Draining Water from Aircraft Fuel Using Nitrogen Enriched Air
Abstract
:1. Introduction
2. Methodology
2.1. Governing Multiphase Equations
2.2. Droplet Dynamics
2.3. Mass Exchange
2.4. Momentum Exchange
2.5. Heat Exchange
2.6. Computational Domain
2.7. Initial and Boundary Conditions
2.8. NEA Inlets
2.9. Grid Convergence
2.10. Validation
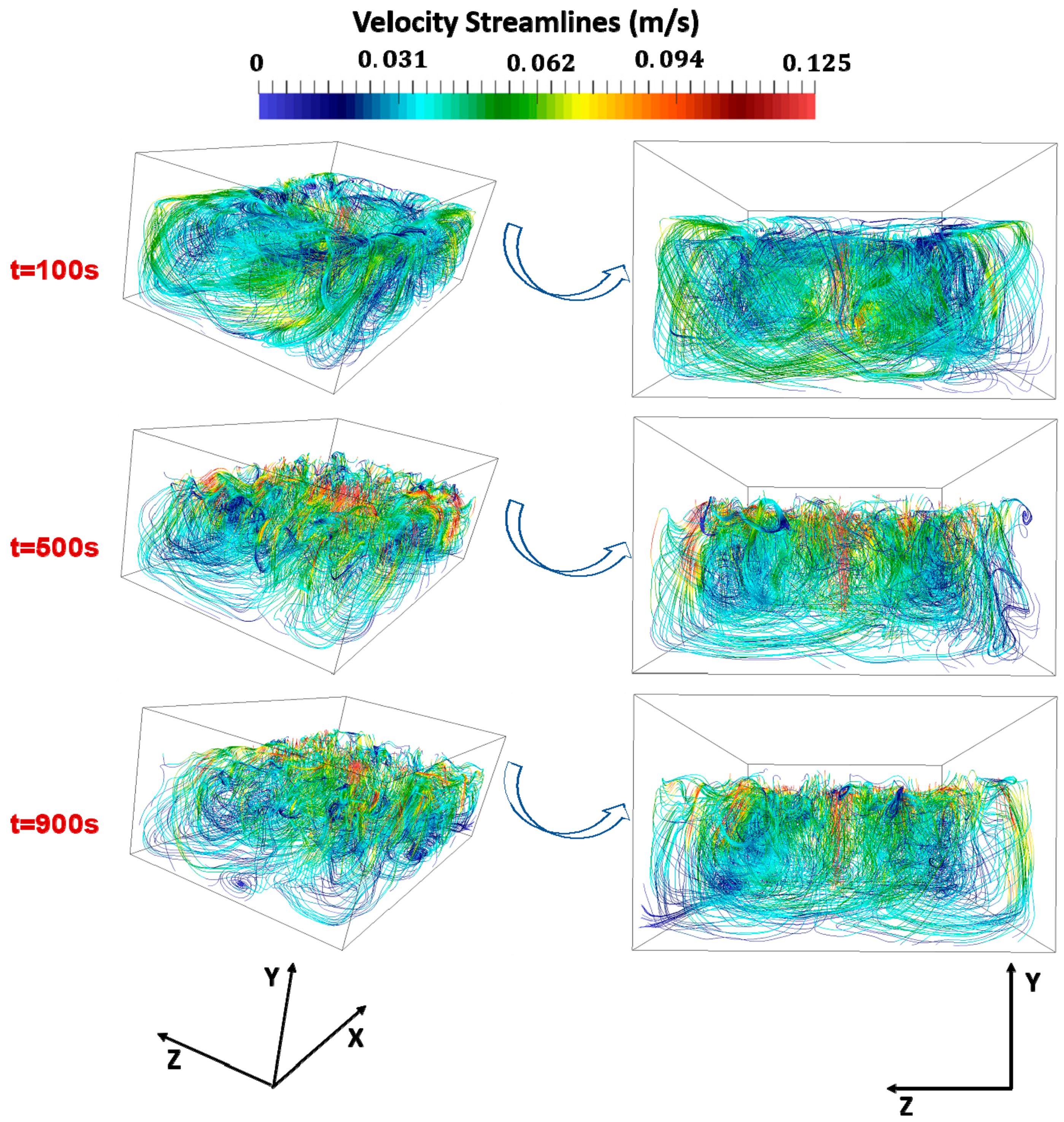
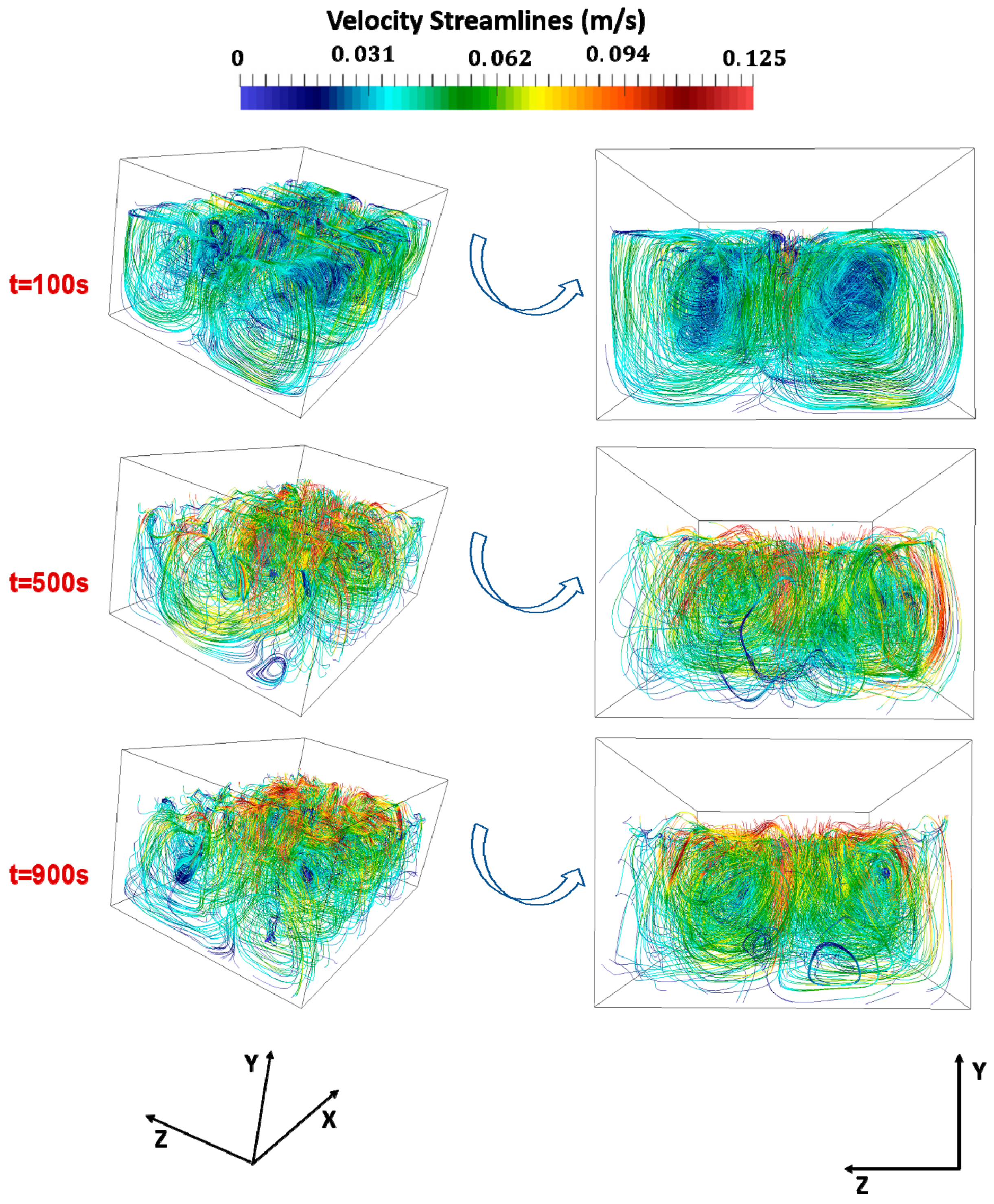
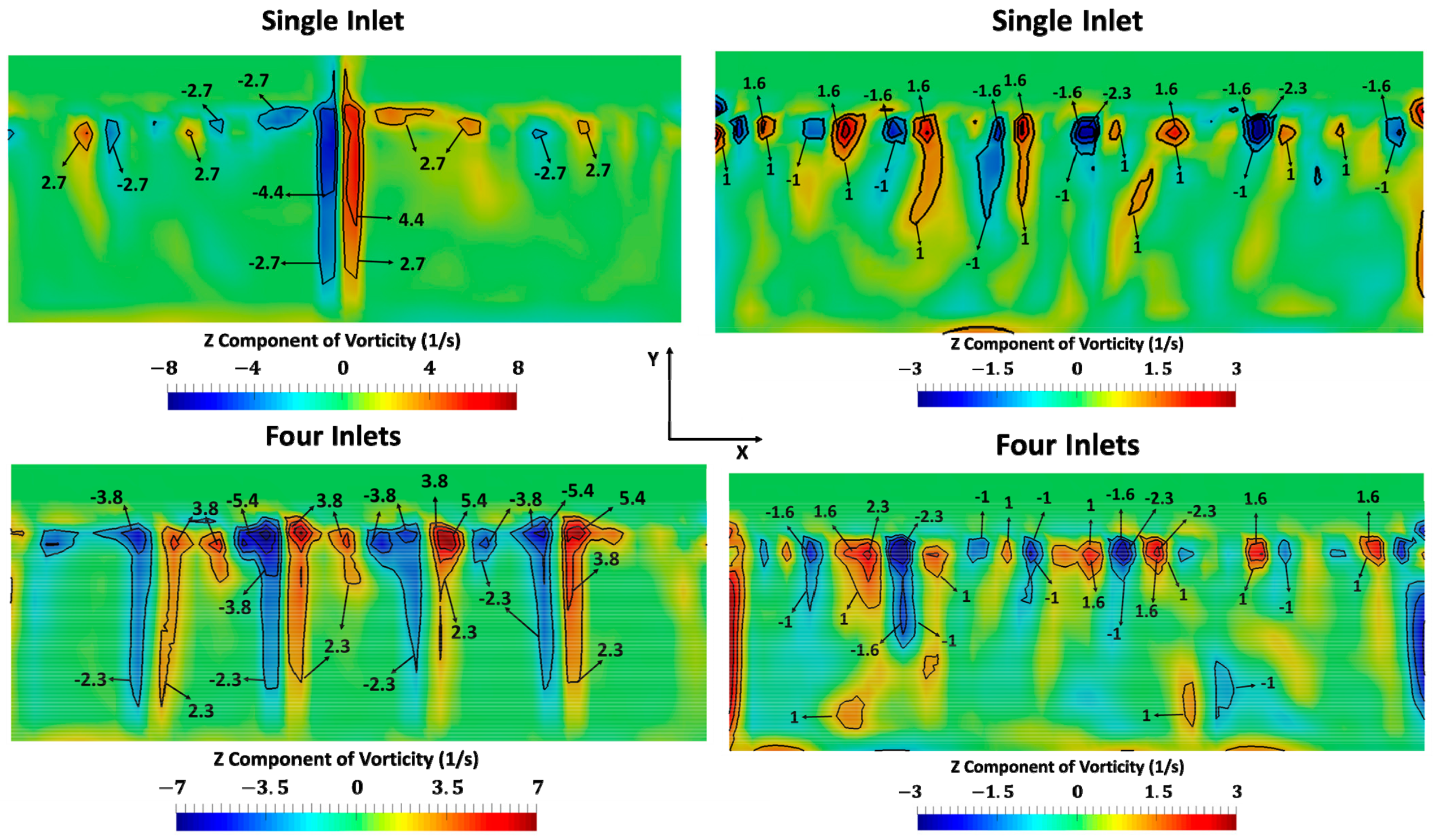
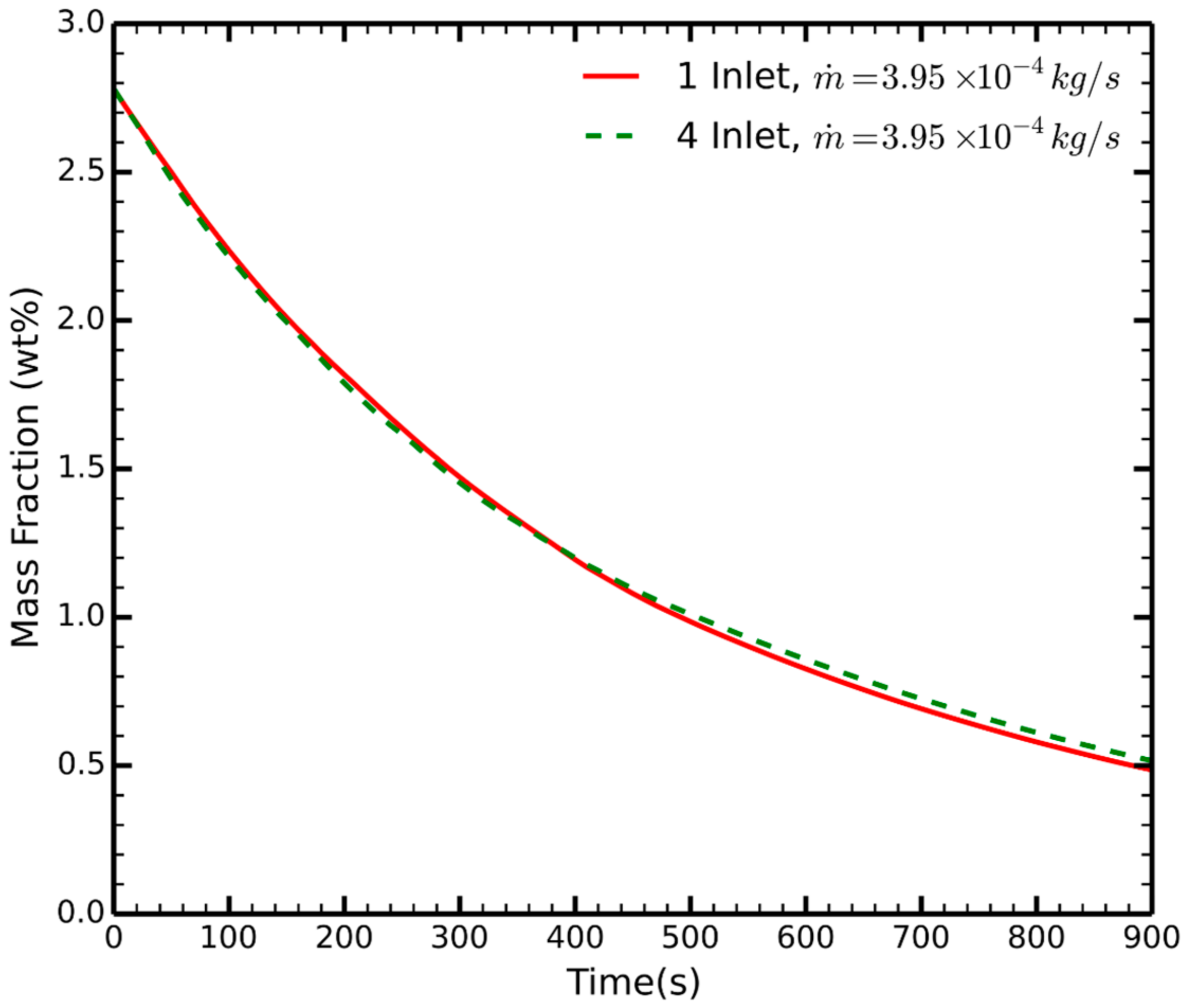
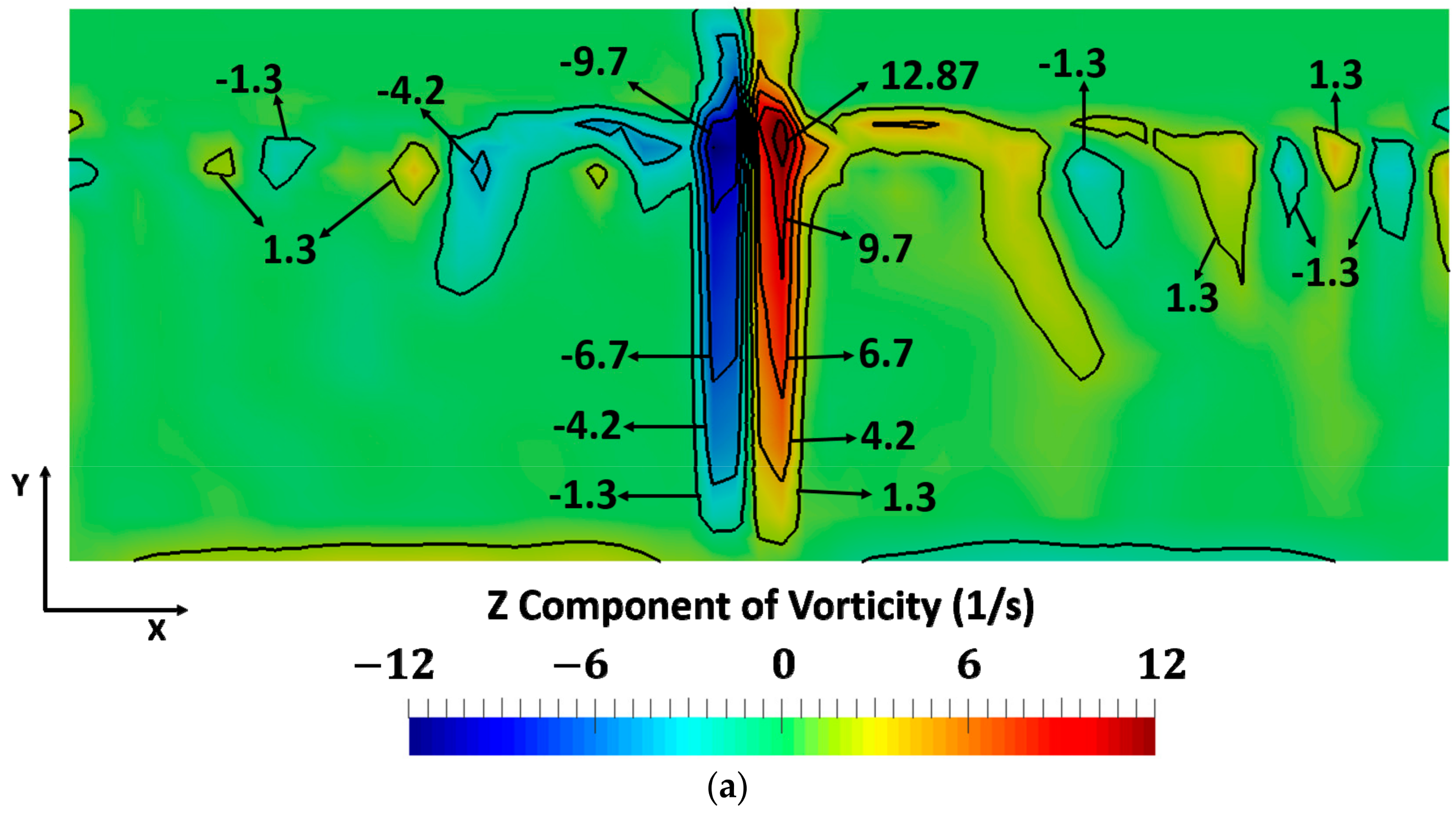
3. Results
4. Conclusions
- (1)
- The rising NEA bubbles absorb a small amount of water from the fuel and remain relatively dry compared to the moist air in the ullage. Therefore, when the NEA enters the ullage it decreases its water content.
- (2)
- Water is released from the fuel’s entire surface, due to the low partial pressure of water in the ullage. The mass of water exchanged directly between the fuel and ullage is much higher than the water that is absorbed by the rising NEA bubbles.
- (3)
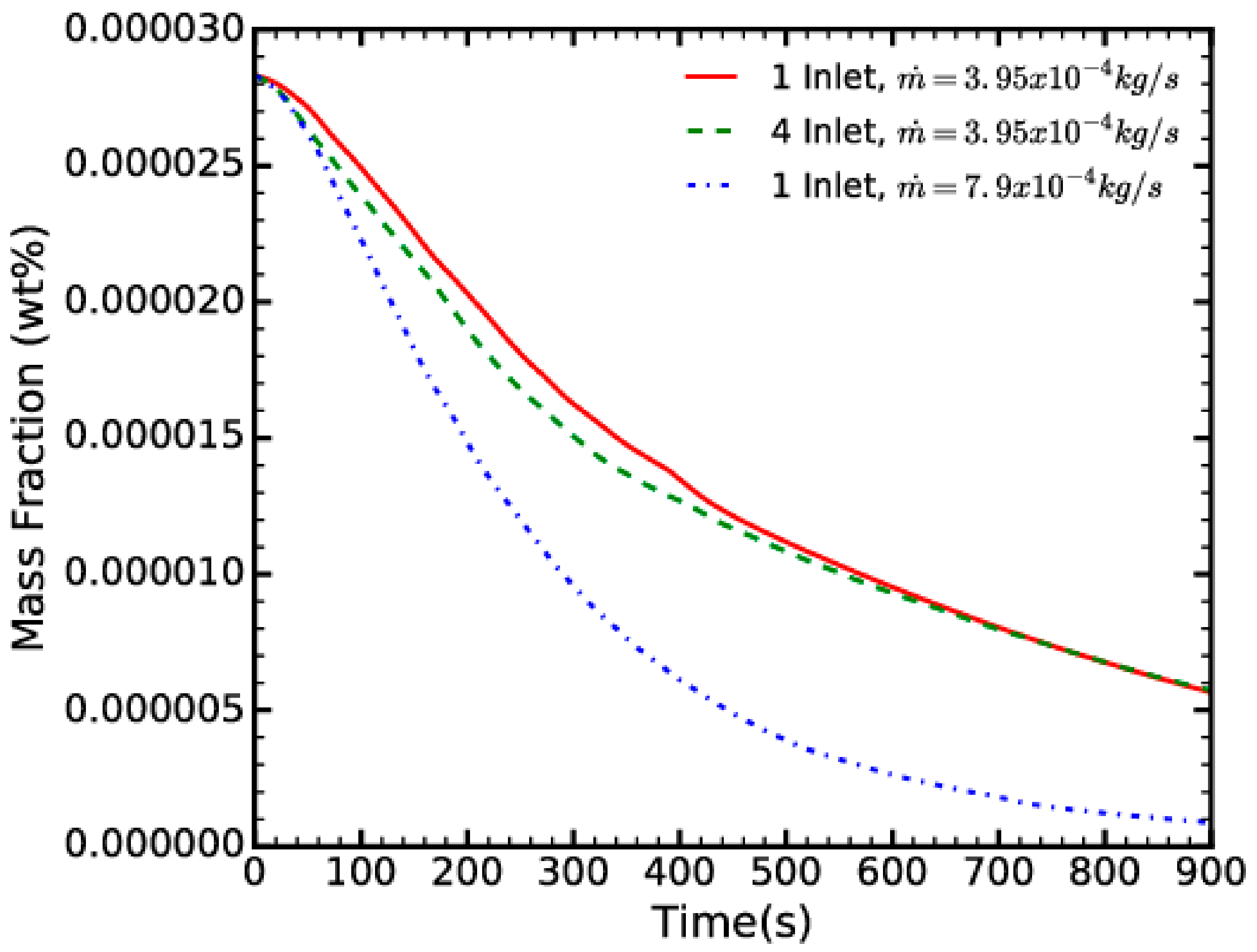
- For a constant mass flow rate, the number of NEA inlets does not significantly affect the timescale of the process.
- (4)
- The main contributor to the dehydration of the fuel is the total mass flow rate: increasing the mass flow rate by a factor of two, i.e., halving the time for NEA pumping.
- (5)
- Removing the dissolved water reduces the formation of water-in-fuel emulsions. We also speculate that entrained water bubbles will be mixed and dissolved in the fuel due to the circulation that is caused by the flow of NEA. Thus, the pumping of NEA will also be able to remove the separated water. Since fuel circulation depends on the number and the position of the inlets, these would be important factors to consider when dealing with emulsions.
Author Contributions
Conflicts of Interest
References
- Edwards, T.; Stricker, J.; Harrison, W., III. Coordinating Support of Fuels and Lubricant Research and Development (R&D) 2; Coordinating Research Council, Inc.: Alpharetta, GA, USA, 2004. [Google Scholar]
- Hemighaus, G.; Boval, T.; Bacha, J.; Barnes, F.; Franklin, M.; Gibbs, L.; Hogue, N.; Jones, J.; Lesnini, D.; Lind, J. Aviation Fuels Technical Review; Chevron Corporation: San Ramon, CA, USA, 2006. [Google Scholar]
- Goodger, E. Transport Fuels and Technology-Mobility for the Millennium; Landfill Press: Norwich, UK, 2000. [Google Scholar]
- Baena-Zambrana, S.; Repetto, S.; Lawson, C.P.; Lam, J.-W. Behaviour of water in jet fuel—A literature review. Prog. Aerosp. Sci. 2013, 60, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Federal Aviation Administration, United States Department of Transportation. Aviation Maintenance Technician Handbook-Airframe; United States Department of Transportation: Washington, DC, USA, 2012.
- Sleight, P.; Carter, R. Report on the Accident to BOEING 777-236ER, G-YMMM, at LONDON Heathrow Airport on 17 January 2008; Air Accidents Investigation Branch: Aldershot, UK, 2010. [Google Scholar]
- Tsanaktsidis, C.G.; Christidis, S.G.; Favvas, E.P. A novel method for improving the physicochemical properties of diesel and jet fuel using polyaspartate polymer additives. Fuel 2013, 104, 155–162. [Google Scholar] [CrossRef]
- Krawczyk, M.A.; Wasan, D.T.; Shetty, C. Chemical demulsification of petroleum emulsions using oil-soluble demulsifiers. Ind. Eng. Chem. Res. 1991, 30, 367–375. [Google Scholar] [CrossRef]
- Smith, M. Aviation Fuels; G. T. Foulis & Co Ltd., Henley-on-Thames: Oxfordshire, UK, 1970. [Google Scholar]
- Bitten, J.F. Coalescence of water droplets on single fibers. J. Colloid Interface Sci. 1970, 33, 265–271. [Google Scholar] [CrossRef]
- Matar, S.; Hatch, L.F. Chemistry of Petrochemical Processes; Gulf Professional Publishing: Houston, TX, USA, 2001. [Google Scholar]
- Tsanaktsidis, C.; Christidis, S.; Tzilantonis, G. Use of bioorganic compounds for reducing the moisture content of diesel fuel to reduce the icing effect. Chem. Technol. Fuels Oils 2010, 46, 211–212. [Google Scholar] [CrossRef]
- Tsanaktsidis, C.G.; Favvas, E.P.; Scaltsoyiannes, A.A.; Christidis, S.G.; Katsidi, E.X.; Scaltsoyiannes, A.V. Natural resins and their application in antifouling fuel technology: Part I: Improving the physicochemical properties of diesel fuel using natural resin polymer as a removable additive. Fuel Process. Technol. 2013, 114, 135–143. [Google Scholar] [CrossRef]
- Tsanaktsidis, C.G.; Favvas, E.P.; Tzilantonis, G.T.; Christidis, S.G.; Katsidi, E.C.; Scaltsoyiannes, A.V. Water removal from biodiesel/diesel blends and jet fuel using natural resin as dehydration agent. Can. J. Chem. Eng. 2015, 93, 1812–1818. [Google Scholar] [CrossRef]
- Terada, Y.; Lawson, C.P.; Shahneh, A.Z. Analytical investigation into the effects of nitrogen enriched air bubbles to improve aircraft fuel system water management. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2017. [Google Scholar] [CrossRef]
- Burns, M.; Cavage, W.M. Inerting of a Vented Aircraft Fuel Tank Test Article with Nitrogen-Enriched Air; FAA William J. Hughes Technical Center, Atlantic City International Airport: Egg Harbor Township, NJ, USA, 2001.
- Cavage, W.M.; Bowman, T. Modeling In-Flight Inert Gas Distribution in a 747 Center Wing Fuel Tank. In Proceedings of the 35th AIAA Fluid Dynamics Conference and Exhibit, Toronto, ON, Canada, 6–9 June 2005. [Google Scholar]
- Burns, M.; Cavage, W.; Morrison, R.; Summer, S. Evaluation of Fuel Tank Flammability and the FAA Inerting System on the NASA 747 SCA; U.S. Department of Transportation, Federal Aviation Administration, Office of Aviation Research: Washington, DC, USA, 2004.
- Cai, Y.; Bu, X.; Lin, G.; Sun, B.; Zeng, Y.; Li, Z. Experimental study of an aircraft fuel tank inerting system. Chin. J. Aeronaut. 2015, 28, 394–402. [Google Scholar] [CrossRef]
- Battistoni, M.; Grimaldi, C.N. Numerical analysis of injector flow and spray characteristics from diesel injectors using fossil and biodiesel fuels. Appl. Energy 2012, 97, 656–666. [Google Scholar] [CrossRef]
- Vujanovic, M.; Petranovic, Z.; Edelbauer, W.; Baleta, J.; Duic, N. Numerical modelling of diesel spray using the Eulerian multiphase approach. Energy Convers. Manag. 2015, 104, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Harlow, F.H.; Amsden, A.A. Numerical calculation of multiphase fluid flow. J. Comput. Phys. 1975, 17, 19–52. [Google Scholar] [CrossRef]
- Ishii, M.; Hibiki, T. Thermo-Fluid Dynamic of Two-Phase Flow; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Sato, Y.; Sekoguchi, K. Liquid velocity distribution in two-phase bubble flow. Int. J. Multiph. Flow 1975, 2, 79–95. [Google Scholar] [CrossRef]
- Guggenheim, E. The theoretical basis of Raoult’s law. Trans. Faraday Soc. 1937, 33, 151–156. [Google Scholar] [CrossRef]
- Karimi, M.; Akdogan, G.; Dellimore, K.H.; Bradshaw, S.M. Comparison of different drag coefficient correlations in the CFD modelling of a laboratory-scale Rushton-turbine flotation tank. In Proceedings of the Ninth International Conference on CFD in the Minerals and Process Industries, Melbourne, Australia, 10–12 December 2012. [Google Scholar]
- Schiller, L.; Naumann, Z. A drag coefficient correlation. Z. Ver. Dtsch. Ing. 1935, 77, 318–320. [Google Scholar]
- Ranz, W.; Marshall, W. Evaporation from drops. Chem. Eng. Prog. 1952, 48, 141–146. [Google Scholar]
- Drikakis, D.; Hahn, M.; Mosedale, A.; Thornber, B. Large eddy simulation using high-resolution and high-order methods. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2009, 367, 2985–2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClure, D.D.; Kavanagh, J.M.; Fletcher, D.F.; Barton, G.W. Oxygen transfer in bubble columns at industrially relevant superficial velocities: Experimental work and CFD modelling. Chem. Eng. J. 2015, 180, 138–146. [Google Scholar] [CrossRef]
- Pfleger, D.; Gomes, S.; Gilbert, N.; Wagner, H.-G. Hydrodynamic simulations of laboratory scale bubble columns fundamental studies of the Eulerian–Eulerian modelling approach. Chem. Eng. Sci. 1999, 54, 5091–5099. [Google Scholar] [CrossRef]







| Component | Fuel (wt %) | Air (wt %) | NEA (wt %) |
|---|---|---|---|
| Decane | 0 | 0 | |
| Nitrogen | 75.4 | 95 | |
| Oxygen | 21.6 | 5 | |
| Water | 2.778 | 0 |
| Grid | Number of Cells | RMS |
|---|---|---|
| 6480 | ||
| 51,840 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, M.; Drikakis, D. Draining Water from Aircraft Fuel Using Nitrogen Enriched Air. Energies 2018, 11, 908. https://doi.org/10.3390/en11040908
Frank M, Drikakis D. Draining Water from Aircraft Fuel Using Nitrogen Enriched Air. Energies. 2018; 11(4):908. https://doi.org/10.3390/en11040908
Chicago/Turabian StyleFrank, Michael, and Dimitris Drikakis. 2018. "Draining Water from Aircraft Fuel Using Nitrogen Enriched Air" Energies 11, no. 4: 908. https://doi.org/10.3390/en11040908





