Lithium-Ion Cell Fault Detection by Single-Point Impedance Diagnostic and Degradation Mechanism Validation for Series-Wired Batteries Cycled at 0 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrochemical Characterization
2.2. Initial Conditioning and Characterization Testing (ICCT) of Li-Ion Cells
3. Results
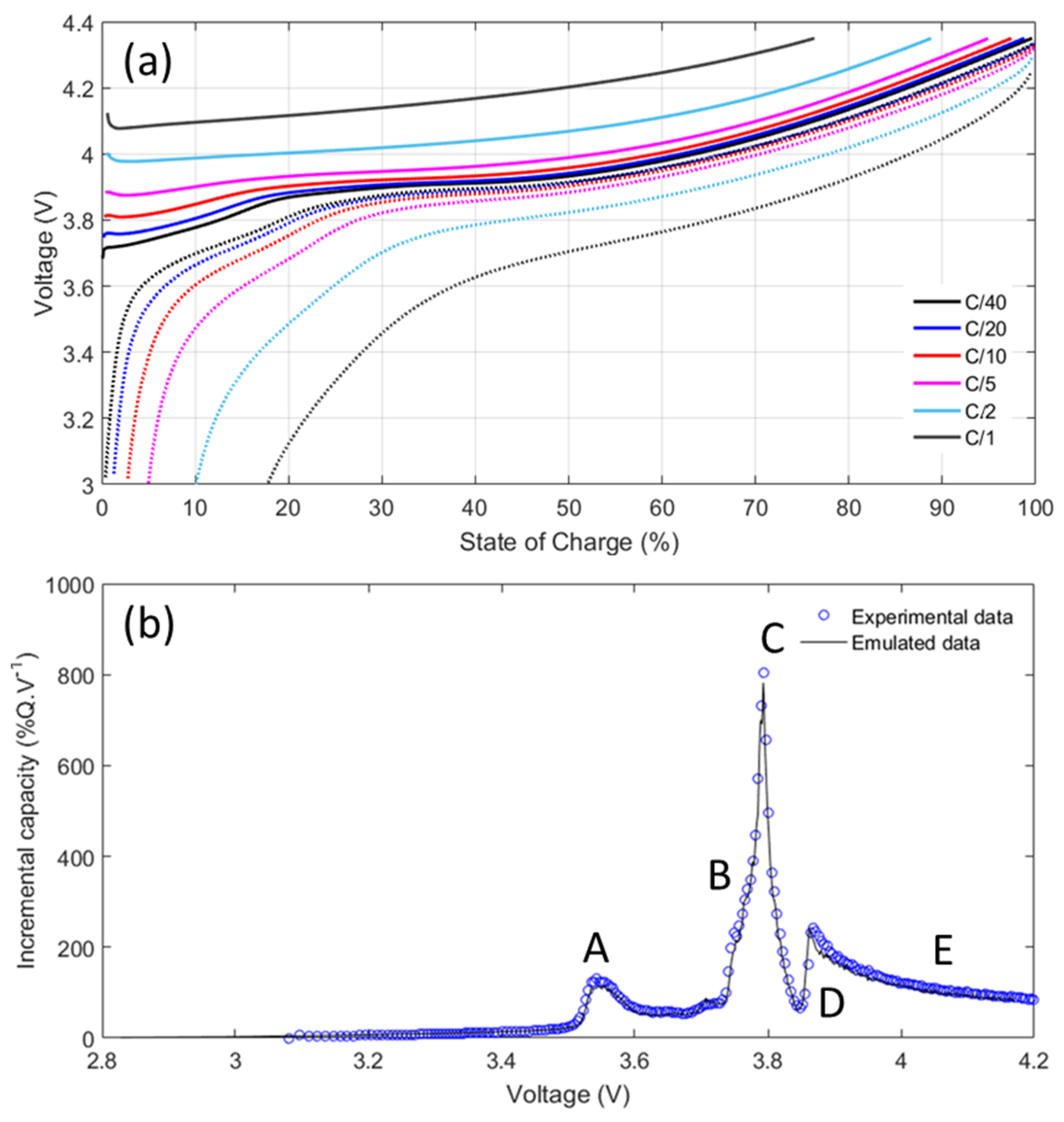
3.1. Single-Point Impedance Diagnostic Parameters
3.1.1. Determination of Current Perturbation Amplitude
3.1.2. Continuity of fSOH with Temperature
3.1.3. Temperature Dependence of Impedance Response
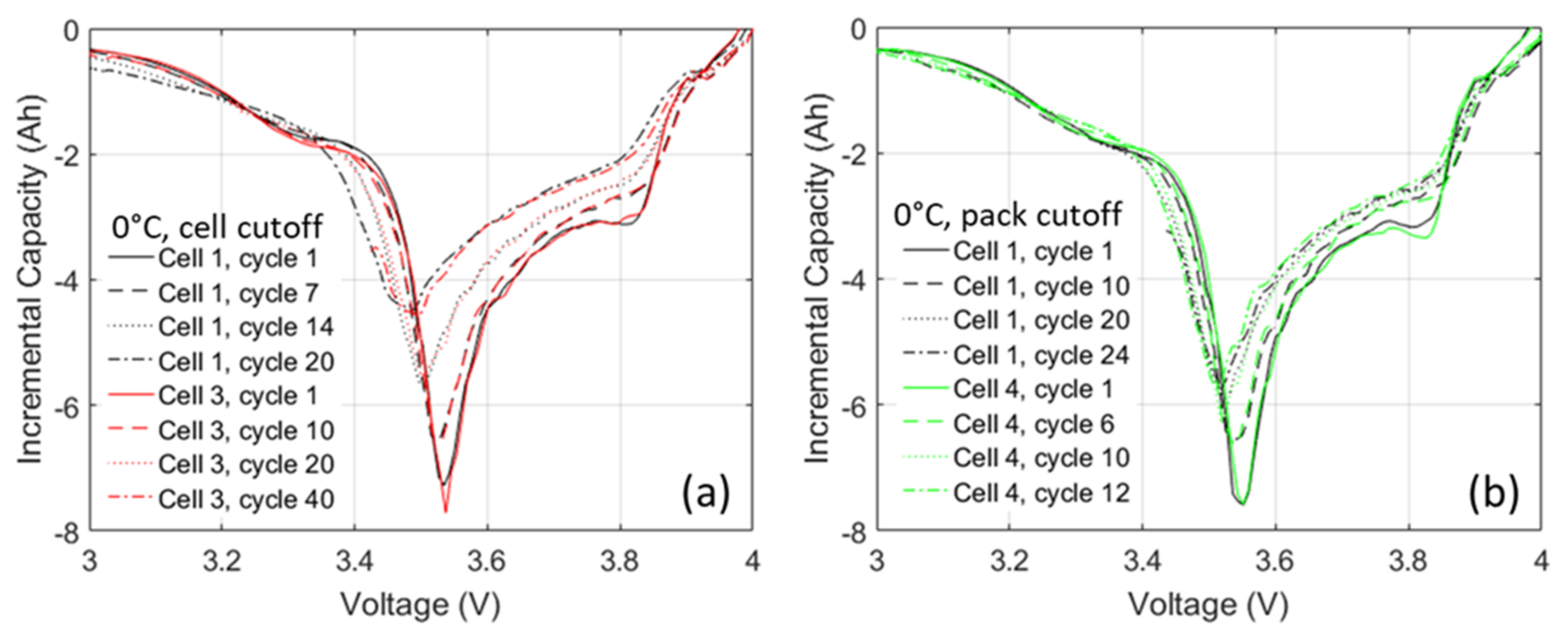
3.2. Pack Level Cycling
3.3. Analysis of Pack-Level EIS Monitoring
3.4. Validation of Specific Degradation Modes from Impedance Diagnostic Early Warning
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| 4S | four cells in series |
| CC | constant current |
| CC-CV | constant current-constant voltage |
| DC | direct current |
| DMC | dimethyl carbonate |
| EC | ethylene carbonate |
| EIS | electrochemical impedance spectroscopy |
| fSOH | state-of-health frequency |
| HNEI | Hawaii Natural Energy Institute |
| IC | incremental capacity |
| ICCT | initial conditioning and characterization testing |
| LAMdeNE | loss of active material on delithiated negative electrode |
| LAMdePE | loss of active material on delithiated positive electrode |
| LAMliNE | loss of active material on lithiated negative electrode |
| LAMliPE | loss of active material on lithiated positive electrode |
| LLI | loss of lithium inventory |
| MIST-BTS | modular one-channel signal treatment-battery testing system |
| NE | negative electrode |
| NRL | U.S. Naval Research Laboratory |
| OCV | open circuit voltage |
| PE | positive electrode |
| PTFE | polytetrafluorethylene |
| RDFNE | rate degradation factor negative electrode |
| RDFPE | rate degradation factor positive electrode |
| RPT | reference performance test |
| SEI | solid electrolyte interphase |
| SOH | state-of-health |
| SOC | state-of-charge |
| VC | vinylene carbonate |
| Zimag | imaginary impedance |
| |Z|mag | impedance magnitude |
| Zreal | real impedance |
References
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, J. A review on prognostics and health monitoring of Li-ion battery. J. Power Sources 2011, 196, 6007–6014. [Google Scholar] [CrossRef]
- Love, C.T.; Virji, M.B.; Rocheleau, R.E.; Swider-Lyons, K.E. State-of-health monitoring of 18650 4S packs with a single-point impedance diagnostic. J. Power Sources 2014, 266, 512–519. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Dubarry, M.; Vuillaume, N.; Liaw, B.Y. Origins and accommodation of cell variations in Li-ion battery pack modeling. Int. J. Energy Res. 2010, 34, 216–231. [Google Scholar] [CrossRef]
- Gao, Z.; Chin, C.S.; Woo, W.L.; Jia, J. Integrated Equivalent Circuit and Thermal Model for Simulation of Temperature-Dependent LiFePO4 Battery in Actual Embedded Application. Energies 2017, 10, 85. [Google Scholar] [CrossRef]
- Takeno, K.; Ichimura, M.; Takano, K.; Yamaki, J.; Okada, S. Quick testing of batteries in lithium-ion battery packs with impedance-measuring technology. J. Power Sources 2004, 128, 67–75. [Google Scholar] [CrossRef]
- Love, C.T.; Swider-Lyons, K. Impedance Diagnostic for Overcharged Lithium-Ion Batteries. Electrochem. Solid State Lett. 2012, 15, A53–A56. [Google Scholar] [CrossRef]
- Senyshyn, A.; Mühlbauer, M.J.; Dolotko, O.; Ehrenberg, H. Low-temperature performance of Li-ion batteries: The behavior of lithiated graphite. J. Power Sources 2015, 282, 235–240. [Google Scholar] [CrossRef]
- Huang, C.K.; Sakamoto, J.S.; Wolfenstine, J.; Surampudi, S. The Limits of Low-Temperature Performance of Li-Ion Cells. J. Electrochem. Soc. 2000, 147, 2893–2896. [Google Scholar] [CrossRef]
- Love, C.T.; Baturina, O.A.; Swider-Lyons, K.E. Observation of Lithium Dendrites at Ambient Temperature and Below. ECS Electrochem. Lett. 2015, 4, A24–A27. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Huang, J.; Liaw, B.Y.; Metzler, V.; Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. The low temperature performance of Li-ion batteries. J. Power Sources 2003, 115, 137–140. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fowler, M.; Fraser, R. Uneven temperature and voltage distributions due to rapid discharge rates and different boundary conditions for series-connected LiFePO4 batteries. Int. Commun. Heat Mass Transf. 2017, 81, 210–217. [Google Scholar] [CrossRef]
- Panchal, S.; Mathew, M.; Fraser, R.; Fowler, M. Electrochemical thermal modeling and experimental measurements of 18650 cylindrical lithium-ion battery during discharge cycle for an EV. Appl. Therm. Eng. 2018, 135, 123–132. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- Bloom, I.; Jansen, A.N.; Abraham, D.P.; Knuth, J.; Jones, S.A.; Battaglia, V.S.; Henriksen, G.L. Differential voltage analyses of high-power, lithium-ion cells: 1. Technique and application. J. Power Sources 2005, 139, 295–303. [Google Scholar] [CrossRef]
- Dubarry, M.; Svoboda, V.; Hwu, R.; Liaw, B.Y. Incremental Capacity Analysis and Close-to-Equilibrium OCV Measurements to Quantify Capacity Fade in Commercial Rechargeable Lithium Batteries. Electrochem. Solid-State Lett. 2006, 9, A454–A457. [Google Scholar] [CrossRef]
- Spinner, N.S.; Love, C.T.; Rose-Pehrsson, S.L.; Tuttle, S.G. Expanding the Operational Limits of the Single-Point Impedance Diagnostic for Internal Temperature Monitoring of Lithium-ion Batteries. Electrochim. Acta 2015, 174, 488–493. [Google Scholar] [CrossRef]
- Williard, N.; Sood, B.; Osterman, M.; Pecht, M. Disassembly methodology for conducting failure analysis on lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2011, 22, 1616. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Alawa Central. Available online: https://www.soest.hawaii.edu/HNEI/alawa/ (accessed on 3 April 2018).
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Devie, A.; Dubarry, M. Durability and Reliability of Electric Vehicle Batteries under Electric Utility Grid Operations. Part 1: Cell-to-Cell Variations and Preliminary Testing. Batteries 2016, 2, 28. [Google Scholar] [CrossRef]
- Spinner, N.; Love, C.T. Coupling Imepadance and Temperature of 18650 Li-ion Cells; U.S Naval Research Laboratory: Washington, DC, USA, 2015; unpublished result. [Google Scholar]
- Beelen, H.P.G.J.; Raijmakers, L.H.J.; Donkers, M.C.F.; Notten, P.H.L.; Bergveld, H.J. A comparison and accuracy analysis of impedance-based temperature estimation methods for Li-ion batteries. Appl. Energy 2016, 175, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Friesen, A.; Horsthemke, F.; Mönnighoff, X.; Brunklaus, G.; Krafft, R.; Börner, M.; Risthaus, T.; Winter, M.; Schappacher, F.M. Impact of cycling at low temperatures on the safety behavior of 18650-type lithium ion cells: Combined study of mechanical and thermal abuse testing accompanied by post-mortem analysis. J. Power Sources 2016, 334, 1–11. [Google Scholar] [CrossRef]
- Li, Q.; Lu, D.; Zheng, J.; Jiao, S.; Luo, L.; Wang, C.M.; Xu, K.; Zhang, J.G.; Xu, W. Li+-Desolvation Dictating Lithium-Ion Battery’s Low-Temperature Performances. ACS Appl. Mater. Interfaces 2017, 9, 42761–42768. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Newman, J. Effect of Anode Film Resistance on the Charge/Discharge Capacity of a Lithium-Ion Battery. J. Electrochem. Soc. 2003, 150, A1416–A1420. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V.; Surampudi, S.; Wang, Y.; Zhang, X.; Greenbaum, S.G.; Hightower, A.; Ahn, C.C.; Fultz, B. Irreversible Capacities of Graphite in Low-Temperature Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 1999, 146, 3963–3969. [Google Scholar] [CrossRef]
- Zhuo, Z.; Lu, P.; Delacourt, C.; Qiao, R.; Xu, K.; Pan, F.; Harris, S.J.; Yang, W. Breathing and oscillating growth of solid-electrolyte-interphase upon electrochemical cycling. Chem. Commun. 2018, 54, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Xu, K.; Jow, T.R. Electrochemical impedance study on the low temperature of Li-ion batteries. Electrochim.Acta 2004, 49, 1057–1061. [Google Scholar] [CrossRef]
- Dubarry, M.; Baure, G.; Devie, A. Durability and Reliability of EV Batteries under Electric Utility Grid Operations: Path Dependence of Battery Degradation. J. Electrochem. Soc. 2018, 165, A773–A783. [Google Scholar] [CrossRef]
- Ouyang, M.; Chu, Z.; Lu, L.; Li, J.; Han, X.; Feng, X.; Liu, G. Low temperature aging mechanism identification and lithium deposition in a large format lithium iron phosphate battery for different charge profiles. J. Power Sources 2015, 286, 309–320. [Google Scholar] [CrossRef]
- Rohr, S.; Müller, S.; Baumann, M.; Kerler, M.; Ebert, F.; Kaden, D.; Lienkamp, M. Quantifying Uncertainties in Reusing Lithium-Ion Batteries from Electric Vehicles. Procedia Manuf. 2017, 8, 603–610. [Google Scholar] [CrossRef]
- Baumhöfer, T.; Brühl, M.; Rothgang, S.; Sauer, D.U. Production caused variation in capacity aging trend and correlation to initial cell performance. J. Power Sources 2014, 247, 332–338. [Google Scholar] [CrossRef]












| Rate Capability | Resistance (without Outliers) | Capacity Ration |
|---|---|---|
| 99.35 (±0.13%) | 71 mΩ (±3.8%) | 2.657 (±0.51%) |
| Cells # in 4S Pack | 25 °C 2.75 V Cell Cutoff | 0 °C 2.75 V Cell Cutoff | 0 °C 11.0 V Pack Cutoff |
|---|---|---|---|
| Cell1 | ~1.5 | 25.0 | 3.5 |
| Cell 2 | ~1.5 | 19.0 | 8.0 |
| Cell 3 | ~1.5 | 7.5 | 4.0 |
| Cell 4 | ~1.5 | 6.5 | 44.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Love, C.T.; Dubarry, M.; Reshetenko, T.; Devie, A.; Spinner, N.; Swider-Lyons, K.E.; Rocheleau, R. Lithium-Ion Cell Fault Detection by Single-Point Impedance Diagnostic and Degradation Mechanism Validation for Series-Wired Batteries Cycled at 0 °C. Energies 2018, 11, 834. https://doi.org/10.3390/en11040834
Love CT, Dubarry M, Reshetenko T, Devie A, Spinner N, Swider-Lyons KE, Rocheleau R. Lithium-Ion Cell Fault Detection by Single-Point Impedance Diagnostic and Degradation Mechanism Validation for Series-Wired Batteries Cycled at 0 °C. Energies. 2018; 11(4):834. https://doi.org/10.3390/en11040834
Chicago/Turabian StyleLove, Corey T., Matthieu Dubarry, Tatyana Reshetenko, Arnaud Devie, Neil Spinner, Karen E. Swider-Lyons, and Richard Rocheleau. 2018. "Lithium-Ion Cell Fault Detection by Single-Point Impedance Diagnostic and Degradation Mechanism Validation for Series-Wired Batteries Cycled at 0 °C" Energies 11, no. 4: 834. https://doi.org/10.3390/en11040834






