CrossRef]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-Negative Biofuels from Low-Input High-Diversity Grassland Biomass. Science (New York, N.Y.) 2006, 314, 1598–1600. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; van Beek, J.; Bellings, L.; Liebert, K.; Muys, B.; Hermy, M. Energy potential for combustion and anaerobic digestion of biomass from low-input high-diversity systems in conservation areas. GCB Bioenergy 2015, 7, 888–898. [Google Scholar] [CrossRef]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Linke, B.; Idler, C.; Amon, T.; Hobbs, P.J. Bioenergy from permanent grassland—A review: 1. Biogas. Bioresour. Technol. 2009, 100, 4931–4944. [Google Scholar] [CrossRef]
- Manning, D.B.; Bemmann, A.; Bredemeier, M.; Ammer, C.; Lamersdorf, N. Bioenergy from Dendromass for the Sustainable Development of Rural Areas; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Richter, F.; Graß, R.; Fricke, T.; Zerr, W.; Wachendorf, M. Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. II. Effects of hydrothermal conditioning and mechanical dehydration on anaerobic digestion of press fluids. Grass Forage Sci. 2009, 64, 354–363. [Google Scholar] [CrossRef]
- Runge, T.; Wipperfurth, P.; Zhang, C. Improving biomass combustion quality using a liquid hot water treatment. Biofuels 2014, 4, 73–83. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Barnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion and Co-Firing; Earthscan: London, UK, 2008. [Google Scholar]
- Sommersacher, P.; Brunner, T.; Obernberger, I. Fuel Indexes: A Novel Method for the Evaluation of Relevant Combustion Properties of New Biomass Fuels. Energy Fuels 2012, 26, 380–390. [Google Scholar] [CrossRef]
- Huyghe, C.; De Vliegher, A.; Van Gils, B.; Peeters, A. Grasslands and Herbivore Production in Europe and Effects of Common Policies; Editions Quae: Versailles Cedex, France, 2014. [Google Scholar]
- Riedl-Narentenau, R.; Obernberger, I. Corrosion and Fouling in Boilers of Biomass Combustion Plants, 1st ed.; Pergamon: Oxford, UK, 1996. [Google Scholar]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef] [Green Version]
- Wachendorf, M.; Richter, F.; Fricke, T.; Graß, R.; Neff, R. Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. I. Effects of hydrothermal conditioning and mechanical dehydration on mass flows of organic and mineral plant compounds, and nutrient balances. Grass Forage Sci. 2009, 64, 132–143. [Google Scholar] [CrossRef]
- Richter, F.; Fricke, T.; Wachendorf, M. Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. III. Effects of hydrothermal conditioning and mechanical dehydration on solid fuel properties and on energy and greenhouse gas balances. Grass Forage Sci. 2010, 65, 185–199. [Google Scholar] [CrossRef]
- Hensgen, F.; Bühle, L.; Donnison, I.; Frasier, M.; Vale, J.; Corton, J.; Heinsoo, K.; Melts, I.; Wachendorf, M. Mineral concentrations in solid fuels from European semi-natural grasslands after hydrothermal conditioning and subsequent mechanical dehydration. Bioresour. Technol. 2012, 118, 332–342. [Google Scholar] [CrossRef]
- Hensgen, F.; Richter, F.; Wachendorf, M. Integrated generation of solid fuel and biogas from green cut material from landscape conservation and private households. Bioresour. Technol. 2011, 102, 10441–10450. [Google Scholar] [CrossRef]
- Bühle, L.; Dürl, G.; Hensgen, F.; Urban, A.; Wachendorf, M. Effects of hydrothermal conditioning and mechanical dewatering on ash melting behaviour of solid fuel produced from European semi-natural grasslands. Fuel 2014, 118, 123–129. [Google Scholar] [CrossRef]
- Bühle, L.; Hensgen, F.; Donnison, I.; Heinsoo, K.; Wachendorf, M. Life cycle assessment of the integrated generation of solid fuel and biogas from biomass (IFBB) in comparison to different energy recovery, animal-based and non-refining management systems. Bioresour. Technol. 2012, 111, 230–239. [Google Scholar] [CrossRef]
- COMBINE. Converting Organic Matters from European Urban and Natural Areas into Storable Bio-Energy. Available online: http://www.combine-nwe.eu/index.php?id=2 (accessed on 25 August 2017).
- DANUBENERGY. DANUBENERGY. Improving Eco-Efficiency of Bio-Energy Production and Supply in Riparian Areas of the Danube River Basin and Other Floodplains in Central Europe. Available online: http://www.danubenergy.eu/index.php?id=2 (accessed on 28 August 2017).
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- DIN. DIN EN 304:2004-01 Heating Boilers—Test Code for Heating Boilers for Atomizing Oil Burners. Available online: https://www.beuth.de/de/norm/din-en-304/67411860 (accessed on 11 September 2017).
- DIN. DIN EN 303-5:2012-10 Heating Boilers—Part 5: Heating Boilers for Solid Fuels, Manually and Automatically Stoked, Nominal Heat Output of Up to 500 kW. Available online: https://www.beuth.de/en/standard/din-en-303-5/148568550 (accessed on 19 September 2017).
- DIN. DIN 51730:2007-09 Testing of Solid Fuels—Determination of Fusibility of Fuel Ash. Available online: https://www.beuth.de/de/norm/din-51730/98893895 (accessed on 19 September 2017).
- ISO. ISO 540:2008: Hard Coal and Coke—Determination of Ash Fusibility. Available online: https://www.iso.org/standard/41484.html (accessed on 15 September 2017).
- DIN. DIN CEN/TS 15404:2007-01 Solid Recovered Fuels—Methods for the Determination of Ash Melting Behaviour by Using Characteristic Temperatures. Available online: https://www.beuth.de/de/vornorm/din-cen-ts-15404/85128767 (accessed on 11 September 2017).
- DIN. DIN CEN/TS 15370-1:2006-12 Solid Biofuels—Method for the Determination of Ash Melting Behaviour—Part 1: Characteristic Temperatures Method. Available online: https://www.beuth.de/de/vornorm/din-cen-ts-15370-1/84836928 (accessed on 19 September 2017).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 12 October 2017).
- Connolly, J.; Wachendorf, M. Developing Multisite Dynamic Models of Mixed Species Plant Communities. Ann. Bot. 2001, 88, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Khalsa, J.; Döhling, F.; Berger, F. Foliage and Grass as Fuel Pellets–Small Scale Combustion of Washed and Mechanically Leached Biomass. Energies 2016, 9, 361. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, G. Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass Bioenergy 2004, 27, 653–669. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, T.; Che, D. Effect of water washing on fuel properties, pyrolysis and combustion characteristics, and ash fusibility of biomass. Fuel Process. Technol. 2013, 106, 712–720. [Google Scholar] [CrossRef]
- King, C.; McEniry, J.; O’Kiely, P.; Richardson, M. The effects of hydrothermal conditioning, detergent and mechanical pressing on the isolation of the fibre-rich press-cake fraction from a range of grass silages. Biomass Bioenergy 2012, 42, 179–188. [Google Scholar] [CrossRef]
- Tonn, B.; Dengler, V.; Thumm, U.; Piepho, H.-P.; Claupein, W. Influence of leaching on the chemical composition of grassland biomass for combustion. Grass Forage Sci. 2011, 66, 464–473. [Google Scholar] [CrossRef]
- Carrillo, M.A.; Staggenborg, S.A.; Pineda, J.A. Washing sorghum biomass with water to improve its quality for combustion. Fuel 2014, 116, 427–431. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Piepenschneider, M.; Nurmatov, N.; Bühle, L.; Hensgen, F.; Wachendorf, M. Chemical Properties and Ash Slagging Characteristics of Solid Fuels from Urban Leaf Litter. Waste Biomass Valor. 2016, 7, 625–633. [Google Scholar] [CrossRef]
- Nitsche, M.; Hensgen, F.; Wachendorf, M. Using Grass Cuttings from Sports Fields for Anaerobic Digestion and Combustion. Energies 2017, 10, 388. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships between Heating Value and Lignin, Moisture, Ash and Extractive Contents of Biomass Fuels. Energy Explor. Exploit. 2002, 20, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, B.M.; Bakker, R.R.; Wei, J.B. On the properties of washed straw. Biomass Bioenergy 1996, 10, 177–200. [Google Scholar] [CrossRef]
- Nussbaumer, T. Combustion and Co-combustion of Biomass: Fundamentals, Technologies, and Primary Measures for Emission Reduction. Energy Fuels 2003, 17, 1510–1521. [Google Scholar] [CrossRef]
- 48.BImSchG. Erste Allgemeine Verwaltungsvorschrift zum Bundes–Immissionsschutzgesetz: (Technische Anleitung zur Reinhaltung der Luft—TA Luft). Available online: http://www.bmub.bund.de/fileadmin/Daten_BMU/Download_PDF/Luft/taluft.pdf (accessed on 15 November 2017).
- Carvalho, L.; Wopienka, E.; Pointner, C.; Lundgren, J.; Verma, V.K.; Haslinger, W.; Schmidl, C. Performance of a pellet boiler fired with agricultural fuels. Appl. Energy 2013, 104, 286–296. [Google Scholar] [CrossRef]
- Sanz, D.; Rojas, E.; Rodríguez-Maroto, J.J.; Ramos, R.; Borjabad, E.; Escalada, R.; García-Alonso, S.; Gutierrez-Canas, C.; Aragon, G.; Mugica, I.; et al. Review of critical parameters in biomass combustion emissions control by means of hybrid filter. IOP Conf. Ser. Earth Environ. Sci. 2015, 28, 12012. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P.; Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Amsterdam, The Netherlands; London, UK, 2012. [Google Scholar]
- Hahn, H.; Hartmann, K.; Bühle, L.; Wachendorf, M. Comparative life cycle assessment of biogas plant configurations for a demand oriented biogas supply for flexible power generation. Bioresour. Technol. 2015, 179, 348–358. [Google Scholar] [CrossRef]
- Hahn, H.; Krautkremer, B.; Hartmann, K.; Wachendorf, M. Review of concepts for a demand-driven biogas supply for flexible power generation. Renew. Sustain. Energy Rev. 2014, 29, 383–393. [Google Scholar] [CrossRef]



| Reference Code | Dominant Species | Country (Region) | Harvest Date | DM Yield (t·ha−1) |
|---|---|---|---|---|
| AC | Agrostis capillaris, Holcus lanatus | Slovakia (Žilina) | 22.06.2013 | 5.66 |
| AE | Arrhenatherum elatius | Belgium (West Flanders) | 05.06.2013 | 1.70 |
| AM | Agrostis x murbeckii | France (Côtes-d’Armor) | n.a | n.a |
| AS | Agrostis stolonifera, Phragmites australis | Austria (Neusiedler See) | 21.06.2013 | 8.56 |
| BS | Bromus erectus, Ahrrenatherum elatius | Czech Republic (Zlin) | 20.06.2013 | 2.75 |
| CD | Carex disticha, Phragmites australis | Germany (Freising) | 27.06.2013 | 3.21 |
| DC | Deschampsia cespitosa | Wales (Caernarfon) | 27.08.2013 | 2.51 |
| JE | Juncus effusus, Carex spec., | Slovenia (Pomurje) | 24.07.2013 | 5.28 |
| PA 1 | Phalaris arundinacea | Austria (Waldviertel) | 11.09.2013 | n.a |
| PA 2 | Phalaris arundinacea, Carex riparia | Poland (Wielko-Polska) | 18.06. 2013 | 10.98 |
| PS | Phragmites australis, Carex elata | Italy (Mantova) | 05.07.2013 | 11.76 |
| Prototype | Commercial | ||
|---|---|---|---|
| Silage throughput | kg·day−1 | 300 | 40,000 |
| Briquette output | kg·day−1 | 90 | 8400 |
| Silage batch size | kg | 20 | 500–3500 |
| Conditioning temperature | °C | 25 | 10 |
| Conditioning time | min | 30 | 40 |
| Silage to water ratio | 1:8 | 1:15 | |
| AC | AE | AM | AS | BS | CD | DC | JE | PA 1 | PA 2 | PS | Mean | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | %FM | SI | 55.18 | 66.01 | 27.32 | 31.67 | 82.54 | 62.23 | 38.42 | 66.32 | 56.35 | 39.54 | 44.11 | 55.91 |
| SF | 31.51 | 28.20 | 29.43 | 77.00 | 24.55 | 33.25 | 32.46 | 26.61 | 30.25 | 31.68 | 29.67 | 29.93 | ||
| LHV | SI | 17.12 | 17.55 | 16.67 | 17.47 | 17.32 | 17.26 | 17.81 | 17.33 | 16.70 | 17.54 | 17.48 | 17.30 | |
| SF | 17.69 | 17.69 | 17.56 | 17.80 | 17.69 | 17.65 | 17.84 | 17.78 | 17.55 | 17.94 | 17.82 | 17.72 | ||
| Ash | %DM | SI | 9.89 | 7.40 | 13.96 | 5.69 | 7.23 | 8.49 | 7.07 | 7.91 | 11.80 | 7.22 | 7.88 | 8.59 |
| SF | 3.59 | 3.88 | 4.40 | 3.59 | 3.76 | 4.74 | 3.54 | 4.42 | 4.52 | 3.54 | 3.74 | 3.97 | ||
| RR | 63.70 | 47.57 | 68.48 | 36.91 | 47.99 | 44.17 | 49.93 | 44.12 | 61.69 | 50.97 | 52.54 | 51.64 *** | ||
| N | %DM | SI | 1.40 | 1.58 | 1.79 | 0.96 | 1.16 | 1.50 | 1.64 | 1.09 | 1.52 | 1.23 | 1.07 | 1.36 |
| SF | 0.68 | 0.89 | 0.66 | 0.73 | 0.76 | 0.96 | 0.79 | 0.86 | 0.70 | 0.70 | 0.58 | 0.76 | ||
| RR | 51.43 | 43.67 | 63.13 | 23.96 | 34.48 | 36.00 | 51.83 | 21.10 | 53.95 | 43.09 | 45.79 | 42.58 *** | ||
| S | %DM | SI | 0.16 | 0.18 | 0.20 | 0.29 | 0.14 | 0.17 | 0.23 | 0.15 | 0.17 | 0.28 | 0.23 | 0.20 |
| SF | 0.06 | 0.07 | 0.06 | 0.09 | 0.06 | 0.08 | 0.08 | 0.07 | 0.07 | 0.09 | 0.07 | 0.07 | ||
| RR | 62.50 | 61.11 | 70.00 | 68.97 | 57.14 | 52.94 | 65.22 | 53.33 | 58.82 | 67.86 | 69.57 | 62.50 *** | ||
| Cl | %DM | SI | 0.22 | 0.45 | 1.24 | 0.44 | 0.21 | 1.01 | 0.86 | 0.50 | 0.54 | 0.61 | 0.64 | 0.61 |
| SF | 0.04 | 0.04 | 0.11 | 0.04 | 0.03 | 0.06 | 0.11 | 0.05 | 0.04 | 0.08 | 0.05 | 0.06 | ||
| RR | 81.82 | 91.11 | 91.13 | 90.91 | 85.71 | 94.06 | 87.21 | 90.00 | 92.59 | 86.89 | 92.19 | 89.42 *** | ||
| K | %DM | SI | 2.81 | 0.95 | 1.96 | 0.65 | 2.08 | 1.33 | 1.02 | 1.86 | 1.16 | 0.82 | 1.15 | 1.44 |
| SF | 0.32 | 0.08 | 0.22 | 0.09 | 0.25 | 0.17 | 0.16 | 0.20 | 0.11 | 0.11 | 0.10 | 0.16 | ||
| RR | 88.61 | 91.58 | 88.78 | 86.15 | 87.98 | 87.22 | 84.31 | 89.25 | 90.52 | 86.59 | 91.30 | 88.39 *** | ||
| Ca | %DM | SI | 0.64 | 0.54 | 0.69 | 0.33 | 0.87 | 0.86 | 0.41 | 0.54 | 0.45 | 1.17 | 0.90 | 0.67 |
| SF | 0.53 | 0.43 | 0.33 | 0.32 | 0.81 | 0.60 | 0.35 | 0.61 | 0.34 | 0.41 | 0.44 | 0.47 | ||
| RR | 17.19 | 20.37 | 52.17 | 3.03 | 6.90 | 30.23 | 14.63 | (12.96) | 24.44 | 64.96 | 51.11 | 24.73 *** | ||
| Mg | %DM | SI | 0.16 | 0.21 | 0.18 | 0.34 | 0.15 | 0.26 | 0.23 | 0.19 | 0.21 | 0.24 | 0.20 | 0.22 |
| SF | 0.06 | 0.06 | 0.04 | 0.09 | 0.08 | 0.08 | 0.08 | 0.07 | 0.05 | 0.05 | 0.05 | 0.06 | ||
| RR | 62.50 | 71.43 | 77.78 | 73.53 | 46.67 | 69.23 | 65.22 | 63.16 | 76.19 | 79.17 | 75.00 | 69.08 *** | ||
| P | %DM | SI | 0.33 | 0.27 | 0.26 | 0.1 | 0.22 | 0.18 | 0.21 | 0.17 | 0.19 | 0.18 | 0.12 | 0.20 |
| SF | 0.05 | 0.05 | 0.06 | 0.04 | 0.08 | 0.06 | 0.05 | 0.05 | 0.06 | 0.04 | 0.04 | 0.05 | ||
| RR | 84.85 | 81.48 | 76.92 | 60.00 | 63.64 | 66.67 | 76.19 | 70.59 | 68.42 | 77.78 | 66.67 | 72.11 *** | ||
| Na | %DM | SI | 0.01 | 0.37 | 0.29 | 0.19 | 0.01 | 0.09 | 0.42 | 0.02 | 0.04 | 0.08 | 0.05 | 0.14 |
| SF | 0.05 | 0.07 | 0.07 | 0.06 | 0.06 | 0.05 | 0.01 | 0.05 | 0.05 | 0.04 | 0.04 | 0.05 | ||
| RR | (400) | 81.08 | 75.86 | 68.42 | (500) | 44.44 | 76.19 | (150) | (25) | 50 | 20 | (59.91) | ||
| 2S/Cl | SI | 1.61 | 0.91 | 0.35 | 1.45 | 1.52 | 0.37 | 0.58 | 0.67 | 0.71 | 1.03 | 0.82 | 0.91 | |
| SF | 3.14 | 4.08 | 1.22 | 5.57 | 4.60 | 2.58 | 1.64 | 3.37 | 3.81 | 2.32 | 2.95 | 3.20 | ||
| Ash Softening Temperature | Ash Flowing Temperature | |||
|---|---|---|---|---|
| °C | °C | |||
| SI | SF | SI | SF | |
| AC | 1070 | 1050 | 1260 | 1297 |
| AE | 1040 | 1097 | 1290 | 1277 |
| AM | 1080 | 1143 | 1300 | 1353 |
| AS | 1023 | 1370 | 1243 | 1467 |
| BS | 1163 | 1170 | 1307 | 1277 |
| CD | 1153 | 1147 | 1280 | 1290 |
| DC | 1067 | 1077 | 1217 | 1273 |
| JE | 1027 | 1147 | 1213 | 1260 |
| PA 1 | 1157 | 1183 | 1377 | 1423 |
| PA 2 | 1167 | 1230 | 1247 | 1377 |
| PS | 1157 | 1180 | 1297 | 1400 |
| Mean | 1100 | 1163 | 1275 | 1336 |
| NOx | CO | SO2 | PM | (λ) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Partial Load | Full Load | Partial Load | Full Load | Partial Load | Full Load | Partial Load | Full Load | Partial Load | Full Load | ||
| AC | mg m−3 | 352.32 | 357.20 | 627.35 | 36.20 | 2.91 | 34.91 | 103.48 | 55.38 | 2.9 | 1.9 |
| AE | mg m−3 | 567.65 | 404.03 | 276.49 | 52.88 | 16.23 | 58.41 | 35.04 | 37.24 | 3.1 | 1.8 |
| AM | mg m−3 | 348.53 | 353.33 | 337.20 | 75.38 | 20.57 | 41.41 | 65.13 | 43.18 | 2.9 | 2 |
| CD | mg m−3 | 438.20 | 467.71 | 497.00 | 173.87 | 26.04 | 40.73 | 65.72 | 49.58 | 3.7 | 2.6 |
| DC | mg m−3 | 399.62 | 405.60 | 407.46 | 38.21 | 10.99 | 58.53 | 77.88 | 50.77 | 3 | 1.8 |
| PA2 | mg m−3 | 359.41 | 349.52 | 169.26 | 94.56 | 48.31 | 70.82 | 26.08 | 30.30 | 2.8 | 1.7 |
| Legal threshold a | 500 | 250 | 350 | 50 | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, B.; Hensgen, F.; Bühle, L.; Wachendorf, M. Solid Fuel Production from Semi-Natural Grassland Biomass—Results from a Commercial-Scale IFBB Plant. Energies 2018, 11, 3011. https://doi.org/10.3390/en11113011
Joseph B, Hensgen F, Bühle L, Wachendorf M. Solid Fuel Production from Semi-Natural Grassland Biomass—Results from a Commercial-Scale IFBB Plant. Energies. 2018; 11(11):3011. https://doi.org/10.3390/en11113011
Chicago/Turabian StyleJoseph, Ben, Frank Hensgen, Lutz Bühle, and Michael Wachendorf. 2018. "Solid Fuel Production from Semi-Natural Grassland Biomass—Results from a Commercial-Scale IFBB Plant" Energies 11, no. 11: 3011. https://doi.org/10.3390/en11113011





