An Experimental and Theoretical Study of the Gasification of Miscanthus Briquettes in a Double-Stage Downdraft Gasifier: Syngas, Tar, and Biochar Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass
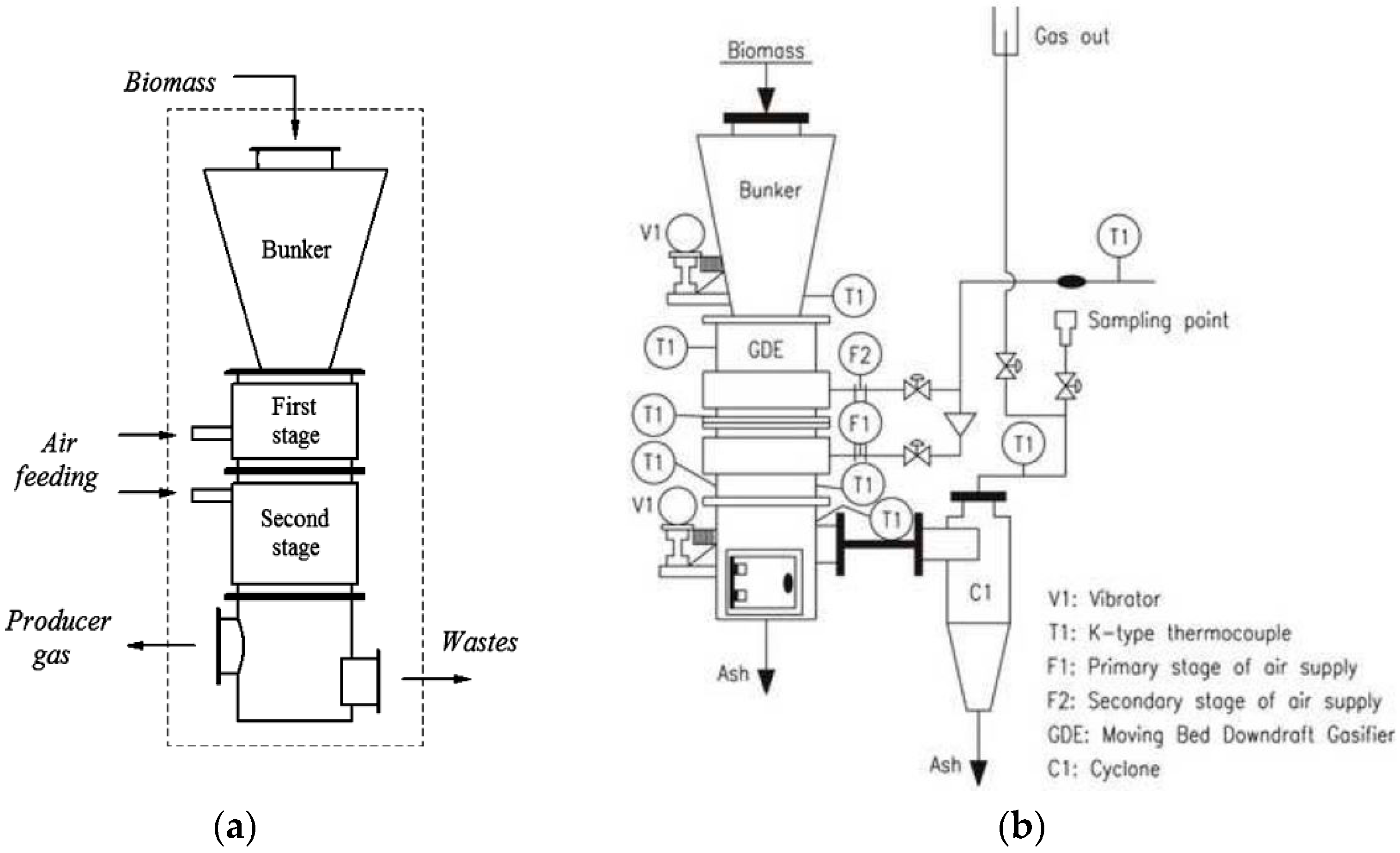
2.2. Double Air Stage Downdraft Gasifier
2.3. Operational Procedures
2.4. Experimental Planning
- The temperature in the different gasifier zones.
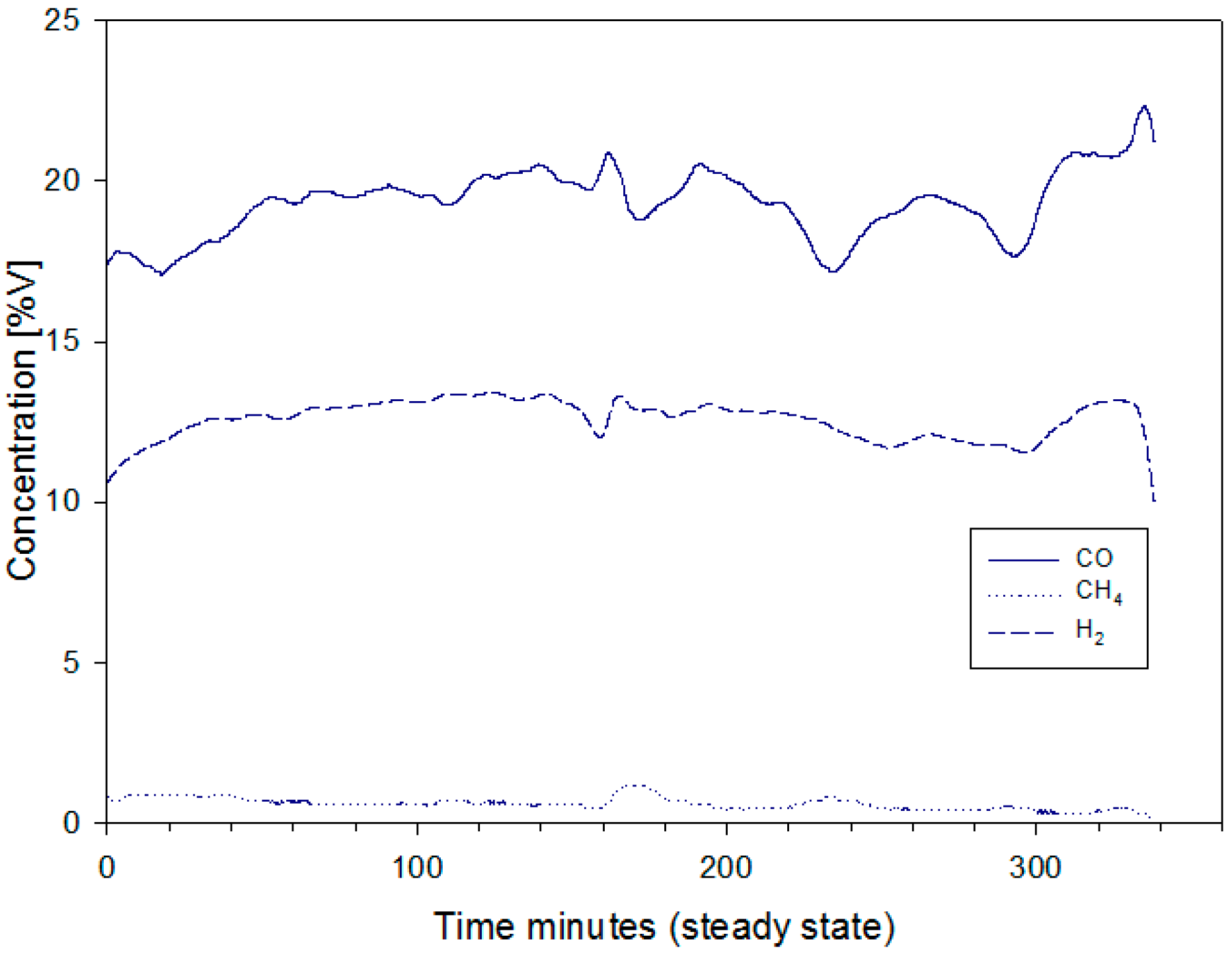
- CO, CH4, and H2 concentrations of the producer gas using the gas analyzer systems: BINOS 100, and HYDROS 100 (Emerson Process Management, Hasselroth, Germany). The uncertainty of BINOS 100 is ±0.2%, while that of HYDROS 100 is ±0.01%
- The syngas content for equivalence ratio between 0.2 to 0.45
- The biochar surface area was analyzed using a Nova 4200 instrument and a Hitachi S-4800 SEM instrument (Emerson Process Management, Hasselroth, Germany)
- Tar was collected using a set of six impingers, five of which contain isopropanol at a temperature of less than 0 °C. The average tar collected at steady state and content is obtained in mg/Nm3.
3. Mass and Energy Balance
3.1. Model Formulation
3.2. Model Validation
4. Results
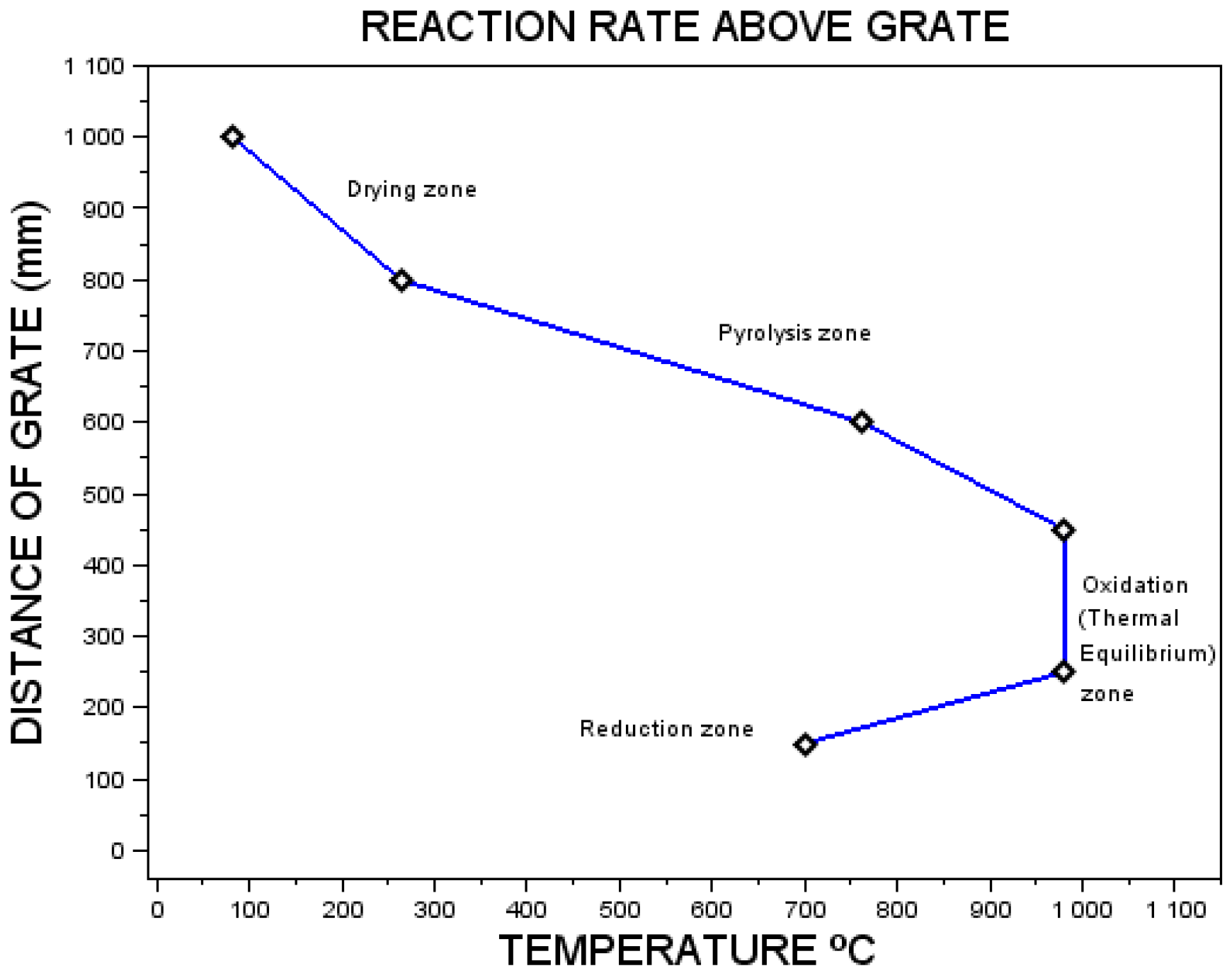
4.1. Gas Composition and Analysis
4.2. Comparison to Simulation Model
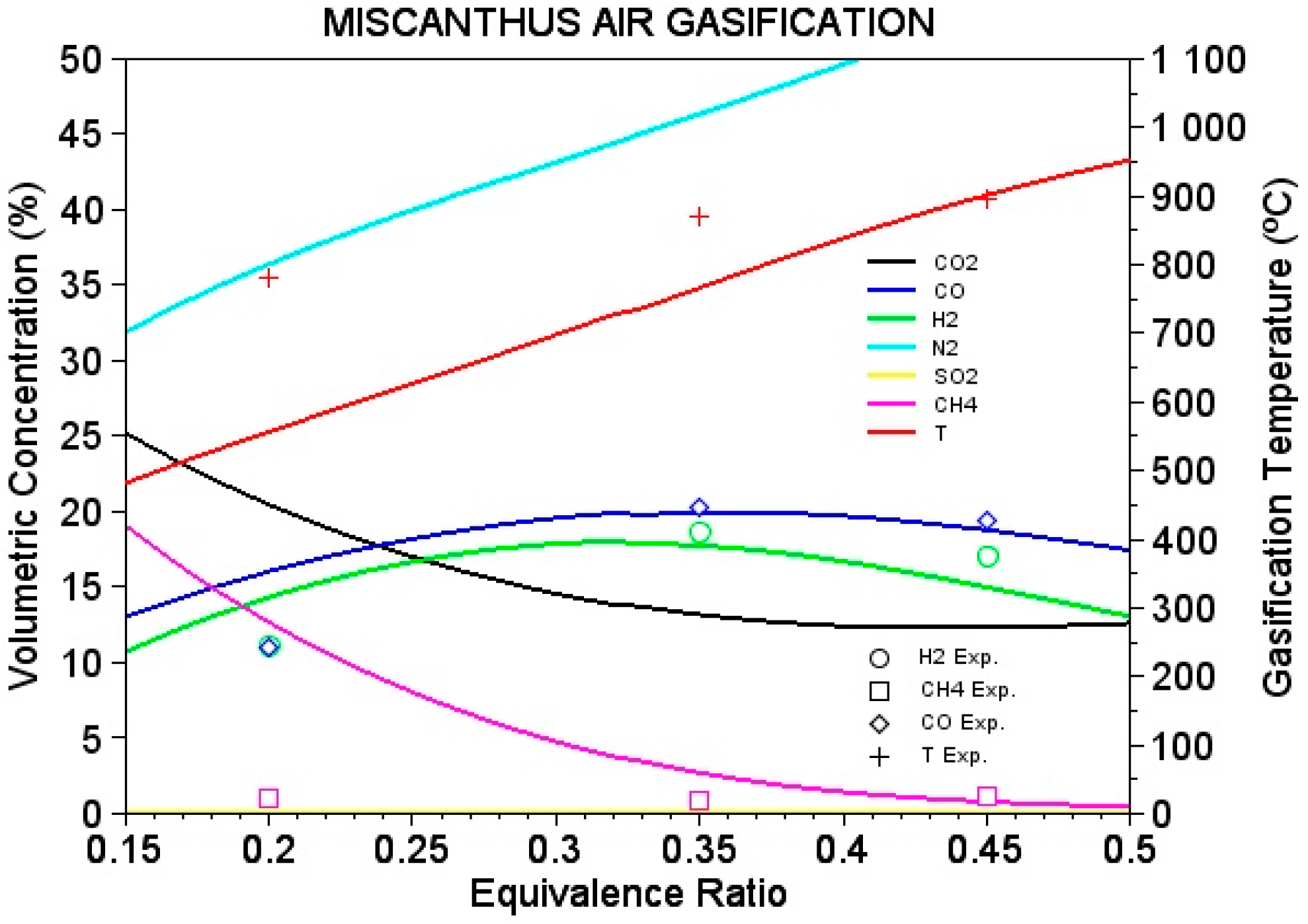
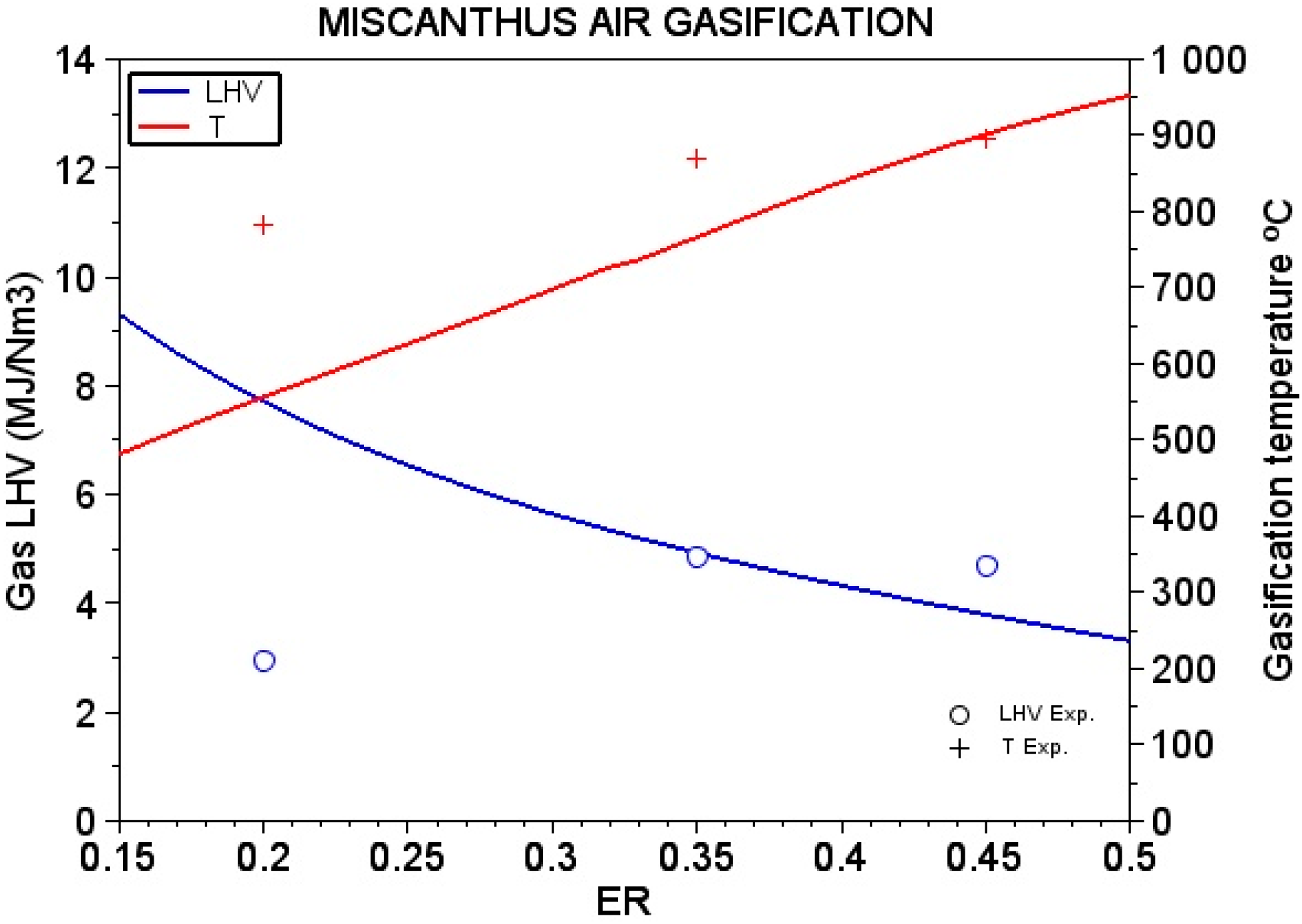
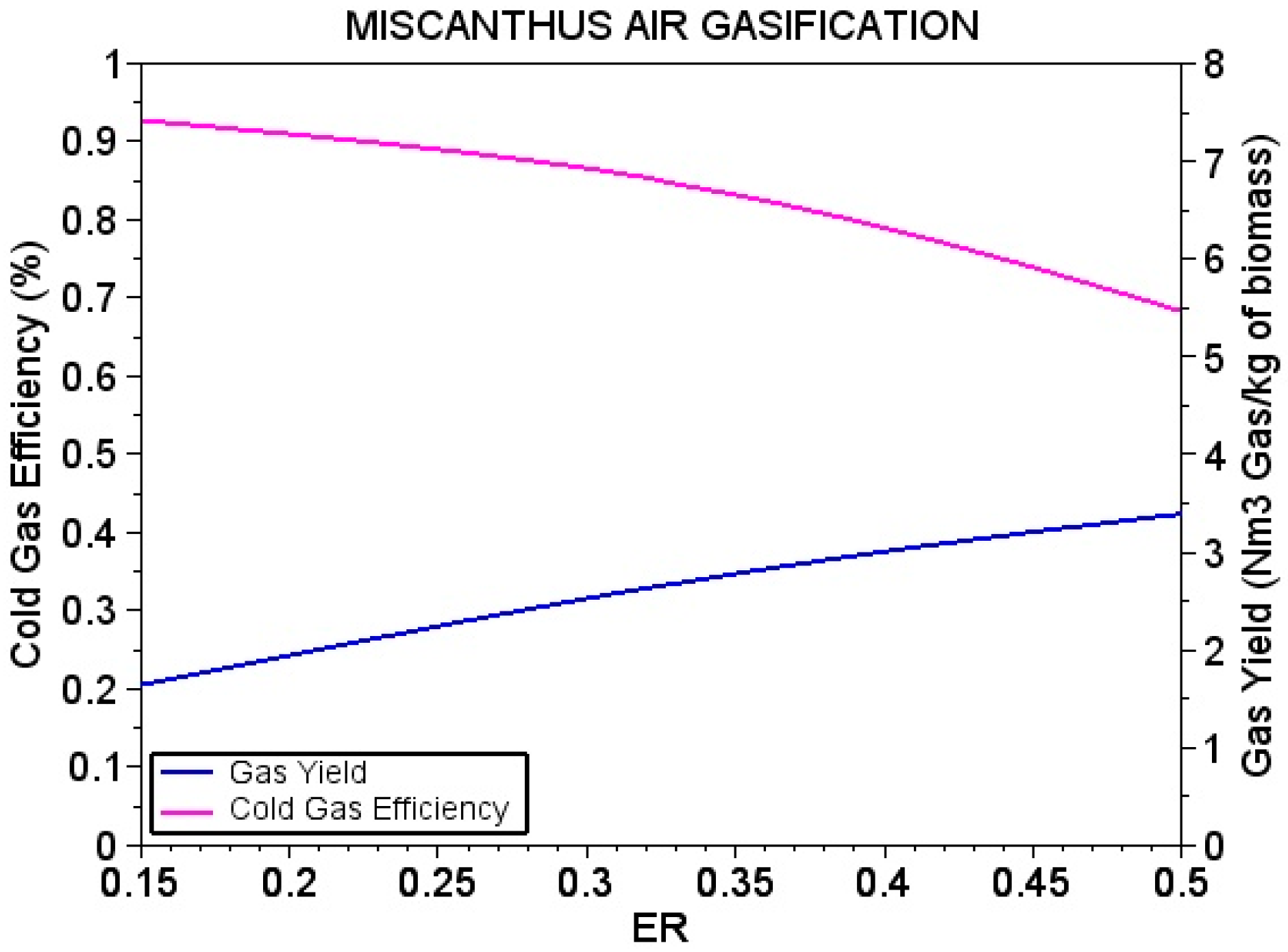
4.3. Performance of Variables
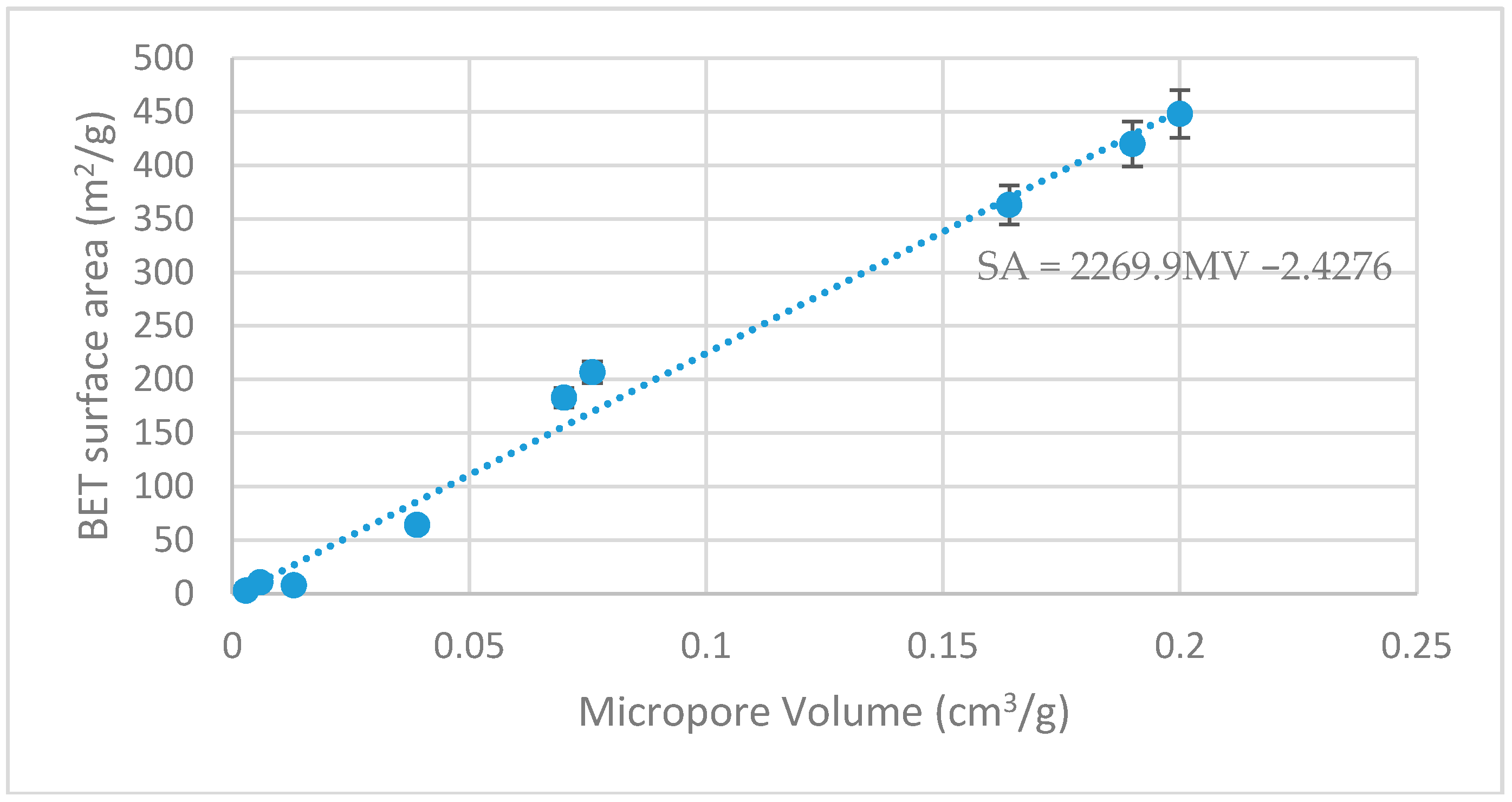
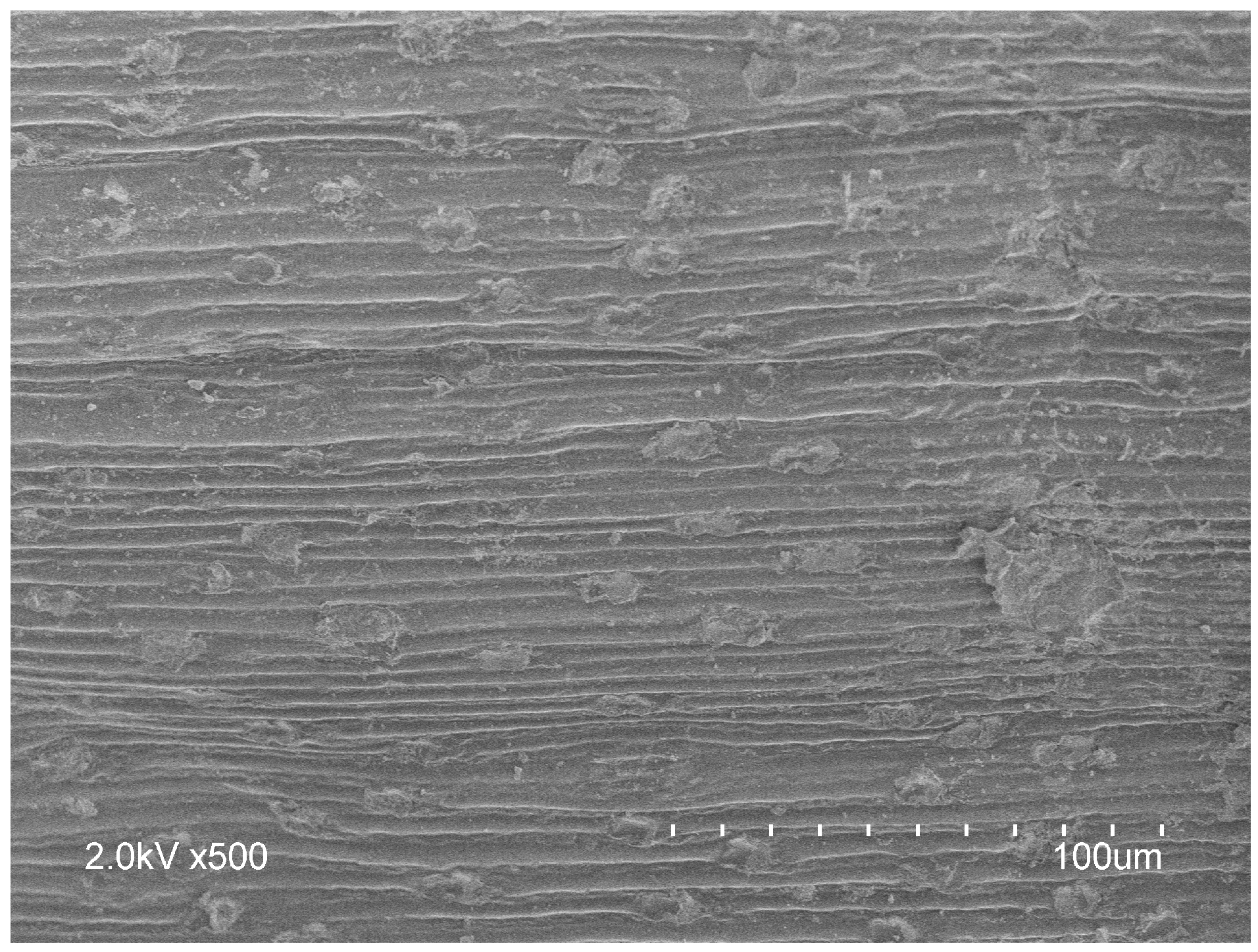
4.4. Biochar
4.5. Tar Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Ash content (%) |
| A/F | Air Fuel |
| a1…a8 | Stoichiometric coefficients of gasification products reaction |
| Carbon | |
| Specific Heat (kJ/kgK) | |
| CGE | Cold gas efficiency (%) |
| c1…c7 | Burcat polynomial coefficients |
| ER | Equivalence ratio |
| Gibbs Equation (kJ/kmol) | |
| Specific enthalpy (kJ/kg) | |
| H | Hydrogen |
| Formation enthalpy (kJ/kmol) | |
| Equilibrium constant | |
| Mass (kg) | |
| M | Molecular mass (kg/kmol) |
| Biomass moisture-umid basis% | |
| N | Nitrogen |
| OP | Oxygen concentration (%) |
| O | Oxygen |
| HV | Heat Value (kJ/kg) |
| LHV | Low heat value (kJ/kg) |
| HHV | High heat value (kJ/kg) |
| SB | Steam biomass ratio |
| S | Sulfur % |
| s | Specific entropy (kJ/kgK) |
| v | Number of sulfur atoms contained in biomass |
| x | Number of carbon atoms contained in biomass |
| Molar fraction | |
| Stoichiometric coefficients | |
| y | Number of hydrogen atoms contained in biomass |
| Gas yield (kg gás/kg biomass) | |
| w | Number of nitrogen atoms contained in biomass |
| z | Number of oxygen atoms contained in biomass |
| Subscripts | |
| db | dry basis |
| bio | biomass |
| drybio | dry biomass |
| c | combustion |
| c | carbon |
| stoich | stoichiometric |
| c | cold |
| g | gas |
| g | gasification |
| h | hydrogen |
| i | component |
| j | product |
| l | líquid |
| lv | líquid vapor |
| m | mass |
| n | nitrogen |
| o | oxygen |
| piro | pyrolysis |
| p | constant pressure |
| p | product |
| r | reagent |
| s | solid |
| s | sulfur |
| T | temperature |
| w | wet |
| v | vapor, volumetric |
| v | stoichiometric coefficient for sulfur |
| x | stoichiometric coefficient for carbon |
| y | stoichiometric coefficient for hydrogen |
| z | stoichiometric coefficient for oxygen |
| w | stoichiometric coefficient for nitrogen |
| Greek letters | |
| α | Molar content of moisture biomass |
| β | Air molar content |
| γ | Molar content of water vapor |
| δ | Nitrogen molar content |
| ν | Matrix of stoichiometric coefficients |
| ω | Percentage in elementary analysis (%) |
References
- Sen, S.; Ganguly, S. Opportunities, barriers and issues with renewable energy development—A discussion. Renew. Sustain. Energy Rev. 2017, 69, 1170–1181. [Google Scholar] [CrossRef]
- International Energy Agency. Africa Energy Outlook 2014; International Energy Agency: Paris, France, 2015. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, CA, USA, 2013. [Google Scholar]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.I.; Yoon, S.J.; Kim, Y.K.; Lee, J.G. Gasification and power generation characteristics of woody biomass utilizing a downdraft gasifier. Biomass Bioenergy 2011, 35, 4215–4220. [Google Scholar] [CrossRef]
- Ravikiran, A.; Renganathan, T.; Pushpavanam, S.; Voolapalli, R.K.; Cho, Y.S. Generalized analysis of gasifier performance using equilibrium modeling. Ind. Eng. Chem. Res. 2011, 51, 1601–1611. [Google Scholar] [CrossRef]
- Martinez, J.D.; Lora, E.E.S.; Andrade, R.V.; Jaén, R.L. Experimental study on biomass gasification in a double air stage downdraft reactor. Biomass Bioenergy 2011, 35, 3465–3480. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Huang, J.; Schmidt, K.G.; Bian, Z. Removal and conversion of tar in syngas from woody biomass gasification for power utilization using catalytic hydrocracking. Energies 2011, 4, 1163–1177. [Google Scholar] [CrossRef]
- Soomro, A.; Chen, S.; Ma, S.; Xiang, W. Catalytic activities of nickel, dolomite, and olivine for tar removal and H2-enriched gas production in biomass gasification process. Energy Environ. 2018, 29, 839–867. [Google Scholar] [CrossRef]
- Shrestha, S.; Ali, B.S.; Hamid, M.D.B. Cold flow model of dual fluidized bed: A review. Renew. Sustain. Energy Rev. 2016, 53, 1529–1548. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Paethanom, A.; Nakahara, S.; Kobayashi, M.; Prawisudha, P.; Yoshikawa, K. Performance of tar removal by absorption and adsorption for biomass gasification. Fuel Process. Technol. 2012, 104, 144–154. [Google Scholar] [CrossRef]
- Bui, T.; Loof, R.; Bhattacharya, S.C. Multi-stage reactor for thermal gasification of wood. Energy 1994, 19, 397–404. [Google Scholar] [CrossRef]
- Galindo, A.L.; Lora, E.S.; Andrade, R.V.; Giraldo, S.Y.; Jaén, R.L.; Cobas, V.M. Biomass gasification in a downdraft gasifier with a two-stage air supply: Effect of operating conditions on gas quality. Biomass Bioenergy 2014, 61, 236–244. [Google Scholar] [CrossRef]
- Reed, T.B.; Walt, R.; Ellis, S.; Das, A.; Deutch, S. Superficial velocity—The key to downdraft gasification. In Biomass—A Growth Opportunity in Green Energy; Overend, R., Chornet, E., Eds.; Pergamon Press: Pergamon, Turkey, 1999. [Google Scholar]
- Peterson, S.C.; Jackson, M.A.; Kim, S.; Palmquist, D.E. Increasing biochar surface area: Optimization of ball milling parameters. Powder Technol. 2012, 228, 115–120. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Kumar, A.; Bellmer, D.D.; Huhnke, R.L. Synthesis and evaluation of biochar-derived catalysts for removal of toluene (model tar) from biomass-generated producer gas. Renew. Energy 2014, 66, 346–353. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of biomass feedstocks and gasification conditions on the physiochemical properties of char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- Atkinson, C.J. Establishing perennial grass energy crops in the UK: A review of current propagation options for Miscanthus. Biomass Bioenergy 2009, 33, 752–759. [Google Scholar] [CrossRef]
- Siedlecki, M.; De Jong, W. Biomass gasification as the first hot step in clean syngas production process-gas quality optimization and primary tar reduction measures in a 100 kW thermal input steam-oxygen blown CFB gasifier. Biomass Bioenergy 2011, 35, S40–S62. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Yim, J.H.; Sohn, J.M.; Park, J.; Kim, S.S.; Ryu, C.; Jeon, J.K.; Park, Y.K. Influence of operation variables on fast pyrolysis of Miscanthus sinensis var. Purpurascens. Bioresour. Technol. 2010, 101, 3672–3677. [Google Scholar] [CrossRef] [PubMed]
- Porbatzki, D.; Stemmler, M.; Müller, M. Release of inorganic trace elements during gasification of wood, straw, and miscanthus. Biomass Bioenergy 2011, 35, S79–S86. [Google Scholar] [CrossRef]
- Michel, R.; Rapagnà, S.; Di Marcello, M.; Burg, P.; Matt, M.; Courson, C.; Gruber, R. Catalytic steam gasification of Miscanthus X giganteus in fluidised bed reactor on olivine based catalysts. Fuel Process. Technol. 2011, 92, 1169–1177. [Google Scholar] [CrossRef]
- De Jong, W.; Andries, J.; Hein, K.R. Coal-biomass gasification in a pressurized fluidized bed gasifier. In Proceedings of the ASME International GT and Aerospace Congress, Stockholm, SE, USA, 2–5 June 1998; pp. 1–7. [Google Scholar]
- Xue, G.; Kwapinska, M.; Horvat, A.; Li, Z.; Dooley, S.; Kwapinski, W.; Leahy, J.J. Gasification of Miscanthus × giganteus in an air-blown bubbling fluidized bed: A preliminary study of performance and agglomeration. Energy Fuels 2014, 28, 1121–1131. [Google Scholar] [CrossRef]
- Kwapinska, M.; Xue, G.; Horvat, A.; Rabou, L.P.; Dooley, S.; Kwapinski, W.; Leahy, J.J. Fluidized Bed Gasification of Torrefied and Raw Grassy Biomass (Miscanthus× gigantenus). The Effect of Operating Conditions on Process Performance. Energy Fuels 2015, 29, 7290–7300. [Google Scholar] [CrossRef]
- Kallis, K.X.; Susini, G.A.; Oakey, J.E. A comparison between Miscanthus and bioethanol waste pellets and their performance in a downdraft gasifier. Appl. Energy 2013, 101, 333–340. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gökalp, I. Pyrolysis, combustion and gasification characteristics of miscanthus and sewage sludge. Energy Convers. Manag. 2015, 89, 83–91. [Google Scholar] [CrossRef]
- Khelfa, A.; Sharypov, V.; Finqueneisel, G.; Weber, J.V. Catalytic pyrolysis and gasification of Miscanthus Giganteus: Haematite (Fe2O3) a versatile catalyst. J. Anal. Appl. Pyrolysis 2009, 84, 84–88. [Google Scholar] [CrossRef]
- Ahmed, T.Y.; Ahmad, M.M.; Yusup, S.; Inayat, A.; Khan, Z. Mathematical and computational approaches for design of biomass gasification for hydrogen production: A review. Renew. Sustain. Energy Rev. 2012, 16, 2304–2315. [Google Scholar] [CrossRef]
- Baruah, D.; Baruah, D.C. Modeling of biomass gasification: A review. Renew. Sustain. Energy Rev. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and analysis of biomass gasification models. Renew. Sustain. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- Widholm, J.A.C.K.; Zabotina, O.A.; McIsaac, G.F.; David, M.B.; Voigt, T.B.; Boersma, N.N.; Long, S.P. Miscanthus: A promising biomass crop. Adv. Bot. Res. 2010, 56, 75–137. [Google Scholar]
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Heaton, E.; Voigt, T.; Long, S.P. A quantitative review comparing the yields of two candidate C 4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenergy 2004, 27, 21–30. [Google Scholar] [CrossRef]
- Termoquip Energia Alternativa Ltd. Gaseificador Tipo Co-Corrente com Dois Estágios; Protocolo de Venda do Equipamento; Termoquip Energia Alternativa Ltd.: São Paulo, Brazil, 2007. [Google Scholar]
- Reed, T.B.; Das, A. Handbook of Biomass Downdraft Gasifier Engine Systems; Biomass Energy Foundation: Golden, CO, USA, 1988. [Google Scholar]
- Souza-Santos, M.L.D. Solid Fuels Combustion and Gasification. Modeling, Simulation and Equipment Operation; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Jarungthammachote, S.; Dutta, A. Thermodynamic equilibrium model and second law analysis of a downdraft waste gasifier. Energy 2007, 32, 1660–1669. [Google Scholar] [CrossRef]
- Itai, Y.; Santos, R.; Branquinho, M.; Malico, I.; Ghesti, G.F.; Brasil, A.M. Numerical and experimentaassessment of a downdraft gasifier for electric power in Amazon using açaí seed (Euterpe oleracea Mart.) as a fuel. Renew. Energy 2014, 66, 662–669. [Google Scholar] [CrossRef]
- Burcat, A. Third Millennium Ideal Gas and Condensed Phase Thermochemical Database for Combustion with Updates from Active Thermochemical Tables; Argonne National Lab.: Argonne, IL, USA, 2005. [Google Scholar]
- Balu, E.; Lee, U.; Chung, J.N. High temperature steam gasification of woody biomass—A combined experimental and mathematical modeling approach. Int. J. Hydrogen Energy 2015, 40, 14101–14115. [Google Scholar] [CrossRef]
- Diyoke, C.; Gao, N.; Aneke, M.; Wang, M.; Wu, C. Modelling of down-draft gasification of biomass—An integrated pyrolysis, combustion and reduction process. Appl. Therm. Eng. 2018, 142, 444–456. [Google Scholar] [CrossRef]
- Campoy, M.; Gómez-Barea, A.; Vidal, F.B.; Ollero, P. Air–steam gasification of biomass in a fluidised bed: Process optimisation by enriched air. Fuel Process. Technol. 2009, 90, 677–685. [Google Scholar] [CrossRef]
- OLIVEIRA, C.A.V.B.D. Estudo experimental da gaseificação do eucalipto em gaseificador co-corrente de duplo estágio com ar, vapor saturado e oxigênio para produção de gás de síntese. Ph.D. Thesis, Universidade Federal de Itajubá, Itajubá, Brazil, 2015. [Google Scholar]
- Dogru, M.; Howarth, C.R.; Akay, G.; Keskinler, B.; Malik, A.A. Gasification of hazelnut shells in a downdraft gasifier. Energy 2002, 27, 415–427. [Google Scholar] [CrossRef]
- Guo, F.; Dong, Y.; Dong, L.; Guo, C. Effect of design and operating parameters on the gasification process of biomass in a downdraft fixed bed: An experimental study. Int. J. Hydrogen Energy 2014, 39, 5625–5633. [Google Scholar] [CrossRef]
- Nascimento, F.R.M. Equilibrium Modeling of a Downdraft Gasifier Using Different Gasification Fluids, Itajubá. Master’s Thesis, Institute of Mechanical Engineering, Federal University of Itajubá, Itajubá, Brazil, 2015. [Google Scholar]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T.F. Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Fellet, G.; Marchiol, L.; Delle Vedove, G.; Peressotti, A. Application of biochar on mine tailings: Effects and perspectives for land reclamation. Chemosphere 2011, 83, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Do, D.D. The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover-and peanut shell-derived biochar properties and TCE adsorption water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Guerrero, M.; Ruiz, M.P.; Millera, Á.; Alzueta, M.U.; Bilbao, R. Characterization of biomass chars formed under different devolatilization conditions: Differences between rice husk and eucalyptus. Energy Fuels 2008, 22, 1275–1284. [Google Scholar] [CrossRef]
- Macias-García, A.; García, M.B.; Díaz-Díez, M.A.; Jiménez, A.H. Preparation of active carbons from a commercial holm-oak charcoal: Study of micro-and meso-porosity. Wood Sci. Technol. 2004, 37, 385–394. [Google Scholar] [CrossRef]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- LI, C.; SUZUKI, K. Tar property, analysis, reforming mechanism and model for biomass gasification—An overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]




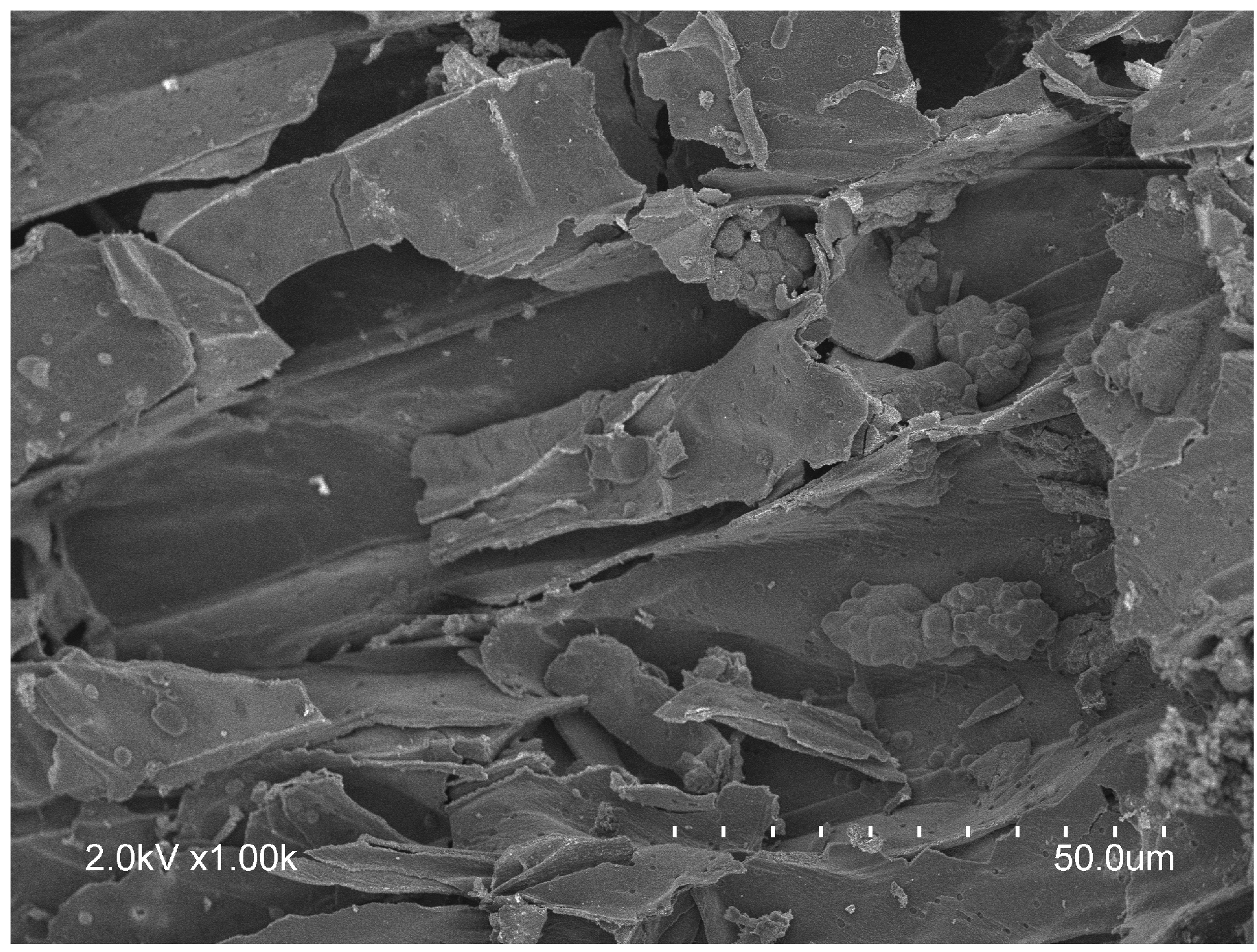
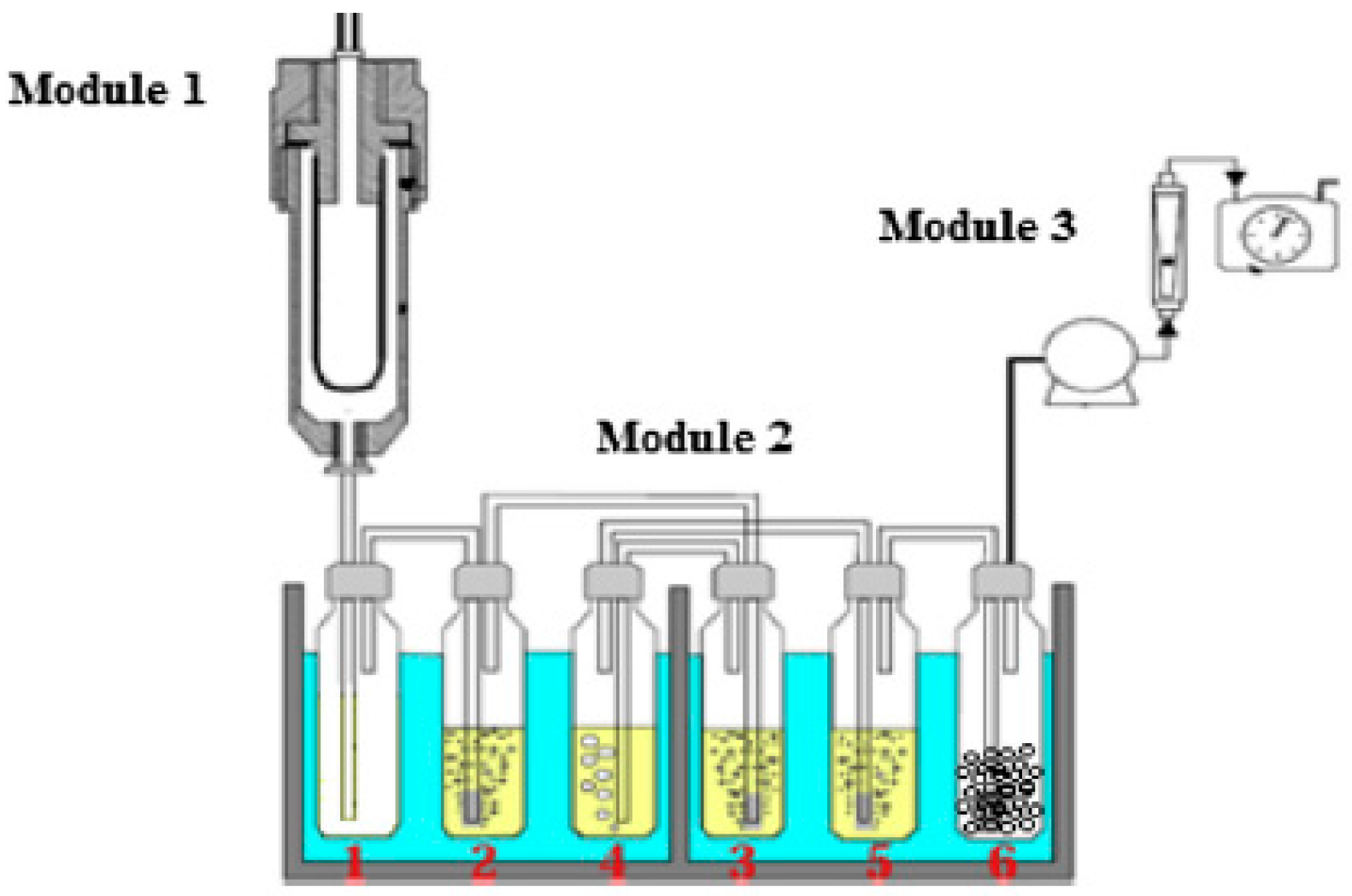
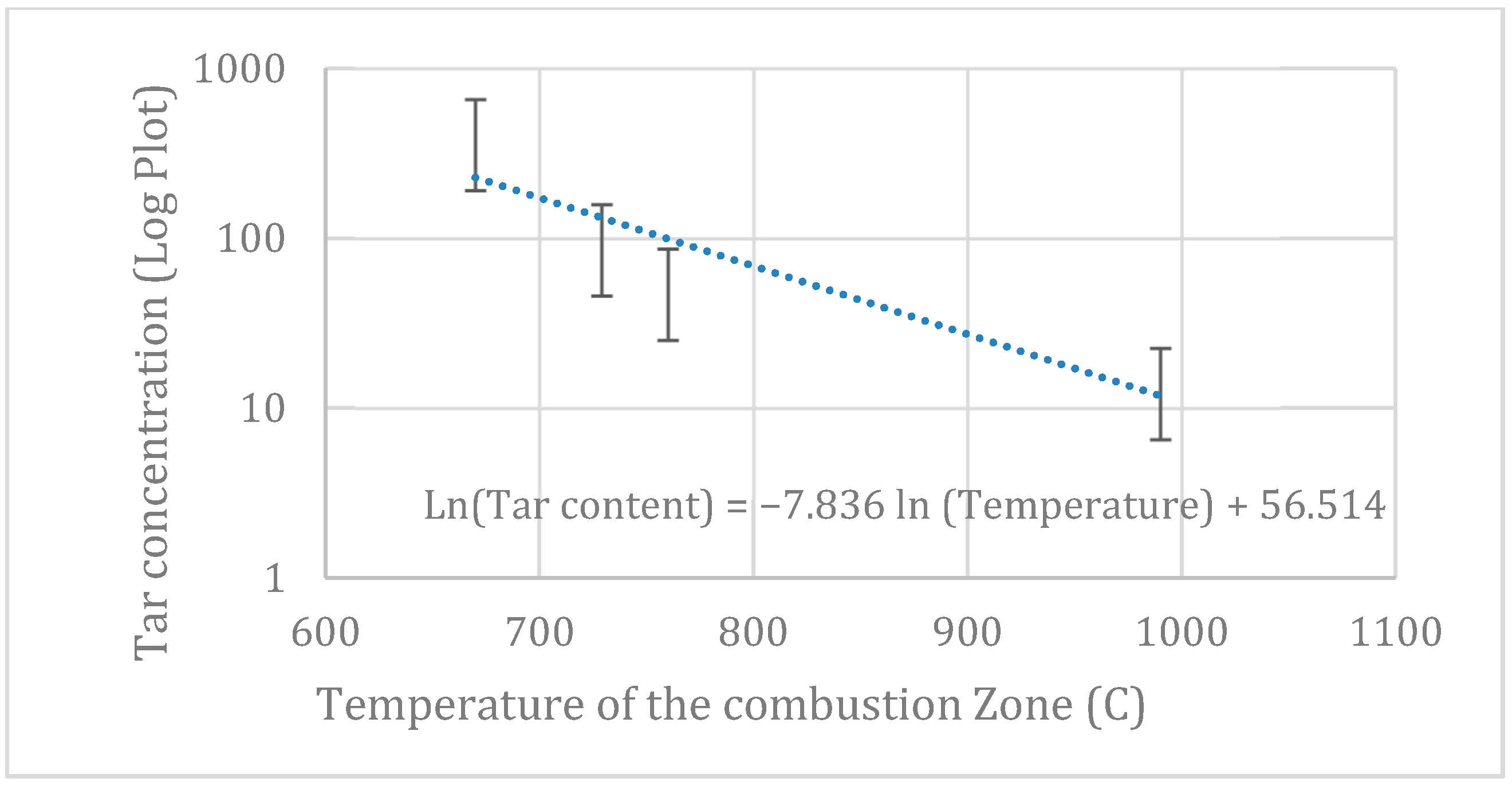
| Proximate Analysis | Ultimate Analysis | ||
|---|---|---|---|
| Moisture | 11.6% | Carbon | 48.6% |
| Volatile Matter | 67.1% | Hydrogen | 6% |
| Fixed Carbon | 16.9% | Nitrogen | 0.3% |
| Ash | 4.4 | Sulfur | 0.1% |
| Total | 100.0% | Oxygen | 45.0% |
| LHV (MJ/kg) | 16.3 | Total | 100% |
| Run | Gasification Parameters | CO | H2 | CO2 | CH4 | RMS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | ER | SB | OP | E | M | E | M | E | M | E | M | - |
| 1 | 0.35 | 0 | 21 | 15.80 | 24.57 | 8.70 | 17.03 | 15.10 | 9.68 | 5.10 | 2.08 | 6.08 |
| 2 | 0.33 | 0.22 | 21 | 15.40 | 19.30 | 11.90 | 19.26 | 15.90 | 13.96 | 4.80 | 3.27 | 3.89 |
| 3 | 0.33 | 0.45 | 21 | 13.80 | 14.46 | 13.30 | 20.12 | 17.00 | 17.56 | 4.60 | 4.12 | 3.09 |
| 4 | 0.27 | 0.23 | 21 | 15.00 | 17.62 | 14.00 | 18.90 | 16.20 | 16.67 | 4.70 | 6.46 | 2.64 |
| 5 | 0.27 | 0.43 | 21 | 11.90 | 12.96 | 16.20 | 19.67 | 18.60 | 20.12 | 5.30 | 7.28 | 1.97 |
| 6 | 0.36 | 0.32 | 30 | 18.90 | 24.24 | 16.40 | 24.20 | 17.60 | 15.42 | 5.50 | 2.00 | 4.63 |
| 7 | 0.35 | 0.60 | 30 | 15.70 | 18.18 | 18.30 | 25.70 | 18.80 | 19.93 | 5.70 | 3.24 | 3.74 |
| 8 | 0.25 | 0.31 | 30 | 20.80 | 21.40 | 20.00 | 24.53 | 15.80 | 19.30 | 6.70 | 7.38 | 2.60 |
| 9 | 0.24 | 0.58 | 30 | 15.30 | 14.39 | 22.30 | 25.24 | 20.30 | 24.66 | 7.10 | 9.29 | 2.61 |
| 10 | 0.38 | 0.33 | 35 | 20.00 | 26.57 | 17.50 | 25.38 | 16.80 | 16.06 | 5.60 | 1.55 | 4.97 |
| 11 | 0.34 | 0.56 | 35 | 17.50 | 21.06 | 21.80 | 27.89 | 18.00 | 20.24 | 6.10 | 3.31 | 3.61 |
| 12 | 0.27 | 0.31 | 35 | 23.90 | 24.86 | 22.40 | 27.06 | 12.60 | 18.35 | 7.30 | 5.74 | 3.46 |
| 13 | 0.26 | 0.63 | 35 | 19.30 | 16.57 | 25.10 | 28.10 | 16.20 | 24.53 | 7.40 | 7.76 | 4.24 |
| 14 | 0.32 | 0.10 | 40 | 27.40 | 35.26 | 18.30 | 26.84 | 16.20 | 12.23 | 7.30 | 2.31 | 5.94 |
| 15 | 0.33 | 0.29 | 40 | 25.10 | 25.54 | 23.10 | 28.37 | 13.70 | 16.15 | 6.50 | 2.58 | 3.23 |
| 16 | 0.35 | 0.36 | 40 | 23.90 | 27.93 | 22.30 | 28.29 | 14.60 | 17.24 | 6.70 | 2.23 | 4.08 |
| 17 | 0.32 | 0.54 | 40 | 20.20 | 22.85 | 24.50 | 29.89 | 16.70 | 20.97 | 6.90 | 3.92 | 3.67 |
| 18 | 0.33 | 0.57 | 40 | 19.30 | 22.51 | 25.70 | 29.87 | 17.00 | 21.15 | 6.70 | 3.61 | 3.44 |
| 19 | 0.26 | 0.30 | 40 | 28.50 | 26.84 | 25.70 | 28.57 | 9.20 | 18.68 | 8.10 | 6.18 | 4.66 |
| 20 | 0.24 | 0.56 | 40 | 23.50 | 18.70 | 27.50 | 29.33 | 14.60 | 24.81 | 7.70 | 8.75 | 5.22 |
| - | Average | 3.89 | ||||||||||
| ER | H2 % | CO% | CH4 % |
|---|---|---|---|
| 0.2 | 11.2 | 10.97 | 1.03 |
| 0.35 | 18.68 | 20.29 | 0.86 |
| 0.45 | 17.07 | 19.43 | 1.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, T.; Yepes Maya, D.M.; M. Nascimento, F.R.; Shi, Y.; Ratner, A.; Silva Lora, E.E.; Mendes Neto, L.J.; Escobar Palacios, J.C.; Vieira Andrade, R. An Experimental and Theoretical Study of the Gasification of Miscanthus Briquettes in a Double-Stage Downdraft Gasifier: Syngas, Tar, and Biochar Characterization. Energies 2018, 11, 3225. https://doi.org/10.3390/en11113225
Sharma T, Yepes Maya DM, M. Nascimento FR, Shi Y, Ratner A, Silva Lora EE, Mendes Neto LJ, Escobar Palacios JC, Vieira Andrade R. An Experimental and Theoretical Study of the Gasification of Miscanthus Briquettes in a Double-Stage Downdraft Gasifier: Syngas, Tar, and Biochar Characterization. Energies. 2018; 11(11):3225. https://doi.org/10.3390/en11113225
Chicago/Turabian StyleSharma, Tejasvi, Diego M. Yepes Maya, Francisco Regis M. Nascimento, Yunye Shi, Albert Ratner, Electo E. Silva Lora, Lourival Jorge Mendes Neto, Jose Carlos Escobar Palacios, and Rubenildo Vieira Andrade. 2018. "An Experimental and Theoretical Study of the Gasification of Miscanthus Briquettes in a Double-Stage Downdraft Gasifier: Syngas, Tar, and Biochar Characterization" Energies 11, no. 11: 3225. https://doi.org/10.3390/en11113225






