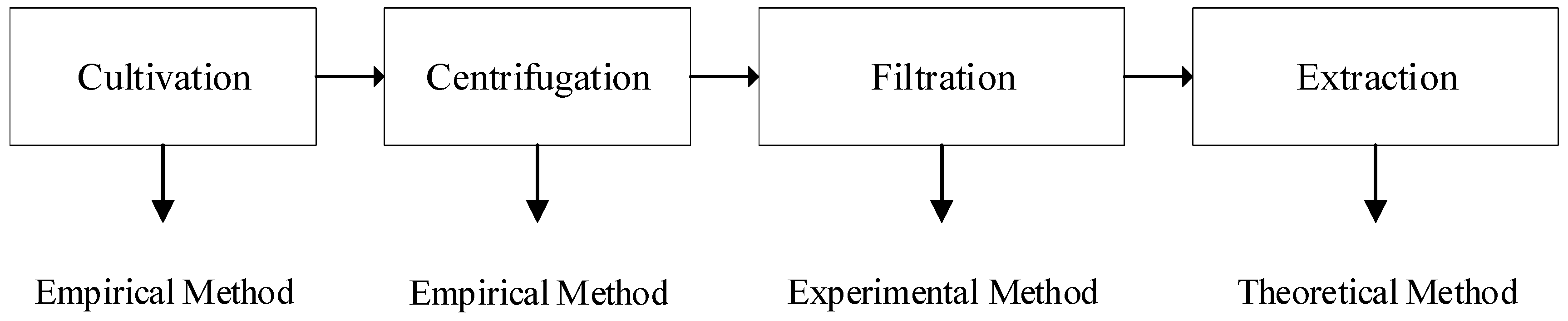
3.2. Cultivation
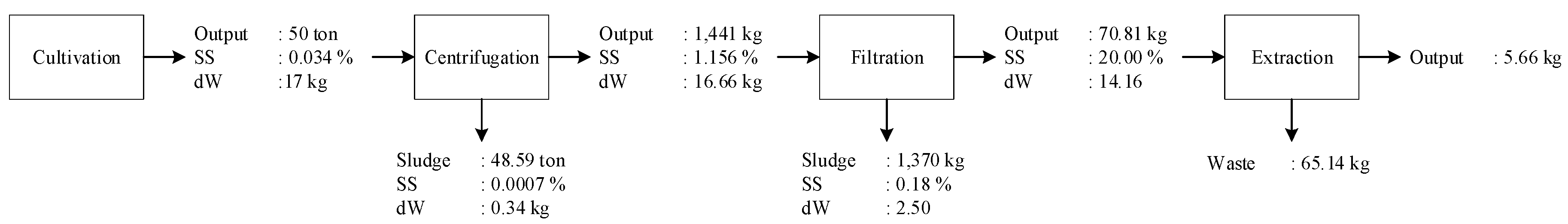
The pilot project in Minamisoma used an ORP to cultivate microalgae. The ORP dimensions were 20 m in width and 50 m in length (1000 m2 area). The cultivation depth was 0.2 m, which made the volume 200 m3 or 200,000 L. The ORP was constructed from concrete.
The main microalgae species cultivated in Minamisoma is
Desmodesmus. The microalgae cultivation had a hydraulic retention time (HRT) of four days with an average harvest concentration of 0.034%. The harvesting volume per day was 50 m
3. Therefore, the everyday cultivation had a yield of 17 kg of microalgae (dry weight) or 0.34 g/L. The primary equipment in this cultivation station was the raceway pond, which utilized an electric motor to drive the paddlewheel.
Table 1 shows the parameters and value for ORP at Minamisoma pilot plant
Nagappan and Verma [
31] used the same species,
Desmodesmus sp., but the highest yield obtained from their laboratory was 77.73 mg/L or 0.08 g/L. The biomass yield obtained in this research was similar to the result from Ji et al., in which the biomass yield was 0.385 g/L [
32]. The yield in this research can be increased by adding anaerobically digested wastewater (ADW), where the yield reached 1.039 g/L, or a more-than-250% increase in biomass; therefore, in the future, the yield potential can still be increased.
Using Equation (2), the calculated
dh value was 0.77 m and, using Equation (1), the power for the paddlewheel was 0.86 MJ. The paddlewheel was operated for 24 h a day, and the energy consumption was 20.53 MJ. In addition, we could reduce the paddlewheel speed at night, when there is no photosynthesis, to further reduce the energy consumption to 15.40 MJ.
Table 2 shows the energy consumption for the two scenarios.
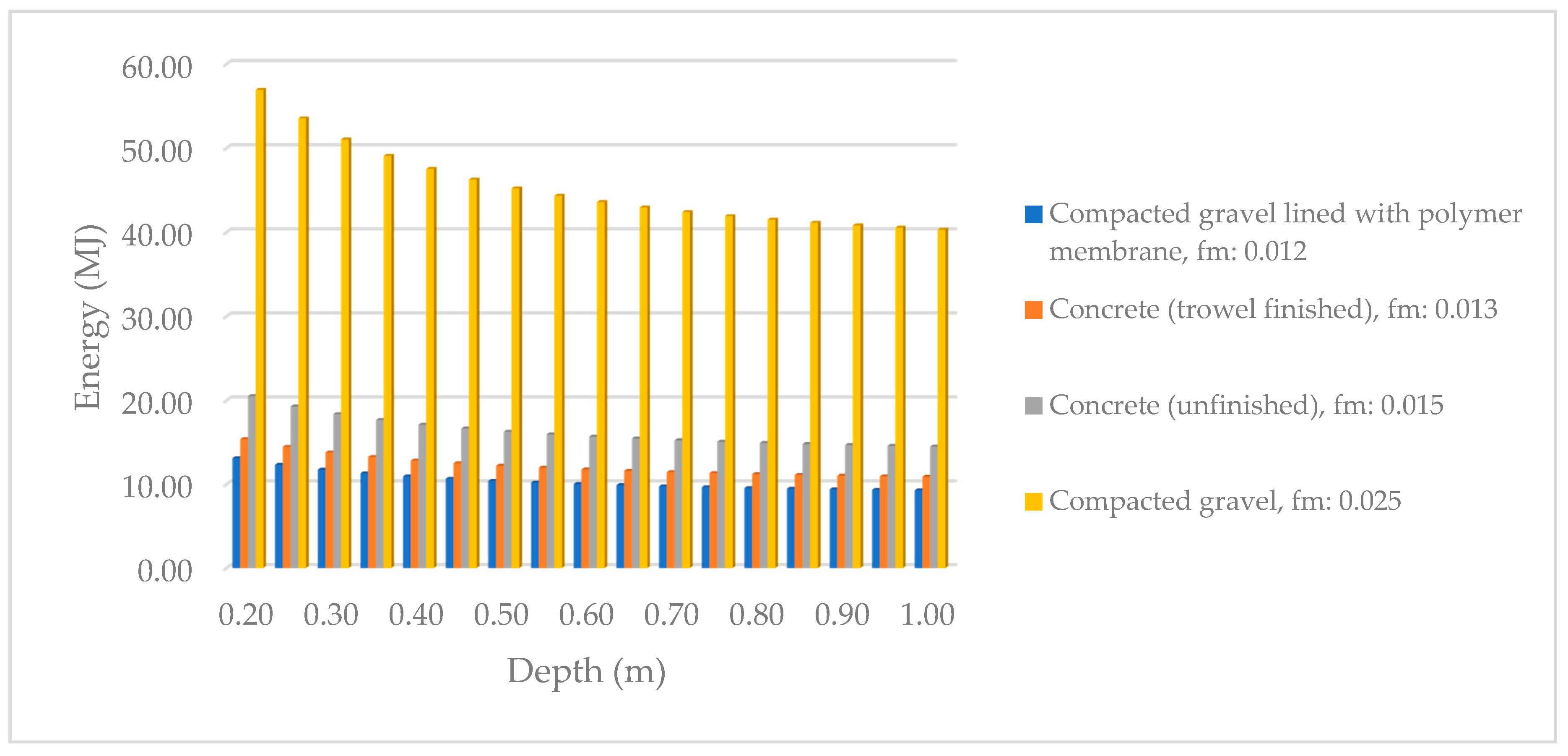
By using calculation based on Equation (1), the power consumption decreases with the increased culture depth. The power consumption is also decreased by lowering the Manning coefficient. The Manning value is related to the pond materials; a smooth surface gives a lower Manning value because the friction is lower, thereby reducing the overall power consumption.
Figure 6 shows the power-consumption changes by depth and material changes.
Additional energy is required if we consider the nutrients used in the cultivation. With an initial weight of 5 kg and a harvest weight of 17 kg, approximately 12 kg of biomass was produced. Using stoichiometry from [
23,
33], we can estimate the nutrients needed for cultivation. The calculation based on the stoichiometry can be seen in
Appendix A, and
Table 3 shows the energy for the nutrients. The energy demand was estimated using the Simapro
® Cumulative Energy Demand Methods, version 1.09.
The nutrients’ energy demand is very high if we rely on the fertilizer. Alternatively, we can integrate the microalgae cultivation with other industries to supply nutrients for the microalgae [
32,
34,
35,
36]. If we could replace the CO
2 and other nutrients, it would be beneficial for the final EPR value.
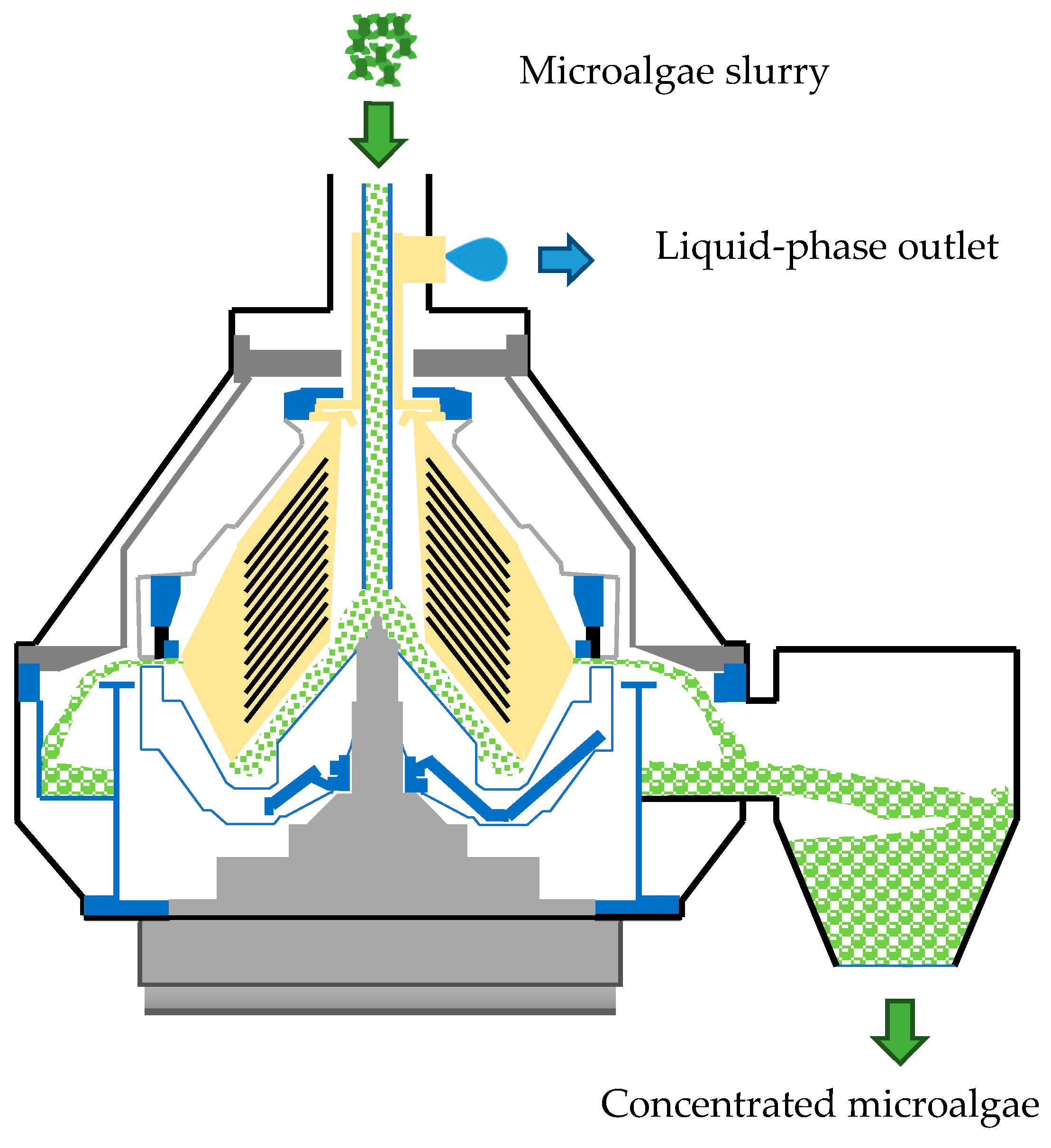
3.3. Centrifugation
A centrifuge was used to accelerate the harvesting time for microalgae cultivation compared to gravity sedimentation. With a 360-L volume and a 270-s residence time, we calculated the volumetric flow rate of 1.33 L/s. The total time to process 50 m
3 input using Equation (4) results in 10.42 h. With a volumetric flow rate of 1.33 L/s, we can compare this value to the typical centrifuge setting to obtain the speed of the centrifuge and the radius of the bucket/bowl from [
33]. The typical setting for a 1.33 L/s input uses a 10,000-rpm speed and a bucket radius of 0.25 m. The later power can be calculated using Equation (5), which results in a value of 4.99 HP or 13.39 MJ.
The calculated theoretical value for the centrifugal action was 13.39 MJ or 0.07 kWh/m
3. This theoretical value was lower than the application in practice because of influences such as machine efficiency. A field measurement of around 1 kWh/m
3 was reported; this value is the same as [
37], which also gave a value of 1 kWh/m
3, but higher than the value from the Alva Laval in [
38], which gave a value of 0.53 kWh/m
3. The parameters to calculate the centrifuge power can be seen in
Table 4.
3.5. Extraction
The native microalgae communities cultivated in Minamisoma were mainly
Desmodesmus. Theoretically, solvent extraction can only extract from fatty-acid content, which is only 13% of the total biomass. This fatty-acid value was lower than the protein and polysaccharide values, which were 45.7% and 21.1%, respectively. Hydrothermal liquefaction (HTL), on the other hand, could convert almost all the biomass into crude oil. Therefore, the yield is expected to be higher than for only oil extraction. Because the purpose is to produce energy, a higher yield is preferable.
Table 5 shows the properties of the algal communities in Minamisoma [
40].
A previous study showed a base for calculating the specific heat of the algal communities [
29].
Table 6 shows the parameters used to calculate the specific heat of the algal communities with
Desmodesmus as the main species.
The specific heat of the microalgae was calculated using the composition given in
Table 5 and
Table 6. Using Equation (7), the specific heat (
Cp) of the microalgae was 1.58 kJ/(kg·°C).
The HTL input was a microalgae slurry with a microalgae concentration of 20%; therefore, for 70.81 kg of input, 56.64 kg was water and 14.16 kg was microalgae. Here, we used the average value required to heat the water from 25 °C to 350 °C. The energy required to heat 56.64 kg of water from 25 °C to 350 °C, according to Equation (8), was 87.02 MJ, and the energy required to heat 14.16 kg of microalgae from 25 °C to 350 °C was 7.28 MJ. Thus, the total energy to heat 70.81 kg of microalgae slurry from 25 °C to 350 °C was 94.29 MJ. The microalgae specific heat (
Cp) value was 1.58 kJ/(kg·°C). This microalgae specific heat value was categorized as average, as [
29] stated that the specific heat for microalgae ranged from 1.2–2 kJ/(kg·°C).
Table 7 shows the parameters used in the theoretical calculation of the energy requirement.
3.6. Energy Profit Ratio (EPR)
The EPR (Energy Profit Ratio) is the ratio of the energy produced to the energy consumed by an energy-production method [
30]. The highest energy demand came from the nutrients, followed by the HTL process, with around 68% and 22%, respectively. Carbon dioxide from flue gas could be used as a CO
2 source in microalgae cultivation [
41]. If we could obtain CO
2 from another industry, the nutrient percentage would decrease to around 40% of the total energy demand. Further, if we could completely substitute the nutrients, the highest energy consumption would be the HTL process with around a 68% share.
Without the nutrients, the HTL energy consumption was the highest, with 94.29 MJ or slightly more than 68%, followed by cultivation with 15.40 MJ or slightly more than 11%. The drum filtration and centrifuge contributed 14.17 MJ and 13.39 MJ or around 10% and 9%, respectively. With a microalgae-oil energy content of 193.27 MJ, the energy profit ratio was found to be 1.41.
Figure 7 shows the EPR value with nutrients, with nutrients but without CO
2, and without nutrients. It was observed that with nutrients, the EPR was very low at only 0.45; the EPR value increased to 0.85 if we excluded the CO
2 from the system. The EPR value would rise more if we could substitute all the needed nutrients. Therefore, an integrated system for microalgae cultivation or overall microalgae oil production is preferable. An integrated microalgae cultivation system was partially designed and a study reported on a photo-bioreactor using wastewater, which resulted in an increased yield [
39,
40]. We expect that the EPR value can be increased with the increased yield.
To determine the EPR value from the increase in biomass yield, we calculated different scenarios, from the existing 0.03% to a concentration of 0.1%. The other parameters are the same, with the paddlewheel running for 18 h and a culture depth of 0.2 m. The energy requirement for cultivation was mostly the same because of the constant paddlewheel operation, and the increased yield produced more energy, thereby helping to increase the EPR value. A similar scenario occurred in a centrifuge, which further increased the EPR value. The results are shown in
Table 8.
The theoretical EPR value can reach more than 1, which indicates a positive energy balance; however, the field applications were typically lower than the theoretical value. Therefore, equipment optimization or an increased yield is still needed. The increasing yields in the scenarios showed an increase in the EPR.
Desmodesmus yields reached 0.8 g/L in our experiment and [
42] also achieved a yield of 0.758 g/L; several other studies were also included [
34,
35,
36].
Figure 8 shows the EPR value increase with different yield scenarios.
Increasing the concentration in the HTL input yielded a higher EPR; however, the HTL equipment design needs further improvement. A higher concentration in the HTL input means more microalgae and less water. Because the energy required to heat water is higher compared to heating microalgae, the energy requirement is expected to decrease. However, the higher the input concentration, the more likely the equipment is to clog and disrupt the HTL process. The HTL equipment in Minamisoma sometimes clogged with a higher input concentration. Therefore, an input concentration higher than 30% in HTL equipment was suggested for future research. Another aspect that needs to be considered is the use of a heat exchanger. The pilot project in Minamisoma did not utilize a heat exchanger; therefore, the energy requirement was higher. By incorporating a heat exchanger, the energy requirement is expected to decrease.
The EPR value in the system for a 0.03% microalgae concentration was 1.41. If we incorporate a heat exchanger, the EPR is expected to increase. The heat exchanger effectiveness was reported to vary between 0.6 and 1 [
43]. We simulated the decreased energy requirement and assumed 10% to 50% energy reductions.
Figure 9 shows the EPR by energy reduction in the four stages for the Minamisoma pilot plant.
Before being used as a biofuel feedstock, microalgae was mostly commercially produced for its nutritional value [
7]. This type of cultivation did not pose problems for energy balance because the product was mostly for food (supplements), feed, or cosmetics. When the purpose was changed to energy production, the EPR was brought into serious consideration. We need to input less energy than the energy produced to make it a sustainable option.
The disadvantage of microalgae is its small size and high water content. These constraints should be overcome through developing equipment and reducing the processing time. On the other hand, the dewatering process requires higher energy if we utilize a centrifuge and filtration, compared to gravity sedimentation. Extraction using HTL also requires higher energy, which, in turn, results in a lower EPR for the overall product line. However, in a gravity-sedimentation system, the required time is longer, and the area is significantly more extensive.
Cultivating microalgae for energy production could be optimized by using traditional methods such as gravity sedimentation to harvest the microalgae; however, the trade-off is the processing time. Time and area could be a trade-off with the energy requirement. The EPR is the decision-making benchmark for the feasibility of converting pilot-type plants to industrial-scale or integrated microalgae production systems. The EPR of this research indicates that the current level of microalgae-cultivation technology for producing bioenergy is still on the border of being feasible. More research on productivity and machine efficiency are still needed.