Passive Fuel Cell Heat Recovery Using Heat Pipes to Enhance Metal Hydride Canisters Hydrogen Discharge Rate: An Experimental Simulation
Abstract
:1. Introduction
2. Thermal Coupling of PEM Fuel Cell and MH Canister: A Literature Review
3. Experimental Investigation
3.1. An Overview
3.2. Heat Pipes to Transfer the Fuel Cell Heat from the Fuel Cell to the MH Canister
3.3. Fuel Cell Heat to Improve Metal Hydride Hydrogen Discharge Rate
3.3.1. Overview
3.3.2. Using Thermal Pads to Mimic the Fuel Cell Heat Transfer towards the MH Canister via Heat Pipes
4. Results and Discussion
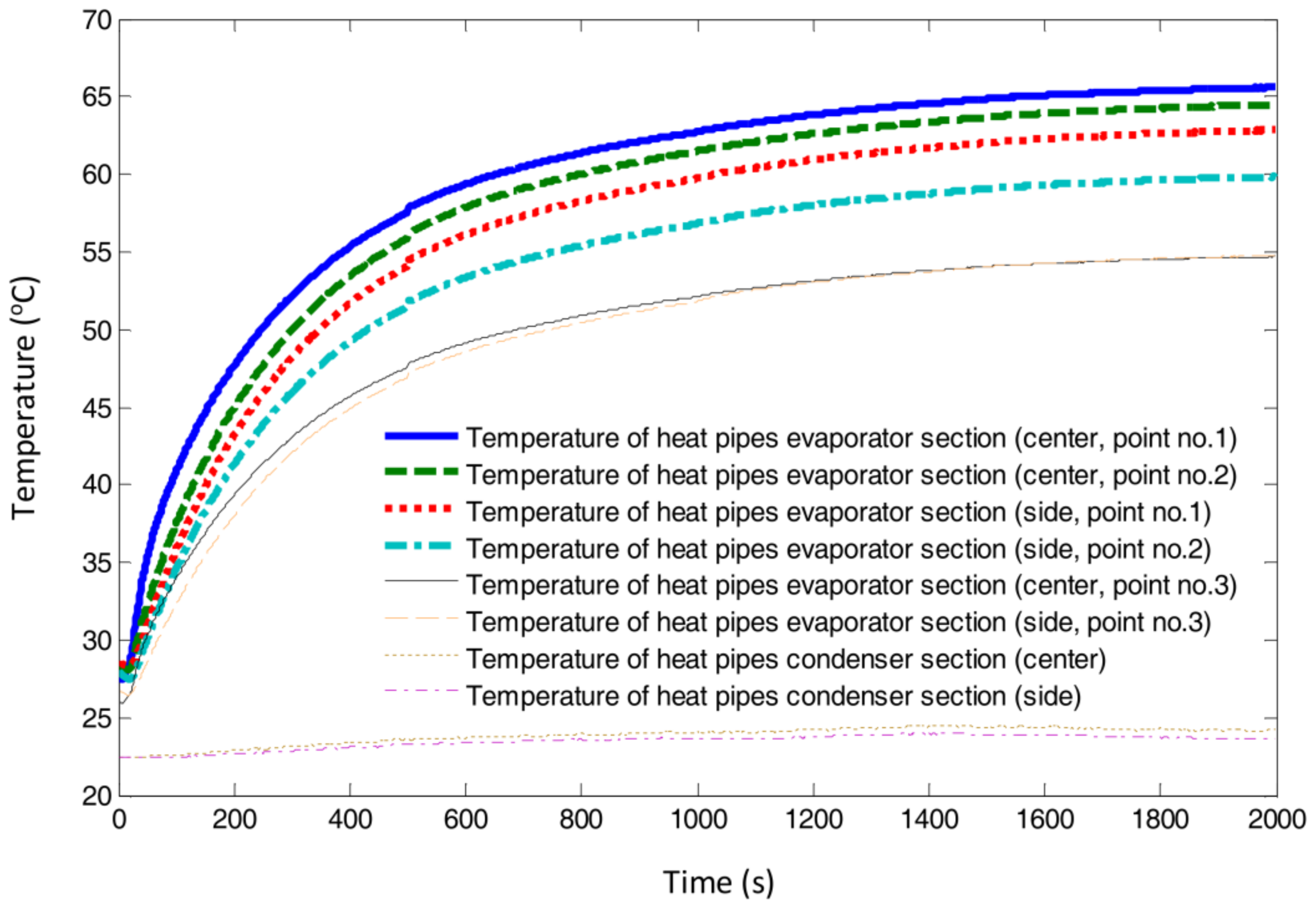
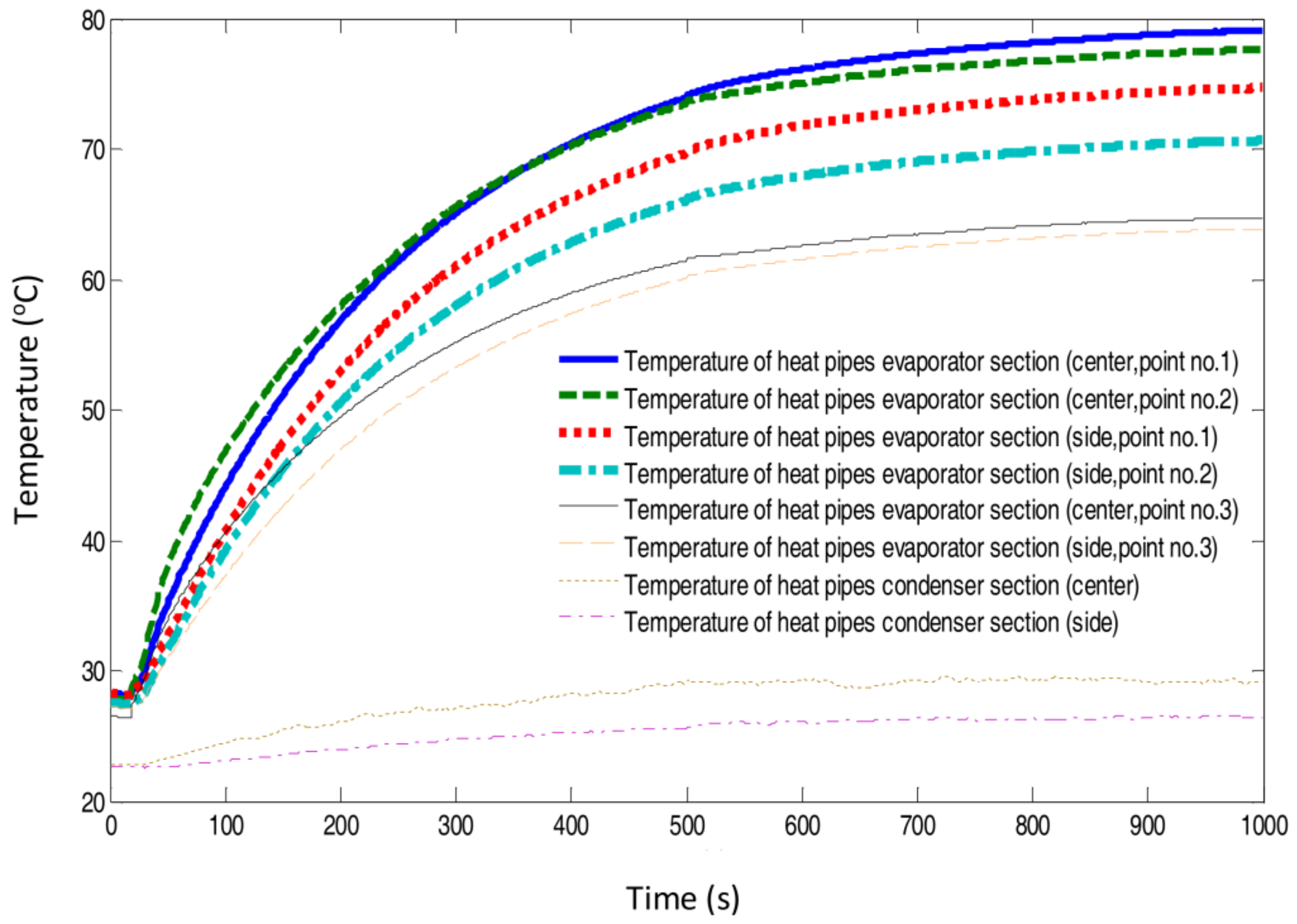
4.1. Heat Pipes to Transfer Heat from the Simulated Fuel Cell
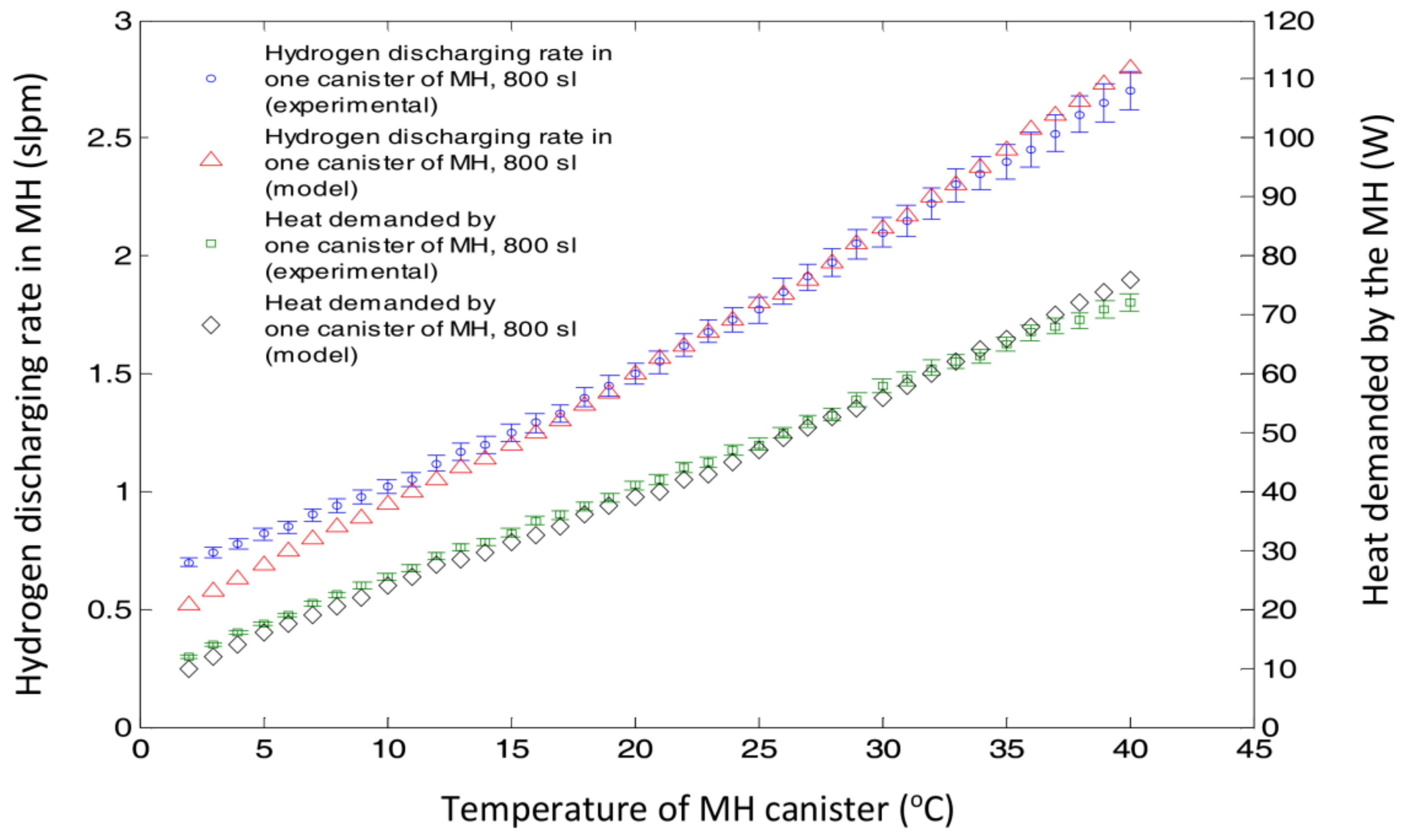
4.2. The Effect of MH Canister Heating on Its Hydrogen Release Rate
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Larminie, J.; Dicks, A. Fuel Cell Systems Explained, 2nd ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Barbir, F. Chapter 1—1. Introduction. In PEM Fuel Cells; Barbir, F., Ed.; Academic Press: Burlington, MA, USA, 2005; pp. 1–16. [Google Scholar]
- Shabani, B.; Andrews, J. An experimental investigation of a pem fuel cell to supply both heat and power in a solar-hydrogen raps system. Int. J. Hydrogen Energy 2011, 36, 5442–5452. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G.; Andrews, J. The potential of using nanofluids in pem fuel cell cooling systems: A review. Renew. Sustain. Energy Rev. 2015, 48, 523–539. [Google Scholar] [CrossRef]
- Sajid Hossain, M.; Shabani, B. Metal foams application to enhance cooling of open cathode polymer electrolyte membrane fuel cells. J. Power Sources 2015, 295, 275–291. [Google Scholar] [CrossRef]
- Zhang, G.; Kandlikar, S.G. A critical review of cooling techniques in proton exchange membrane fuel cell stacks. Int. J. Hydrogen Energy 2012, 37, 2412–2429. [Google Scholar] [CrossRef]
- Pei, P.; Chen, H. Main factors affecting the lifetime of proton exchange membrane fuel cells in vehicle applications: A review. Appl. Energy 2014, 125, 60–75. [Google Scholar] [CrossRef]
- Chen, H.; Pei, P.; Song, M. Lifetime prediction and the economic lifetime of proton exchange membrane fuel cells. Appl. Energy 2015, 142, 154–163. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Nanofluids to improve the performance of pem fuel cell cooling systems: A theoretical approach. Appl. Energy 2016, 178, 660–671. [Google Scholar] [CrossRef]
- Ramousse, J.; Lottin, O.; Didierjean, S.; Maillet, D. Heat sources in proton exchange membrane (PEM) fuel cells. J. Power Sources 2009, 192, 435–441. [Google Scholar] [CrossRef]
- Shabani, B.; Andrews, J.; Watkins, S. Energy and cost analysis of a solar-hydrogen combined heat and power system for remote power supply using a computer simulation. Solar Energy 2010, 84, 144–155. [Google Scholar] [CrossRef]
- Shabani, B.; Andrews, J.; Badwal, S. Fuel cell heat recovery, electrical load management, and the economics of solar-hydrogen systems. Int. J. Power Energy Syst. 2010, 30, 256–263. [Google Scholar] [CrossRef]
- Assaf, J.; Shabani, B. Transient simulation modelling and energy performance of a standalone solar-hydrogen combined heat and power system integrated with solar-thermal collectors. Appl. Energy 2016, 178, 66–77. [Google Scholar] [CrossRef]
- Assaf, J.; Shabani, B. Economic analysis and assessment of a standalone solar-hydrogen combined heat and power system integrated with solar-thermal collectors. Int. J. Hydrogen Energy 2016, 41, 18389–18404. [Google Scholar] [CrossRef]
- Assaf, J.; Shabani, B. Multi-objective sizing optimisation of a solar-thermal system integrated with a solar-hydrogen combined heat and power system, using genetic algorithm. Energy Convers. Manag. 2018, 164, 518–532. [Google Scholar] [CrossRef]
- Shabani, B.; Andrews, J. Hydrogen and fuel cells. In Energy Sustainability through Green Energy; Sharma, A., Kar, S.K., Eds.; Springer: New Delhi, India, 2015; pp. 453–491. [Google Scholar]
- Aris, A.M.; Shabani, B. Sustainable power supply solutions for off-grid base stations. Energies 2015, 8, 10904–10941. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Aris, A.M.; Shabani, B. Pem fuel cell heat recovery for preheating inlet air in standalone solar-hydrogen systems for telecommunication applications: An exergy analysis. Int. J. Hydrogen Energy 2016, 41, 2987–3003. [Google Scholar] [CrossRef]
- Shabani, B.; Biju, M. Theoretical modelling methods for thermal management of batteries. Energies 2015, 8, 10153–10177. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, S.-M.; Hong, B.-S. Experimental study on a passive fuel cell/battery hybrid power system. Energies 2013, 6, 6413–6422. [Google Scholar] [CrossRef]
- Han, J.; Charpentier, J.-F.; Tang, T. An energy management system of a fuel cell/battery hybrid boat. Energies 2014, 7, 2799–2820. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Chang, H.; Shu, S.; Wang, Y.; Tang, H. A review on cold start of proton exchange membrane fuel cells. Energies 2014, 7, 3179–3203. [Google Scholar] [CrossRef]
- Odeim, F.; Roes, J.; Heinzel, A. Power management optimization of an experimental fuel cell/battery/supercapacitor hybrid system. Energies 2015, 8, 6302–6327. [Google Scholar] [CrossRef]
- Shabani, B.; Andrews, J. Standalone solar-hydrogen systems powering fire contingency networks. Int. J. Hydrogen Energy 2015, 40, 5509–5517. [Google Scholar] [CrossRef]
- Førde, T.; Eriksen, J.; Pettersen, A.G.; Vie, P.J.S.; Ulleberg, Ø. Thermal integration of a metal hydride storage unit and a pem fuel cell stack. Int. J. Hydrogen Energy 2009, 34, 6730–6739. [Google Scholar] [CrossRef]
- Kaplan, Y. Effect of design parameters on enhancement of hydrogen charging in metal hydride reactors. Int. J. Hydrogen Energy 2009, 34, 2288–2294. [Google Scholar] [CrossRef]
- MacDonald, B.D.; Rowe, A.M. Impacts of external heat transfer enhancements on metal hydride storage tanks. Int. J. Hydrogen Energy 2006, 31, 1721–1731. [Google Scholar] [CrossRef]
- Møller, K.; Sheppard, D.; Ravnsbæk, D.; Buckley, C.; Akiba, E.; Li, H.-W.; Jensen, T. Complex metal hydrides for hydrogen, thermal and electrochemical energy storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C., Jr. Metal hydride hydrogen compressors: A review. Int. J. Hydrogen Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef]
- Dornheim, M. Chapter 33: Thermodynamics of metal hydrides: Tailoring reaction enthalpies of hydrogen storage materials. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; Moreno-Pirajan, J.C., Ed.; Intech: London, UK, 2011. [Google Scholar]
- David, E. An overview of advanced materials for hydrogen storage. J. Mater. Process. Technol. 2005, 162–163, 169–177. [Google Scholar] [CrossRef]
- Libowitz, G.G. Metallic hydrides; fundamental properties and applications. J. Phys. Chem. Solids 1994, 55, 1461–1470. [Google Scholar] [CrossRef]
- Rönnebro, E.; Whyatt, G.; Powell, M.; Westman, M.; Zheng, F.; Fang, Z. Metal hydrides for high-temperature power generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef]
- Yang, F.S.; Wang, G.X.; Zhang, Z.X.; Meng, X.Y.; Rudolph, V. Design of the metal hydride reactors—A review on the key technical issues. Int. J. Hydrogen Energy 2010, 35, 3832–3840. [Google Scholar] [CrossRef]
- Yang, F.S.; Wang, G.X.; Zhang, Z.X.; Rudolph, V. Investigation on the influences of heat transfer enhancement measures in a thermally driven metal hydride heat pump. Int. J. Hydrogen Energy 2010, 35, 9725–9735. [Google Scholar] [CrossRef]
- MacDonald, B.D.; Rowe, A.M. Experimental and numerical analysis of dynamic metal hydride hydrogen storage systems. J. Power Sources 2007, 174, 282–293. [Google Scholar] [CrossRef]
- MacDonald, B.D.; Rowe, A.M. A thermally coupled metal hydride hydrogen storage and fuel cell system. J. Power Sources 2006, 161, 346–355. [Google Scholar] [CrossRef]
- Melnichuk, M.; Silin, N.; Peretti, H.A. Optimized heat transfer fin design for a metal-hydride hydrogen storage container. Int. J. Hydrogen Energy 2009, 34, 3417–3424. [Google Scholar] [CrossRef]
- Lee, H.-S.; Cho, C.-W.; Seo, J.-H.; Lee, M.-Y. Cooling performance characteristics of the stack thermal management system for fuel cell electric vehicles under actual driving conditions. Energies 2016, 9, 320. [Google Scholar] [CrossRef]
- Tetuko, A.P.; Shabani, B.; Andrews, J. Thermal coupling of pem fuel cell and metal hydride hydrogen storage using heat pipes. Int. J. Hydrogen Energy 2016, 41, 4264–4277. [Google Scholar] [CrossRef]
- Wilson, P.R.; Bowman, R.C., Jr.; Mora, J.L.; Reiter, J.W. Operation of a pem fuel cell with LaNi4.8Sn0.2 hydride beds. J. Alloys Compd. 2007, 446–447, 676–680. [Google Scholar] [CrossRef]
- Jiang, Z.; Dougal, R.A.; Liu, S.; Gadre, S.A.; Ebner, A.D.; Ritter, J.A. Simulation of a thermally coupled metal-hydride hydrogen storage and fuel cell system. J. Power Sources 2005, 142, 92–102. [Google Scholar] [CrossRef]
- Pfeifer, P.; Wall, C.; Jensen, O.; Hahn, H.; Fichtner, M. Thermal coupling of a high temperature pem fuel cell with a complex hydride tank. Int. J. Hydrogen Energy 2009, 34, 3457–3466. [Google Scholar] [CrossRef]
- Yiotis, A.G.; Kainourgiakis, M.E.; Kosmidis, L.I.; Charalambopoulou, G.C.; Stubos, A.K. Thermal coupling potential of solid oxide fuel cells with metal hydride tanks: Thermodynamic and design considerations towards integrated systems. J. Power Sources 2014, 269, 440–450. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Parsons, A.; Smith, F.; Sita, C.; Linkov, V. Performance of electric forklift with low-temperature polymer exchange membrane fuel cell power module and metal hydride hydrogen storage extension tank. J. Power Sources 2016, 316, 239–250. [Google Scholar] [CrossRef]
- Hwang, J.J.; Chang, W.R. Characteristic study on fuel cell/battery hybrid power system on a light electric vehicle. J. Power Sources 2012, 207, 111–119. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Bu, Q.; Guzy, C.J.; Li, Q.; Chen, W.; Wang, C. Novel fuel cell stack with coupled metal hydride containers. J. Power Sources 2016, 328, 329–335. [Google Scholar] [CrossRef]
- Odabaee, M.; Mancin, S.; Hooman, K. Metal foam heat exchangers for thermal management of fuel cell systems—An experimental study. Exp. Therm. Fluid Sci. 2013, 51, 214–219. [Google Scholar] [CrossRef]
- Kukkapalli, V.; Kim, S. Optimization of internal cooling fins for metal hydride reactors. Energies 2016, 9, 447. [Google Scholar] [CrossRef]
- Faghri, A. Heat pipes: Review, opportunities and challenges. Front. Heat Pipes 2014, 5. [Google Scholar] [CrossRef]
- Vasiliev, L.L. Micro and miniature heat pipes—Electronic component coolers. Appl. Therm. Eng. 2008, 28, 266–273. [Google Scholar] [CrossRef]
- Vasiliev, L.; Lossouarn, D.; Romestant, C.; Alexandre, A.; Bertin, Y.; Piatsiushyk, Y.; Romanenkov, V. Loop heat pipe for cooling of high-power electronic components. Int. J. Heat Mass Transf. 2009, 52, 301–308. [Google Scholar] [CrossRef]
- Greco, A.; Cao, D.; Jiang, X.; Yang, H. A theoretical and computational study of lithium-ion battery thermal management for electric vehicles using heat pipes. J. Power Sources 2014, 257, 344–355. [Google Scholar] [CrossRef]
- Tran, T.-H.; Harmand, S.; Sahut, B. Experimental investigation on heat pipe cooling for hybrid electric vehicle and electric vehicle lithium-ion battery. J. Power Sources 2014, 265, 262–272. [Google Scholar] [CrossRef]
- Zhao, R.; Gu, J.; Liu, J. An experimental study of heat pipe thermal management system with wet cooling method for lithium ion batteries. J. Power Sources 2015, 273, 1089–1097. [Google Scholar] [CrossRef]
- Liu, F.; Lan, F.; Chen, J. Dynamic thermal characteristics of heat pipe via segmented thermal resistance model for electric vehicle battery cooling. J. Power Sources 2016, 321, 57–70. [Google Scholar] [CrossRef]
- Silva, A.P.; Galante, R.M.; Pelizza, P.R.; Bazzo, E. A combined capillary cooling system for fuel cells. Appl. Therm. Eng. 2012, 41, 104–110. [Google Scholar] [CrossRef]
- Clement, J.; Wang, X. Experimental investigation of pulsating heat pipe performance with regard to fuel cell cooling application. Appl. Therm. Eng. 2013, 50, 268–274. [Google Scholar] [CrossRef]
- Supra, J.; Janben, H.; Lehnert, W.; Stolten, D. Design and experimental investigation of a heat pipe supported external cooling system for HT-PEFC stacks. J. Fuel Cell Sci. Technol. 2013, 10, 051002. [Google Scholar] [CrossRef]
- Firat, E.; Bandlamudi, G.; Beckhaus, P.; Heinzel, A. Heat Pipe Assisted Thermal Management of a HT PEMFC Stack. In Proceeding of the 2012 COMSOL Conference, Milan, Italy, 10–12 October 2012; pp. 1–6. [Google Scholar]
- Reay, D.; Kew, P. Heat Pipes: Theory, Design and Applications, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar]
- Shabany, Y. Heat Transfer: Thermal Management of Electronics; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Gupta, N.K.; Tiwari, A.K.; Ghosh, S.K. Heat transfer mechanisms in heat pipes using nanofluids—A review. Exp. Therm. Fluid Sci. 2018, 90, 84–100. [Google Scholar] [CrossRef]
- Naresh, Y.; Shri Vignesh, K.; Balaji, C. Experimental investigations of the thermal performance of self-rewetting fluids in internally finned wickless heat pipes. Exp. Therm. Fluid Sci. 2018, 92, 436–446. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Zhang, G.; Zhang, M. Experimental investigations of flat plate heat pipes with interlaced narrow grooves or channels as capillary structure. Exp. Therm. Fluid Sci. 2013, 48, 222–229. [Google Scholar] [CrossRef]
- Faghri, A. Review and advances in heat pipe science and technology. J. Heat Transf. 2012, 134, 123001. [Google Scholar] [CrossRef]
- Loh, C.K.; Harris, E.; Chou, D.J. Comparative Study of Heat Pipes Performances in Different Orientations. In Proceedings of the IEEE Twenty First Annual IEEE Semiconductor Thermal Measurement and Management Symposium, San Jose, CA, USA, 15–17 March 2005; pp. 191–195. [Google Scholar]
- Yang, C.; Song, C.; Shang, W.; Tao, P.; Deng, T. Flexible heat pipes with integrated bioinspired design. Prog. Nat. Sci. Mater. Int. 2015, 25, 51–57. [Google Scholar] [CrossRef]
- Xuan, Y.; Hong, Y.; Li, Q. Investigation on transient behaviors of flat plate heat pipes. Exp. Therm. Fluid Sci. 2004, 28, 249–255. [Google Scholar] [CrossRef]
- Khaitan, S.K.; Raju, M. Discharge dynamics of coupled fuel cell and metal hydride hydrogen storage bed for small wind hybrid systems. Int. J. Hydrogen Energy 2012, 37, 2344–2352. [Google Scholar] [CrossRef]
- Song, C.; Klebanoff, L.E.; Johnson, T.A.; Chao, B.S.; Socha, A.F.; Oros, J.M.; Radley, C.J.; Wingert, S.; Breit, J.S. Using metal hydride H2 storage in mobile fuel cell equipment: Design and predicted performance of a metal hydride fuel cell mobile light. Int. J. Hydrogen Energy 2014, 39, 14896–14911. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Peil, S.; Bathen, D.; Heßke, C.; Burfeind, J.; Hauschild, K.; Felderhoff, M.; Schüth, F. HT-PEM fuel cell system with integrated complex metal hydride storage tank. Fuel Cells 2011, 11, 911–920. [Google Scholar] [CrossRef]
- Weiss-Ungethüm, J.; Bürger, I.; Schmidt, N.; Linder, M.; Kallo, J. Experimental investigation of a liquid cooled high temperature proton exchange membrane (HT-PEM) fuel cell coupled to a sodium alanate tank. Int. J. Hydrogen Energy 2014, 39, 5931–5941. [Google Scholar] [CrossRef]
- Kim, S.H.; Miesse, C.M.; Lee, H.B.; Chang, I.W.; Hwang, Y.S.; Jang, J.H.; Cha, S.W. Ultra compact direct hydrogen fuel cell prototype using a metal hydride hydrogen storage tank for a mobile phone. Appl. Energy 2014, 134, 382–391. [Google Scholar] [CrossRef]
- Faghri, A. Heat Pipe Science and Technology; Taylor & Francis Inc.: London, UK, 1995. [Google Scholar]
- Narcy, M.; Lips, S.; Sartre, V. Experimental investigation of a confined flat two-phase thermosyphon for electronics cooling. Exp. Therm. Fluid Sci. 2018, in press. [Google Scholar] [CrossRef]
- Zhao, J.; Lv, P.; Rao, Z. Experimental study on the thermal management performance of phase change material coupled with heat pipe for cylindrical power battery pack. Exp. Therm. Fluid Sci. 2017, 82, 182–188. [Google Scholar] [CrossRef]
- Krishna, J.; Kishore, P.S.; Solomon, A.B. Heat pipe with nano enhanced-PCM for electronic cooling application. Exp. Therm. Fluid Sci. 2017, 81, 84–92. [Google Scholar] [CrossRef]
- Faghri, A.; Guo, Z. Integration of heat pipe into fuel cell technology. Heat Transf. Eng. 2008, 29, 232–238. [Google Scholar] [CrossRef]
- Joung, W.; Yu, T.; Lee, J. Experimental study on the loop heat pipe with a planar bifacial wick structure. Int. J. Heat Mass Transf. 2008, 51, 1573–1581. [Google Scholar] [CrossRef]
- Chung, C.A.; Yang, S.-W.; Yang, C.-Y.; Hsu, C.-W.; Chiu, P.-Y. Experimental study on the hydrogen charge and discharge rates of metal hydride tanks using heat pipes to enhance heat transfer. Appl. Energy 2013, 103, 581–587. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Prasad, A.K.; Advani, S.G. Role of heat pipes in improving the hydrogen charging rate in a metal hydride storage tank. Int. J. Hydrogen Energy 2014, 39, 10552–10563. [Google Scholar] [CrossRef]
- Chung, C.A.; Chen, Y.-Z.; Chen, Y.-P.; Chang, M.-S. CFD investigation on performance enhancement of metal hydride hydrogen storage vessels using heat pipes. Appl. Therm. Eng. 2015, 91, 434–446. [Google Scholar] [CrossRef]
- Do, K.H.; Ha, H.J.; Jang, S.P. Thermal resistance of screen mesh wick heat pipes using the water-based Al2O3 nanofluids. Int. J. Heat Mass Transf. 2010, 53, 5888–5894. [Google Scholar] [CrossRef]
- Putra, N.; Septiadi, W.N.; Rahman, H.; Irwansyah, R. Thermal performance of screen mesh wick heat pipes with nanofluids. Exp. Therm. Fluid Sci. 2012, 40, 10–17. [Google Scholar] [CrossRef]
- Franchi, G.; Huang, X. Development of composite wicks for heat pipe performance enhancement. Heat Transf. Eng. 2008, 29, 873–884. [Google Scholar] [CrossRef]
- Solomon, A.B.; Sekar, M.; Yang, S.H. Analytical expression for thermal conductivity of heat pipe. Appl. Therm. Eng. 2016, 100, 462–467. [Google Scholar] [CrossRef]
- Asirvatham, L.G.; Nimmagadda, R.; Wongwises, S. Heat transfer performance of screen mesh wick heat pipes using silver–water nanofluid. Int. J. Heat Mass Transf. 2013, 60, 201–209. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
P. Tetuko, A.; Shabani, B.; Andrews, J. Passive Fuel Cell Heat Recovery Using Heat Pipes to Enhance Metal Hydride Canisters Hydrogen Discharge Rate: An Experimental Simulation. Energies 2018, 11, 915. https://doi.org/10.3390/en11040915
P. Tetuko A, Shabani B, Andrews J. Passive Fuel Cell Heat Recovery Using Heat Pipes to Enhance Metal Hydride Canisters Hydrogen Discharge Rate: An Experimental Simulation. Energies. 2018; 11(4):915. https://doi.org/10.3390/en11040915
Chicago/Turabian StyleP. Tetuko, Anggito, Bahman Shabani, and John Andrews. 2018. "Passive Fuel Cell Heat Recovery Using Heat Pipes to Enhance Metal Hydride Canisters Hydrogen Discharge Rate: An Experimental Simulation" Energies 11, no. 4: 915. https://doi.org/10.3390/en11040915



