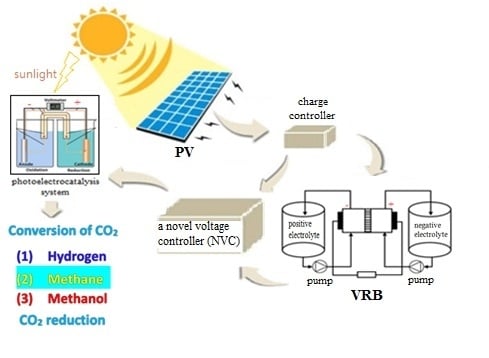
Design of a Novel Voltage Controller for Conversion of Carbon Dioxide into Clean Fuels Using the Integration of a Vanadium Redox Battery with Solar Energy
Abstract
:1. Introduction
2. Design of the Novel Control System for Conversion of Carbon Dioxide
2.1. Integration of Vanadium Redox Battery with PV System
- Positive electrode:
- VO2+ + H2 O ⇄ VO2+ + 2H+ + e−
- Negative electrode:
- V3+ + e− ⇄ V2+
- (→: charge, ←: discharge)
2.2. Design of a Novel Voltage Controller
3. Proposed Optimal External Bias Voltage Control Method
- Anode reaction:
- 2H2O → O2 + 4H+ + 4e−
- Cathode reaction:
- CO2 + 8H++ 8e− → CH4 + 2H2 O or
- CO2 + 6H+ + 6e− → CH3OH + H2 O
3.1. Design of a Novel Three-Loop Voltage Controller
3.2. Optimization Technique
4. Case Studies
5. Conclusions
Acknowledgments
Conflicts of Interest
Appendix A
References
- De León, C.P.; Frias-Ferrer, A.; González-García, J.; Szánto, D.; Walsh, F.C. Redox flow cells for energy conversion. J. Power Sources 2006, 160, 716–732. [Google Scholar] [CrossRef]
- Joerissen, L.; Garche, J.; Fabjan, C.; Tomazic, G. Possible use of vanadium redox-flow batteries for energy storage in small grids and stand-alone photovoltaic systems. J. Power Sources 2004, 127, 98–104. [Google Scholar] [CrossRef]
- Garcia-Martinez, J. Nanotechnology for the Energy Challenge; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Robinson, A.B.; Robinson, N.E.; Soon, A. Environmental effects of increased atmospheric carbon dioxide. J. Am. Phys. Surg. 2007, 12, 79–90. [Google Scholar]
- Centi, G.; Perathoner, S. CO2-based energy vectors for the storage of solar energy. Greenh. Gas Sci. Technol. 2011, 1, 21–35. [Google Scholar] [CrossRef]
- Herron, J.A.; Maravelias, C.T. Assessment of solar-to-fuels strategies: Photocatalysis and electrocatalytic reduction. Energy Technol. 2016, 4, 1369–1391. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Saveant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Boston, D.J.; Xu, C.; Armstrong, D.W.; Myung, N.; MacDonnell, F.M. Photochemical reduction of carbon dioxide to methanol and formate in a homogeneous system with pyridinium catalysts. J. Am. Chem. Soc. 2013, 135, 16252–16255. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Basu, S. An Integrated Device for Converting Water, Carbon Dioxide and Light into Electricity and Organics. J. Electrochem. Soc. 2017, 164, E3406–E3417. [Google Scholar] [CrossRef]
- Tuller, H.L. Solar to fuels conversion technologies: a perspective. Mater. Renew. Sustain. Energy 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. Electrochemical conversion of CO2 to formic acid utilizing Sustainion membranes. J. CO2 Util. 2017, 20, 208–217. [Google Scholar] [CrossRef]
- Chahwan, J.; Abbey, C.; Joos, G. VRB Modelling for the Study of Output Terminal Voltages, Internal Losses and Performance. In Proceedings of the IEEE Electrical Power Conference (EPC’07), Montreal, QC, Canada, 25–26 October 2007; pp. 387–392. [Google Scholar]
- Zheng, Q.; Li, X.F.; Cheng, Y.H.; Ning, G.L.; Xing, F.; Zhang, H.M. Development and perspective in vanadium flow battery modeling. Appl. Energy 2014, 132, 254–266. [Google Scholar] [CrossRef]
- Ou, T.C.; Hong, C.M. Dynamic operation and control of microgrid hybrid power systems. Energy 2014, 66, 314–323. [Google Scholar] [CrossRef]
- Mohamed, M.R.; Ahmad, H.; Abu Seman, M.N.; Razali, S.; Najib, M.S. Electrical circuit model of a vanadium redox flow battery using extended Kalman filter. J. Power Sources 2013, 239, 284–293. [Google Scholar] [CrossRef]
- Li, M.H.; Funaki, T.; Hikihara, T. A Study of Output Terminal Voltage Modeling for Redox Flow Battery Based on Charge and Discharge Experiments. In Proceedings of the Power Conversion Conference, Nagoya, Japan, 2–5 April 2007; IEEE Press: New York, NY, USA, 2007; pp. 221–225. [Google Scholar]
- Qiu, X.; Nguyen, T.A.; Crow, M.L.; Guggenburger, J.; Elmore, A.C. A field validated model of a vanadium redox flow battery for microgrids. IEEE Trans. Smart Grid 2014, 5, 1592–1601. [Google Scholar] [CrossRef]
- Vynnycky, M. Analysis of a model for the operation of a vanadium redox battery. Energy 2011, 36, 2242–2256. [Google Scholar] [CrossRef]
- Gong, Q.; Lei, J. Design of a Bidirectional Energy Storage System for a Vanadium Redox Flow Battery in a Microgrid with SOC Estimation. Sustainability 2017, 9, 441. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Qiu, X.; Guggenberger, J.D., II; Crow, M.L.; Elmore, A.C. Performance Characterization for Photovoltaic-Vanadium Redox Battery Microgrid Systems. IEEE Trans. Sustain. Energy 2014, 5, 1379–1388. [Google Scholar] [CrossRef]
- Wang, G.; Ciobotaru, M.; Agelidis, V.G. Integration of Vanadium Redox Battery with PV Systems: Modeling and Operational Characteristics. In Proceedings of the IEEE International Symposium on Industrial Electronics, Hangzhou, China, 28–31 May 2012; pp. 1598–1603. [Google Scholar]
- Fathima, A.H.; Palanismay, K. Modeling and Operation of a Vanadium Redox Flow Battery for PV Applications. Energy Procedia 2017, 117, 607–614. [Google Scholar] [CrossRef]
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Ritter, S.K. What can we do with carbon dioxide? Scientists are trying to find ways to convert the plentiful greenhouse gas into fuels and other value added products. Chem. Eng. News 2007, 85, 11–17. [Google Scholar]
- Hori, Y. Electrochemical CO2 reduction on metal electrodes. In Modern Aspects of Electrochemistry; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Ganesh, I. Conversion of carbon dioxide into several potential chemical commodities following different pathways—A review. Mater. Sci. Forum 2013, 764, 1–82. [Google Scholar] [CrossRef]
- Pettinau, A.; Mureddu, M.; Ferrara, F. Carbon dioxide conversion into liquid fuels by hydrogenation and photoelectrochemical reduction: Project description and preliminary experimental results. Energy Procedia 2017, 114, 6893–6904. [Google Scholar] [CrossRef]
- Millar, G.J.; Rochester, C.H.; Waugh, K.C. An in situ high pressure FT-IR study of carbon dioxide/hydrogen interactions with model zinc oxide/silica, copper/silica and copper/zinc oxide/silica methanol synthesis catalysts. Catal. Lett. 1992, 14, 289–295. [Google Scholar] [CrossRef]
- Barber, J. Biological solar energy. Philos. Trans. A Math Phys. Eng. Sci. 2007, 365, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Graves, C.R. Recycling CO2 into Sustainable Hydrocarbon Fuels: Electrolysis of CO2 and H2O. Ph.D. Thesis, Columbia University, New York, NY, USA, 2010. [Google Scholar]
- Barton Cole, E.; Lakkaraju, P.S.; Rampulla, D.M.; Morris, A.J.; Abelev, E.; Bocarsly, A.B. Using a one-electron shuttle for the multielectron reduction of CO2 to methanol: Kinetic, mechanistic, and structural insights. J. Am. Chem. Soc. 2010, 132, 11539–11551. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Doi, R. Hydrogen production from H2O and conversion of CO2 to useful chemicals by room temperature photoelectrocatalysis. Catal. Today 1996, 27, 271–277. [Google Scholar] [CrossRef]









| Case 1 | Case 2 | |||
|---|---|---|---|---|
| k = 1.0 | k = 1.2 | Scenario-I | Scenario-II | |
| Hydrogen | F1, 1-1 | F1, 1-2 | F1, 2-1 | F1, 2-2 |
| Methane | F2, 1-1 | F2, 1-2 | F2, 2-1 | F2, 2-2 |
| Methanol | F3, 1-1 | F3, 1-2 | F3, 2-1 | F3, 2-2 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, T.-C. Design of a Novel Voltage Controller for Conversion of Carbon Dioxide into Clean Fuels Using the Integration of a Vanadium Redox Battery with Solar Energy. Energies 2018, 11, 524. https://doi.org/10.3390/en11030524
Ou T-C. Design of a Novel Voltage Controller for Conversion of Carbon Dioxide into Clean Fuels Using the Integration of a Vanadium Redox Battery with Solar Energy. Energies. 2018; 11(3):524. https://doi.org/10.3390/en11030524
Chicago/Turabian StyleOu, Ting-Chia. 2018. "Design of a Novel Voltage Controller for Conversion of Carbon Dioxide into Clean Fuels Using the Integration of a Vanadium Redox Battery with Solar Energy" Energies 11, no. 3: 524. https://doi.org/10.3390/en11030524