Exhaust Emissions and Physicochemical Properties of n-Butanol/Diesel Blends with 2-Ethylhexyl Nitrate (EHN) or Hydrotreated Used Cooking Oil (HUCO) as Cetane Improvers
Abstract
:1. Introduction
2. Fuels
3. Experimental Set-Up and Procedures
3.1. Apparatus Used
3.2. Experimental Procedure
3.2.1. Ignition Quality Investigation Procedure
3.2.2. Exhaust Gas Investigation Procedure
4. Results and Discussion
4.1. Ignition Quality Investigation
4.1.1. Effect of EHN on Ignition Quality
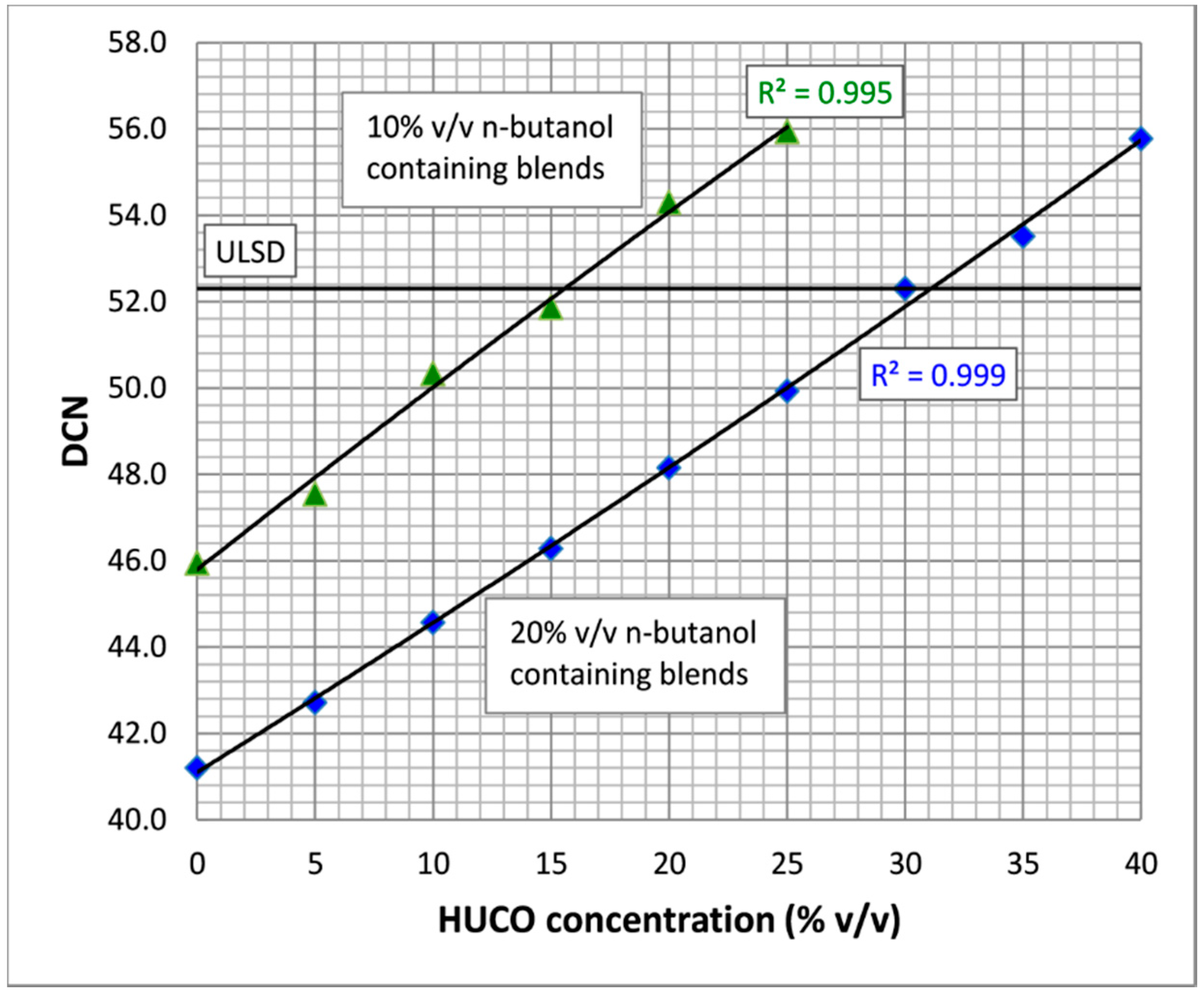
4.1.2. Effect of HUCO on Ignition Quality
4.2. Physicochemical Properties
4.2.1. Samples Studied
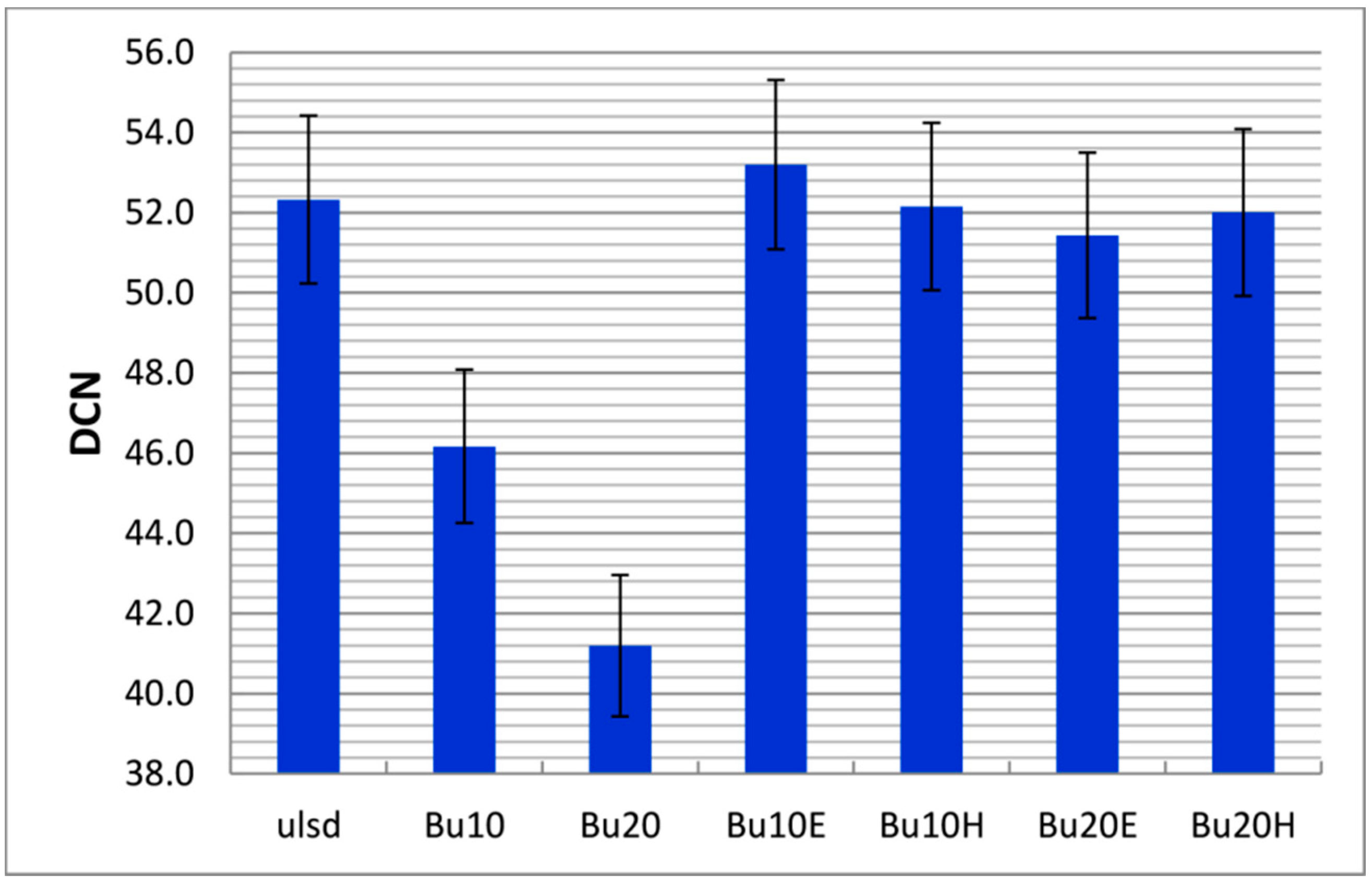
4.2.2. Ignition Quality
4.2.3. Density and Viscosity
4.2.4. Distillation Properties
4.2.5. Cold Filter Plugging Point
4.2.6. Flash Point
4.3. Engine Results
4.3.1. Fuel Consumption
4.3.2. Carbon Monoxide
4.3.3. Hydrocarbons
4.3.4. Nitrogen Oxides
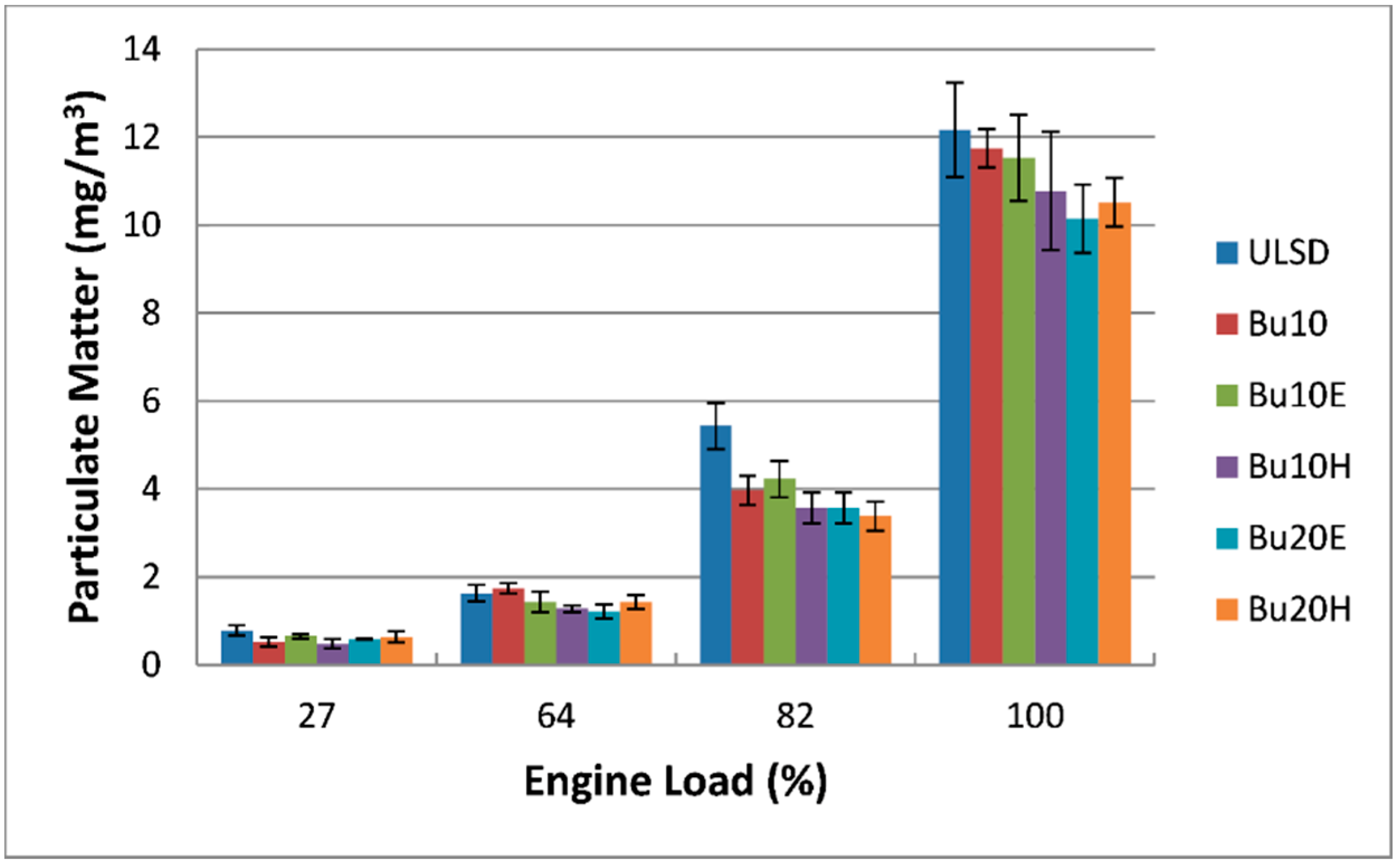
4.3.5. Particulate Matter
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mollenhauer, K.; Tschoeke, H. Handbook of Diesel Engines; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Rakopoulos, C.D.; Giakoumis, E.G. Diesel Engine Transient Operation; Springer: London, UK, 2009. [Google Scholar]
- Knothe, G.; Krahl, J.; Gerpen, J. The Biodiesel Handbook, 2nd ed.; Academic Press: Cambridge, MA, USA; AOCS Press: Urbana, IL, USA, 2010; ISBN 9780983507260. [Google Scholar]
- Fayyazbakhsh, A.; Pirouzfar, V. Comprehensive overview on diesel additives to reduce emissions, enhance fuel properties and improve engine performance. Renew. Sustain. Energy Rev. 2017, 74, 891–901. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Thomson, M.J. A review of the combustion and emissions properties of advanced transportation biofuels and their impact on existing and future engines. Renew. Sustain. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Lapuerta, M.; Villajos, M.; Agudelo, J.R.; Boehman, A.L. Key properties and blending strategies of hydrotreated vegetable oil as biofuel for diesel engines. Fuel Process. Technol. 2011, 92, 2406–2411. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in bio-fuels. Prog. Energy Combust. Sci. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Hountalas, D.T.; Zannis, T.C.; Levendis, Y.A. Operational and Environmental Evaluation of Diesel Engines Burning Oxygen-Enriched Intake Air or Oxygen-Enriched Fuels: A Review; SAE Technical Paper 2004-01-2924; SAE International: Warrendale, PA, USA, 2004. [Google Scholar]
- Bhardwaj, O.; Omari, A.; Nuottimäki, J.; Hervé, R. Optimizing Engine Calibration for Renewable Diesel Fuels. MTZ Worldw. 2018, 79, 26–33. [Google Scholar] [CrossRef]
- Ozgur, C.; Kadir, A. Effects of SCR System on NOx Reduction in Heavy Duty Diesel Engine Fuelled with Diesel and Alcohol Blends. Adv. Automob. Eng. 2016, 5, 139. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Li, Y. Effects of diesel injection parameters on the rapid combustion and emissions of an HD common-rail diesel engine fueled with diesel-methanol dual-fuel. Appl. Therm. Eng. 2016, 108, 1214–1225. [Google Scholar] [CrossRef]
- Shi, Y.; Reitz, R.D. Optimization of a heavy-duty compression–ignition engine fueled with diesel and gasoline-like fuels. Fuel 2010, 89, 3416–3430. [Google Scholar] [CrossRef]
- Abu-Jrai, A.; Rodríguez-Fernández, J.; Tsolakis, A.; Megaritis, A.; Theinnoi, K.; Cracknell, R.F.; Clark, R.H. Performance, combustion and emissions of a diesel engine operated with reformed EGR. Comparison of diesel and GTL fuelling. Fuel 2009, 88, 1031–1041. [Google Scholar] [CrossRef] [Green Version]
- Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S. Hydrotreated Vegetable Oil (HVO) as a Renewable Diesel Fuel: Trade-off between NOx, Particulate Emission, and Fuel Consumption of a Heavy Duty Engine. SAE Int. J. Engines 2009, 1, 1251–1262. [Google Scholar] [CrossRef]
- Carmona, H.P.; Horáček, J.; Alayón, A.B.; Hernández, J.J.M. Suitability of used frying oil for co-processing with atmospheric gas oil. Fuel 2018, 214, 165–173. [Google Scholar] [CrossRef]
- Tyrovola, T.; Dodos, G.; Kalligeros, S.; Zannikos, F. The Introduction of Biofuels in Marine Sector. J. Environ. Sci. Eng. A 2017, 6, 415–421. [Google Scholar] [CrossRef]
- Peter Baladincz, P.; Hancsók, J. Fuel from waste animal fats. Chem. Eng. J. 2015, 282, 152–160. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Buffi, M.; Rizzo, A.M.; Prussi, M.; Martelli, F. Bio-Hydrocarbons through Catalytic Pyrolysis of Used Cooking Oils: Towards sustainable jet and road fuels. Energy Procedia 2015, 82, 343–349. [Google Scholar] [CrossRef]
- Bezergianni, S. Catalytic Hydroprocessing of Liquid Biomass for Biofuels Production. Liquid, Gaseous and Solid Biofuels—Conversion Techniques; INTECH: London, UK, 2013; Chapter 9; pp. 299–326. ISBN 978-953-51-1050-7. [Google Scholar]
- Naik, S.N.; Goud, V.V.; Rout, O.J.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Yusri, I.M.; Mamat, R.; Najafi, G.; Razman, A.; Awad, O.I.; Azmi, W.H.; Ishak, W.F.W.; Shaiful, A.I.M. Alcohol based automotive fuels from first four alcohol family in compression and spark ignition engine: A review on engine performance and exhaust emissions. Renew. Sustain. Energy Rev. 2017, 77, 169–181. [Google Scholar] [CrossRef]
- Lapuerta, M.; Contreras, R.G.; Fernández, J.C.; Dorado, M.P.D. Stability, Lubricity, Viscosity, and Cold-Flow Properties of Alcohol-Diesel Blends. Energy Fuels 2010, 24, 4497–4502. [Google Scholar] [CrossRef]
- Ecklund, E.E.; Bechtold, R.L.; Timbario, T.J.; McCallum, P.W. State-of-the-Art Report on the Use of Alcohols in Diesel Engines; SAE Technical Paper 840118; SAE International: Warrendale, PA, USA, 1984. [Google Scholar]
- Zahos-Siagos, I.; Antonerias, A.; Karonis, D. Impact of Ethanol and N-Butanol Addition on Fuel Properties and Exhaust Emissions of a Stationary Diesel Engine. J. Energy Eng. 2018, 144. [Google Scholar] [CrossRef]
- Iannuzzi, S.E.; Barro, C.; Boulouchos, K.; Burger, J. Combustion behavior and soot formation/oxidation of oxygenated fuels in a cylindrical constant volume chamber. Fuel 2016, 167, 49–59. [Google Scholar] [CrossRef]
- Delfort, B.; Durand, I.; Jaecker-Voirol, A.; Lacôme, T.; Paillé, F.; Montagne, X. Oxygenated Compounds and Diesel Engine Pollutant Emissions Performances of New Generation of Products; SAE Technical Paper 2002-01-2582; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Naegeli, D.W.; Moulton, S.; Owens, E.C.; Frame, E.A. Oxygenates for Advanced Petroleum-Based Diesel Fuels; InterimReport, TFLRF No.357; U.S. Army TARDEC Fuels and Lubricants Research Facility, Southwest Research Institute: San Antonio, TX, USA, 2001.
- Giakoumis, E.G.; Rakopoulos, C.D. Exhaust emissions with ethanol or n-butanol diesel fuel blends during transient operation: A review. Renew. Sustain. Energy Rev. 2013, 17, 170–190. [Google Scholar] [CrossRef]
- Hansen, A.C.; Zhang, Q.; Lyne, P.Q.L. Ethanol-diesel fuel blends: A review. Bioresour. Technol. 2005, 96, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Corkwell, K.C.; Jackson, M.; Daly, D.T. Review of Exhaust Emissions of Compression Ignition Engines Operating on E Diesel Fuel Blends; SAE Technical Paper 2003-01-3283; SAE International: Warrendale, PA, USA, 2003. [Google Scholar]
- Verhelst, S.; Turner, J.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Chao, J.; Mingfa, Y.; Haifeng, L.; Chia-fon, L.; Jing, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Dodos, G.; Tsesmeli, C.; Zahos-Siagos, I.; Tyrovola, T.; Karonis, D.; Zannikos, F. Evaluation of the Stability and Ignition Quality of Diesel-Biodiesel-Butanol Blends; SAE Technical Paper 2017-01-2320; SAE International: Warrendale, PA, USA, 2017. [Google Scholar] [CrossRef]
- Torres-Jimenez, E.; Jerman, M.S.; Gregorc, A.; Lisec, I.; Dorado, M.P.; Kegl, B. Physical and chemical properties of ethanol–diesel fuel blends. Fuel 2011, 90, 795–802. [Google Scholar] [CrossRef]
- Horng-Jang, L.; Yi-Huah, L.; Chia-Ling, T.; Hua-Hsuan, H.; Jia-Huey, L. A mathematical model for predicting the flash point of binary solutions. J. Loss Prev. Process Ind. 2002, 15, 429–438. [Google Scholar] [CrossRef]
- Nylund, N.O.; Erkkilä, K.; Ahtiainen, M.; Murtonen, T.; Saikkonen, P.; Amberla, A.; Aatola, H. Optimized Usage of NExBTL Renewable Diesel Fuel; VTT Tiedotteita—Research Notes 2604; OPTIBIO: Espoo, Finland, 2011; ISBN 978-951-38-7794-1. [Google Scholar]
- Shivananda, S.A.; Dasappa, S. Biomass to liquid transportation fuel via Fischer Tropsch synthesis—Technology review and current scenario. Renew. Sustain. Energy Rev. 2016, 58, 267–286. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer–Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Zahos-Siagos, I.; Karonis, D. Exhaust Emissions and Physicochemical Properties of Hydrotreated Used Cooking Oils in Blends with Diesel Fuel. Int. J. Chem. Eng. 2018, 2018, 4308178. [Google Scholar] [CrossRef]
- BIOFUELS-2G—Demonstration of a Sustainable & Effective 2nd Generation Biofuels Application in an Urban Environment. Available online: http://biofuels2g.gr/index.php?lang=en (accessed on 20 September 2018).
- Bezergianni, S.; Dimitriadis, A.; Sfetsas, T.; Kalogianni, A. Hydrotreating of waste cooking oil for biodiesel production. Part II: Effect of temperature on hydrocarbon composition. Bioresour. Technol. 2010, 101, 7658–7660. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Standardization (CEN). Automotive Fuels—Diesel—Requirements and Test Methods; EN 590:2013; NSAI: Dublin, Ireland, 2017. [Google Scholar]
- Claudy, P.; Létoffé, J.M.; Bonardi, B.; Vassilakis, D.; Damin, B. Interactions between N-Alkanes and Cloud Point-Cold Filter Plugging Point Depressants in a Diesel Fuel. A Thermodynamic Study. Fuel 1993, 72, 821–827. [Google Scholar] [CrossRef]
- Yanowitz, J.; Murphy, M.J.; Taylor, J.D.; Mccormick, R.L. Compendium of Experimental Cetane Number Data; Technical Report NREL/TP-5400-61693; NREL: Golden, CO, USA, 2014. [Google Scholar]









| Property | ULSD | HUCO | n-Butanol | Bu10 | Bu10E | Bu10H | Bu20E | Bu20H | Unit | EN 590 Limits | EN 15940 Limits (Class A) | Method | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | |||||||||||
| Oxygen content | 0 | 0 | 22 | 2.2 | 2.2 | 2.2 | 4.3 | 4.3 | % (w/w) | |||||
| Density | 824.7 ± 0.10 | 790.8 ± 0.08 | 813.6 ± 0.11 | 823.1 ± 0.09 | 823.0 ± 0.12 | 816.3 ± 0.10 | 822.3 ± 0.08 | 808.8 ± 0.11 | kg/m3, 15 °C | 820.0 | 845.0 | 765.0 | 800.0 | EN ISO 12185 |
| Viscosity | 2.567 ± 0.002 | 3.704 ± 0.003 | 2.267 ± 0.001 | 2.305 ± 0.002 | 2.299 ± 0.002 | 2.474 ± 0.002 | 2.213 ± 0.002 | 2.499 ± 0.002 | mm2/s, 40 °C | 2.000 | 4.500 | 2.000 | 4.500 | ASTM D7042 |
| IBP a | 173.4 ± 1.4 | 254.8 ± 1.0 | 116.7 ± 0.8 | 116.7 ± 0.9 | 117.5 ± 1.0 | 116.7 ± 1.0 | 115.7 ± 1.2 | °C | EN ISO 3405 | |||||
| T10 b | 199.9 ± 1.3 | 292.0 ± 0.8 | 171.3 ± 1.3 | 172.4 ± 1.2 | 127.2 ± 1.2 | 130.0 ± 1.0 | 118.6 ± 1.1 | °C | EN ISO 3405 | |||||
| T50 b | 269.8 ± 0.8 | 298.1 ± 0.7 | 260.8 ± 0.8 | 260.9 ± 0.8 | 274.1 ± 0.7 | 251.6 ± 0.7 | 279.7 ± 0.7 | °C | EN ISO 3405 | |||||
| T90 b | 336.3 ± 1.0 | 310.9 ± 0.9 | 333.2 ± 1.0 | 332.9 ± 0.9 | 326.1 ± 0.9 | 332.9 ± 1.0 | 317.8 ± 1.1 | °C | EN ISO 3405 | |||||
| T95 b | 354.9 ± 1.2 | 322.2 ± 1.1 | 350.8 ± 1.1 | 350.5 ± 1.2 | 345.4 ± 1.1 | 350.5 ± 1.1 | 340.0 ± 1.0 | °C | 360 | 360 | EN ISO 3405 | |||
| FBP c | 362.0 ± 1.8 | 349.1 ± 1.3 | 359.1 ± 1.4 | 359.3 ± 1.4 | 354.4 ± 1.5 | 359.3 ± 1.5 | 347.6 ± 1.6 | °C | EN ISO 3405 | |||||
| Boiling point | 118 | °C | ||||||||||||
| DCN d | 52.3 ± 0.3 | 102.7 ± 0.5 | ~25 | 46.2 ± 0.3 | 53.2 ± 0.5 | 52.2 ± 0.3 | 51.4 ± 0.0 | 52.0 ± 0.2 | 51.0 | 70.0 | ASTM D7170 | |||
| LHV e | 43.3 ± 0.10 | 43.7 ± 0.09 | 33.1 ± 0.10 | 42.1 ± 0.11 | 42.1 ± 0.12 | 42.3 ± 0.11 | 41.1 ± 0.12 | 41.4 ± 0.12 | MJ/kg | ASTM D240 | ||||
| CFPP f | −8 ± 0.5 | 21 ± 0.5 | <−50 | −9 ± 0.8 | −8 ± 0.8 | −8 ± 1.0 | −8 ± 0.8 | 2 ± 0.5 | °C | −5 | −5 | EN 116 | ||
| Flash point | 67 ± 0.6 | >100 | 35 ± 0.5 | 37 ± 0.5 | 37 ± 0.5 | 37 ± 0.5 | 36 ± 0.6 | 36 ± 1.0 | °C | 55.0 | 55.0 | EN ISO 2719 | ||
| Carbon Number | Normal Paraffins | Iso-Paraffins |
|---|---|---|
| C15 | 7.8 | 0.0 |
| C16 | 7.0 | 5.9 |
| C17 | 41.8 | 5.0 |
| C18 | 30.6 | 1.0 |
| Description | Types, Value and Unit |
|---|---|
| Fuel injection | Direct |
| Number of cylinders | 2, inline |
| Aspiration | Natural |
| Displacement | 0.93 liters |
| Compression ratio | 18.5:1 |
| Speed | 1500 rpm |
| Cooling | Liquid-cooled |
| Alternator | Brushless |
| Electrical output power | 5.6 kW, 50 Hz |
| Measured Variable | Resolution | Measurement Range | Accuracy |
|---|---|---|---|
| Exhaust gas temperature | 0.1 °C | 0–1100 °C | 1.0 °C ± 0.3% |
| CO | 1 ppm | 0–2000 ppm | ±5 ppm when <100 ppm ±5% when >100 ppm and <2000 ppm ±10% when >2000 ppm |
| CO2 | 0.1% | 0–10% | ±5% |
| HC | 1 ppm | 0–5000 ppm | ±5% |
| NO | 1 ppm | 0–1000 ppm | ±5 ppm when <100 ppm ±5% when >100 ppm and <1000 ppm ±10% when >1000 ppm |
| NO2 | 1 ppm | 0–100 ppm | ±5 ppm when <100 ppm ±10% when >100 ppm |
| Load | Torque (N·m) | Speed (rpm) | Power (kW) |
|---|---|---|---|
| Low | 9.6 | 1500 | 1.5 |
| Medium | 22.3 | 1500 | 3.5 |
| Medium–high | 28.7 | 1500 | 4.5 |
| Full | 35.0 | 1500 | 5.5 |
| Property | Bu10 | Bu10E | Bu10H | Bu20E | Bu20H | Unit |
|---|---|---|---|---|---|---|
| n-Butanol content | 10 | 10 | 10 | 20 | 20 | % (v/v) |
| EHN content | 0 | 0.13 | 0 | 0.52 | 0 | % (v/v) |
| HUCO content | 0 | 0 | 16 | 0 | 31 | % (v/v) |
| ULSD content | 90 | ~90 | 74 | ~80 | 49 | % (v/v) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahos-Siagos, I.; Karathanassis, V.; Karonis, D. Exhaust Emissions and Physicochemical Properties of n-Butanol/Diesel Blends with 2-Ethylhexyl Nitrate (EHN) or Hydrotreated Used Cooking Oil (HUCO) as Cetane Improvers. Energies 2018, 11, 3413. https://doi.org/10.3390/en11123413
Zahos-Siagos I, Karathanassis V, Karonis D. Exhaust Emissions and Physicochemical Properties of n-Butanol/Diesel Blends with 2-Ethylhexyl Nitrate (EHN) or Hydrotreated Used Cooking Oil (HUCO) as Cetane Improvers. Energies. 2018; 11(12):3413. https://doi.org/10.3390/en11123413
Chicago/Turabian StyleZahos-Siagos, Iraklis, Vlasios Karathanassis, and Dimitrios Karonis. 2018. "Exhaust Emissions and Physicochemical Properties of n-Butanol/Diesel Blends with 2-Ethylhexyl Nitrate (EHN) or Hydrotreated Used Cooking Oil (HUCO) as Cetane Improvers" Energies 11, no. 12: 3413. https://doi.org/10.3390/en11123413





