Experimental and Computational Demonstration of a Low-Temperature Waste to By-Product Conversion of U.S. Oil Shale Semi-Coke to a Flue Gas Sorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Activation
2.2. Characterization of Activated Semi-Coke Samples
2.3. Application of Activated Semi-Coke to Flue Gas Treatment
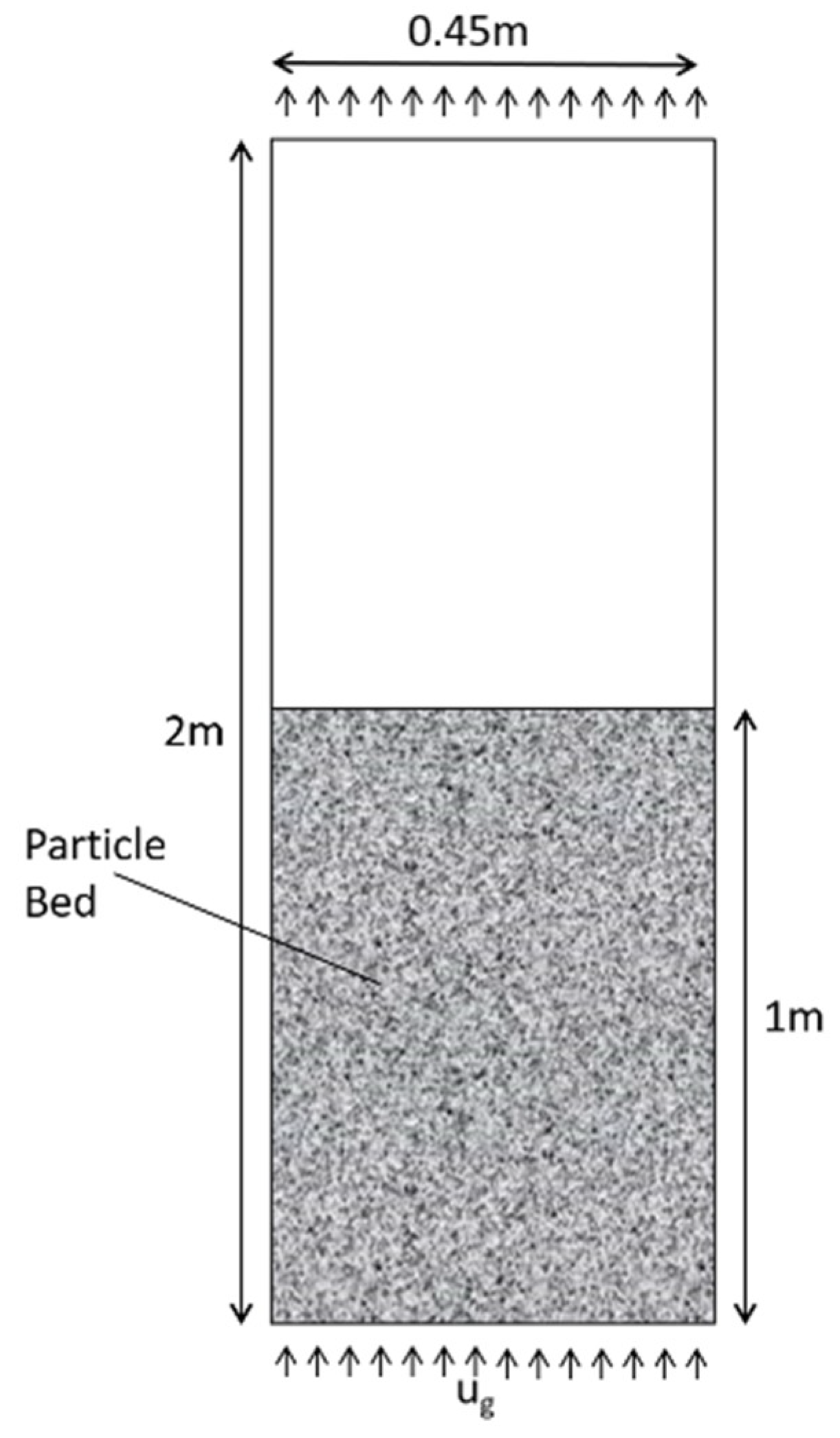
2.4. Computational Modeling of SO2 Adsorption at a Pilot Scale
3. Results and Discussion
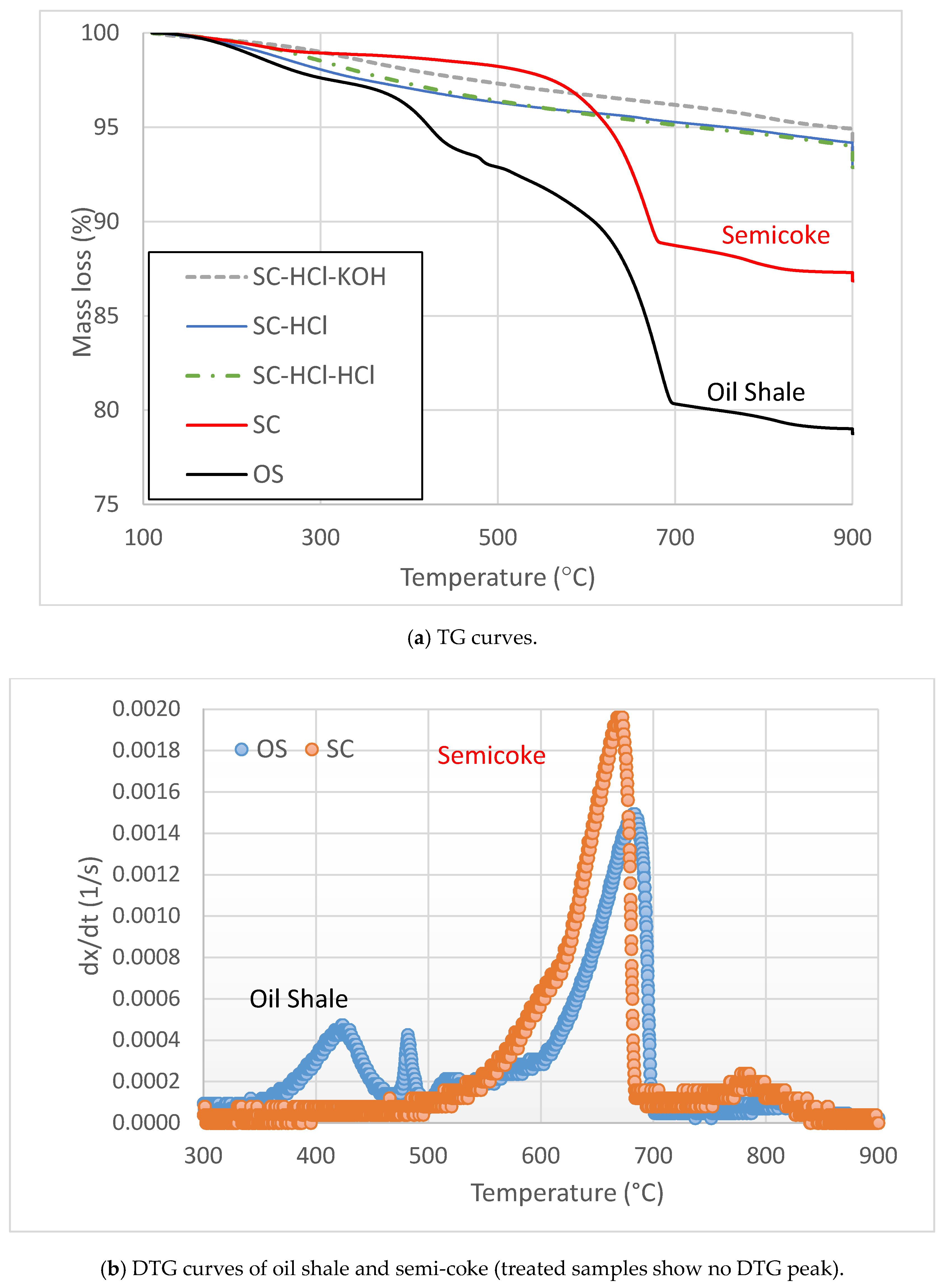
3.1. Impact of Activation on Semi-Coke
3.2. Ability of Semi-Coke Sorbents to Adsorb SO2
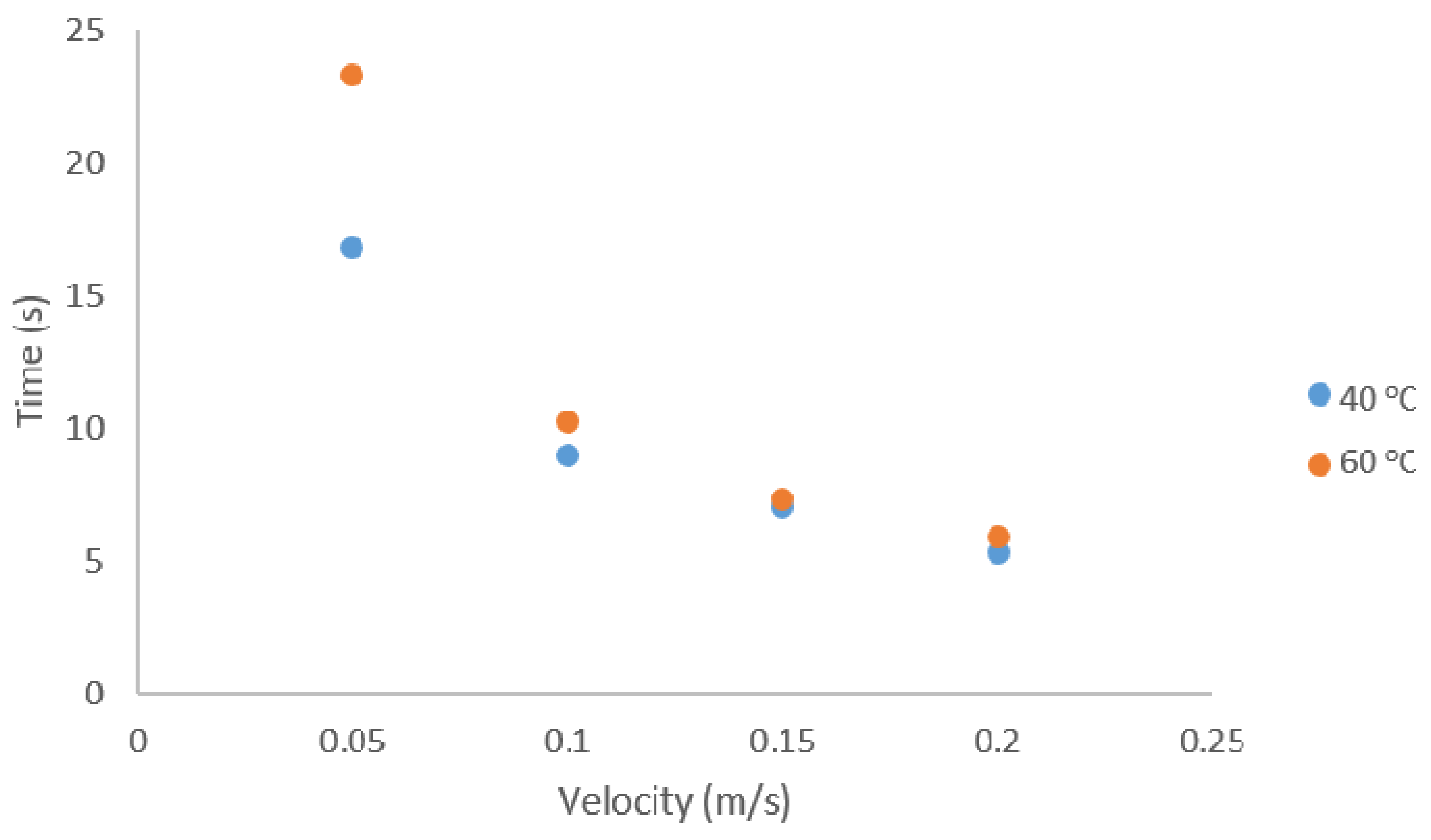
3.3. Simulated Pilot Scale Removal of SO2 Using Semi-Coke Sorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vyas, A.; Xue, J.; Goldfarb, J.L. Improving the Environmental and Economic Viability of U.S. Oil Shale via Waste-to-Byproduct Conversion of Semicoke to Sorbents. Energy Fuels 2016, 30, 188–195. [Google Scholar] [CrossRef]
- Brendow, K. Global Oil Shale Issues and Perspectives. In Proceedings of the Synthesis of the Symposium on Oil Shale, Tallinn, Estonia, 18–19 November 2002. [Google Scholar]
- Datangel, B.; Goldfarb, J.J.L. Heavy Metals in Colorado and Chinese Oil Shale Semicoke: Disposal Issues, Impediments to Byproduct Conversion. Energy Fuels 2011, 25, 3522–3529. [Google Scholar] [CrossRef]
- Han, X.X.; Jiang, X.M.; Cui, Z.G. Study of the Combustion Mechanism of Oil Shale Semicoke in a Thermogravimetric Analyzer. J. Therm. Anal. Calorim. 2008, 92, 595–600. [Google Scholar] [CrossRef]
- Kahru, A.; Põllumaa, L. Environmental Hazard of the Waste Streams of Estonian Oil Shale Industry: An Excotoxicological Review. Oil Shale 2006, 23, 53–93. [Google Scholar]
- Trikkel, A.; Kuusik, R.; Martins, A.; Pihu, T.; Stencel, J.M. Utilization of Estonian Oil Shale Semicoke. Fuel Process. Technol. 2008, 89, 756–763. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, B.; Wu, X.; Bai, J.; Sun, J. Influence of Retorting Temperature on Combustion Characteristics and Kinetic Parameters of Oil Shale Semicoke. Oil Shale 2006, 23, 328–339. [Google Scholar]
- Goldfarb, J.L.; D’Amico, A.; Culin, C.; Suuberg, E.M.; Külaots, I. Oxidation Kinetics of Oil Shale Semicokes: Reactivity as a Function of Pyrolysis Temperature and Shale Origin. Energy Fuels 2013, 27, 666–672. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Shawaqfeh, A.T.; Lafi, W.K. Adsorption of Pesticides from Aqueous Solutions Using Oil Shale Ash. Desalination 2007, 208, 294–305. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Banat, F.; Masad, A. Physical and Chemical Activation of Pyrolyzed Oil Shale Residue for the Adsorption of Phenol from Aqueous Solutions. Environ. Geol. 2003, 44, 333–342. [Google Scholar] [CrossRef]
- Külaots, I.; Goldfarb, J.L.; Suuberg, E.M. Characterization of Chinese, American and Estonian Oil Shale Semicokes and Their Sorptive Potential. Fuel 2010, 89, 3300–3306. [Google Scholar] [CrossRef]
- Nicolini, J.; Pereira, B.F.; Pillon, C.N.; Machado, V.G.; Lopes, W.A.; de Andrade, J.B.; Mangrich, A.S. Characterization of Brazilian Oil Shale Byproducts Planned for Use as Soil Conditioners for Food and Agro-Energy Production. J. Anal. Appl. Pyrolysis. 2011, 90, 112–117. [Google Scholar] [CrossRef]
- Liu, Q.; Li, C.; Li, Y. SO2 Removal from Flue Gas by Activated Semi-Cokes: 1. The Preparation of Catalysts and Determination of Operating Conditions. Carbon 2003, 41, 2217–2223. [Google Scholar] [CrossRef]
- Kaljuvee, T.; Kuusik, R.; Trikkel, A. SO2 Binding into the Solid Phase during Thermooxidation of Blendsestonian Oil Shale Semicoke. J. Therm. Anal. Calorim. 2003, 72, 393–404. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, L.; Zhang, Y.; Liang, J.; Wang, J.; Zhang, Z.; Wang, X. Activated Semi-Coke in SO2 removal from Flue Gas: Selection of Activation Methodology and Desulfurization Mechanism Study. Energy Fuels 2013, 27, 3080–3089. [Google Scholar] [CrossRef]
- Li, J.; Kobayashi, N.; Hu, Y. The Activated Coke Preparation for SO2 Adsorption by Using Flue Gas from Coal Power Plant. Chem. Eng. Process. Process Intensif. 2008, 47, 118–127. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.S.; Li, J.; Li, C. SO2 Removal from Flue Gas by Activated Semi-Cokes: 2. Effects of Physical Structures and Chemical Properties on SO2 Removal Activity. Carbon 2003, 41, 2225–2230. [Google Scholar] [CrossRef]
- Shangguan, J.; Li, C.H.; Miao, M.Q.; Yang, Z. Surface Characterization and SO2 Removal Activity of Activated Semi-Coke with Heat Treatment. New Carbon Mater. 2008, 23, 37–43. [Google Scholar] [CrossRef]
- Self, J.G.; Brownfield, M.E.; Johnson, R.C.; Mercier, T.J. Fischer Assay Histograms of Oil Shale Drill Cores and Cuttings from the Uinta Basin, Utah and Colorado. Available online: https://www.google.com.tw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwiworSMu9DeAhWKgrwKHfiDBXYQ7gEwAHoECAkQCA&url=https%3A%2F%2Ftranslate.google.com.tw%2Ftranslate%3Fhl%3Dzh-TW%26sl%3Den%26u%3Dhttps%3A%2F%2Fpubs.usgs.gov%2Fdds%2Fdds-069%2Fdds-069-bb%2FREPORTS%2F69_BB_CH_5.pdf%26prev%3Dsearch&usg=AOvVaw2zVMPts0VZ6mIlrN8UHXT1 (accessed on 27 September 2018).
- Patnaik, A.S.; Goldfarb, J.L. Continuous Activation Energy Representation of the Arrhenius Equation for the Pyrolysis of Cellulosic Materials: Feed Corn Stover and Cocoa Shell Biomass. Cellul. Chem. Technol. 2016, 50, 311–320. [Google Scholar]
- Williams, P.T.; Ahmad, N. Influence of Process Conditions on the Pyrolysis of Pakistani Oil Shales. Fuel 1999, 78, 653–662. [Google Scholar] [CrossRef]
- Richter, E. Carbon Catalysts for Pollution Control. Catal. Today 1990, 7, 93–112. [Google Scholar] [CrossRef]
- Weller, H.; Greenshields, C.; de Rouvray, C. OpenFOAM v.4.1. 2016. Available online: https://openfoam.org/version/4-1/ (accessed on 27 September 2018).
- Hoomans, B.P.B.; Kuipers, J.A.M.; Briels, W.J.; van Swaaij, W.P.M. Discrete Particle Simulation of Bubble and Slug Formation in a Tow-Dimensional Gas-Fluidised Bed: A Hard Sphere Approach. Chem. Eng. Sci. 1996, 51, 99–118. [Google Scholar] [CrossRef]
- van der Hoef, M.A.; van Sint Annaland, M.; Kuipers, J.A.M. Computational Fluid Dynamics for Dense Gas–Solid Fluidized Beds: A Multi-Scale Modeling Strategy. Chem. Eng. Sci. 2004, 59, 5157–5165. [Google Scholar] [CrossRef]
- Ergun, S. Fluid Flow through Packed Columns. Chem. Eng. Progr. 1952, 48, 89–94. [Google Scholar]
- Gidaspow, D. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Descriptions; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Wen, C.Y.; Yu, Y.H. A Generalized Method for Predicting the Minimum Fluidization Velocity. AIChE J. 1966, 610–612. [Google Scholar] [CrossRef]
- Miao, Z.; Wu, G.; Li, P.; Zhao, N.; Wang, P.; Meng, X. Combustion Characteristics of Daqing Oil Shale and Oil Shale Semi-Cokes. Min. Sci. Technol. 2009, 19, 380–384. [Google Scholar] [CrossRef]
- Sun, B.Z.; Wang, Q.; Shen, P.Y.; Qin, H.; Li, S.H. Kinetic Analysis of Co-Combustion of Oil Shale Semi-Coke with Bituminous Coal. Oil Shale 2012, 29, 63–75. [Google Scholar] [CrossRef]
- Jing, Z.; Cai, K.; Li, Y.; Fan, J.; Zhang, Y.; Miao, J.; Chen, Y.; Jin, F. Hydrothermal Synthesis of Pollucite, Analcime and Their Solid Solutions and Analysis of Their Properties. J. Nucl. Mater. 2017, 488, 63–69. [Google Scholar] [CrossRef]
- Martyniuk, H.; Wieckowska, J. The Effect of Coal Rank and Carbonization Temperature on SO2 Adsorption Properties of Coal Chars. Fuel 1997, 76, 563–565. [Google Scholar] [CrossRef]
- Davini, P. Adsorption of Sulphur Dioxide on Thermally Treated Active Carbon. Fuel 1989, 68, 145–148. [Google Scholar] [CrossRef]
- Lisovskii, A.; Shter, G.; Semiat, R.; Aharoni, C. Adsorption of Sulfur Dioxide by Active Carbon Treated by Nitric Acid: II. Effect of Preheating on the Adsorption Properties. Carbon 1997, 35, 1645–1648. [Google Scholar] [CrossRef]
- Kisiela, A.M.; Czajka, K.M.; Moroń, W.; Rybak, W.; Andryjowicz, C. Unburned Carbon from Lignite Fly Ash as an Adsorbent for SO2 Removal. Energy 2016, 116, 1454–1463. [Google Scholar] [CrossRef]
- Jastrząb, K. Properties of Activated Cokes Used for Flue Gas Treatment in Industrial Waste Incineration Plants. Fuel Process. Technol. 2012, 101, 16–22. [Google Scholar] [CrossRef]






| Sample | BET Surface Area m2/g | Volatile Carbon wt% | Fixed Carbon wt% | Ash wt% |
|---|---|---|---|---|
| OS | 8.2 ± 0.3 | 21.24 ± 0.40 | 0.28 ± 0.01 | 78.48 ± 1.49 |
| SC | 15.4 ± 1.3 | 13.50 ± 0.26 | 0.36 ± 0.03 | 86.14 ± 1.64 |
| SC-HCl | 89.0 ± 1.5 | 9.37 ± 0.17 | 0.30 ± 0.01 | 90.33 ± 1.11 |
| SC-HCl-HCl | 159.2 ± 3.2 | 9.77 ± 0.88 | 1.12 ± 0.03 | 89.11 ± 8.50 |
| SC-HCl-KOH | 123.7 ± 4.1 | 7.34 ± 0.49 | 1.03 ± 0.07 | 91.63 ± 6.12 |
| Adsorption Temperature °C | SC-HCl | SC-HCl-HCl | SC-HCl-KOH | |||
|---|---|---|---|---|---|---|
| Adsorption Uptake Rate mg/(gC)/min | SO2 Adsorbed mg/(gC) | Adsorption Uptake Rate mg/(gC)/min | SO2 Adsorbed mg/(gC) | Adsorption Uptake Rate mg/(gC)/min | SO2 Adsorbed mg/(gC) | |
| 40 | 27 ± 0.3 | 53 ± 1.064 | 28 ± 0.2 | 71 ± 0.9 | 34 ± 0.1 | 68 ± 0.488 |
| 60 | 30 ± 0.7 | 30 ± 1.106 | 30 ± 0.7 | 60 ± 2.3 | 64 ± 1.3 | 45 ± 1.543 |
| 80 | 10 ± 0.5 | 28 ± 2.44 | 17 ± 0.2 | 42 ± 0.9 | 13 ± 0.6 | 38 ± 2.862 |
| 110 | 9.2 ± 0.5 | 13 ± 1.159 | 11 ± 0.7 | 19 ± 2.2 | 9.1 ± 0.4 | 19 ± 1.494 |
| 150 | 2.1 ± 0.1 | 5.3 ± 0.496 | 2.3 ± 0.2 | 5.7 ± 0.9 | 2.9 ± 0.1 | 6.2 ± 0.409 |
| Adsorption Temperature °C | qe, Exp mg/(gC) | qe, Model mg/(gC) | k (gC)/mg/min |
|---|---|---|---|
| SC-HCl | |||
| 40 | 76 ± 2 | 79 | 1.3 × 10−2 ± 1.1 × 10−3 |
| 60 | 60 ± 3 | 63 | 9.9 × 10−3 ± 2.4 × 10−3 |
| 80 | 36 ± 4 | 36 | 6.3 × 10−2 ± 7.3 × 10−4 |
| 110 | 21 ± 2 | 22 | 6.7 × 10−2 ± 1.8 × 10−3 |
| Ea | 28.9 ± 0.7 kJ/mol | ||
| SC-HCl-HCl | |||
| 40 | 115 ± 3 | 122 | 3.9 × 10−3 ± 9.1 × 10−4 |
| 60 | 84 ± 1 | 86 | 1.4 × 10−2 ± 1.2 × 10−3 |
| 80 | 56 ± 2 | 57 | 2.6 × 10−2 ± 4.4 × 10−4 |
| 110 | 28 ± 1 | 26 | 4.0 × 10−2 ± 7.1 × 10−4 |
| Ea | 32.5±1.3 kJ/mol | ||
| SC-HCl-KOH | |||
| 40 | 88 ± 1 | 91 | 1.6 × 10−2 ± 5.2 × 10−4 |
| 60 | 70 ± 3 | 72 | 1.2 × 10−2 ± 7.3 × 10−4 |
| 80 | 45 ± 2 | 46 | 4.4 × 10−2 ± 8.3 × 10−4 |
| 110 | 25 ± 2 | 27 | 4.2 × 10−2 ± 1.0 × 10−3 |
| Ea | 17.3 ± 1.1 kJ/mol | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupre, K.; Ryan, E.M.; Suleimenov, A.; Goldfarb, J.L. Experimental and Computational Demonstration of a Low-Temperature Waste to By-Product Conversion of U.S. Oil Shale Semi-Coke to a Flue Gas Sorbent. Energies 2018, 11, 3195. https://doi.org/10.3390/en11113195
Dupre K, Ryan EM, Suleimenov A, Goldfarb JL. Experimental and Computational Demonstration of a Low-Temperature Waste to By-Product Conversion of U.S. Oil Shale Semi-Coke to a Flue Gas Sorbent. Energies. 2018; 11(11):3195. https://doi.org/10.3390/en11113195
Chicago/Turabian StyleDupre, Kathleen, Emily M. Ryan, Azat Suleimenov, and Jillian L. Goldfarb. 2018. "Experimental and Computational Demonstration of a Low-Temperature Waste to By-Product Conversion of U.S. Oil Shale Semi-Coke to a Flue Gas Sorbent" Energies 11, no. 11: 3195. https://doi.org/10.3390/en11113195




