Sustainable Management of Oleaginous Trees as a Source for Renewable Energy Supply and Climate Change Mitigation: A Case Study in China
Abstract
:1. Introduction
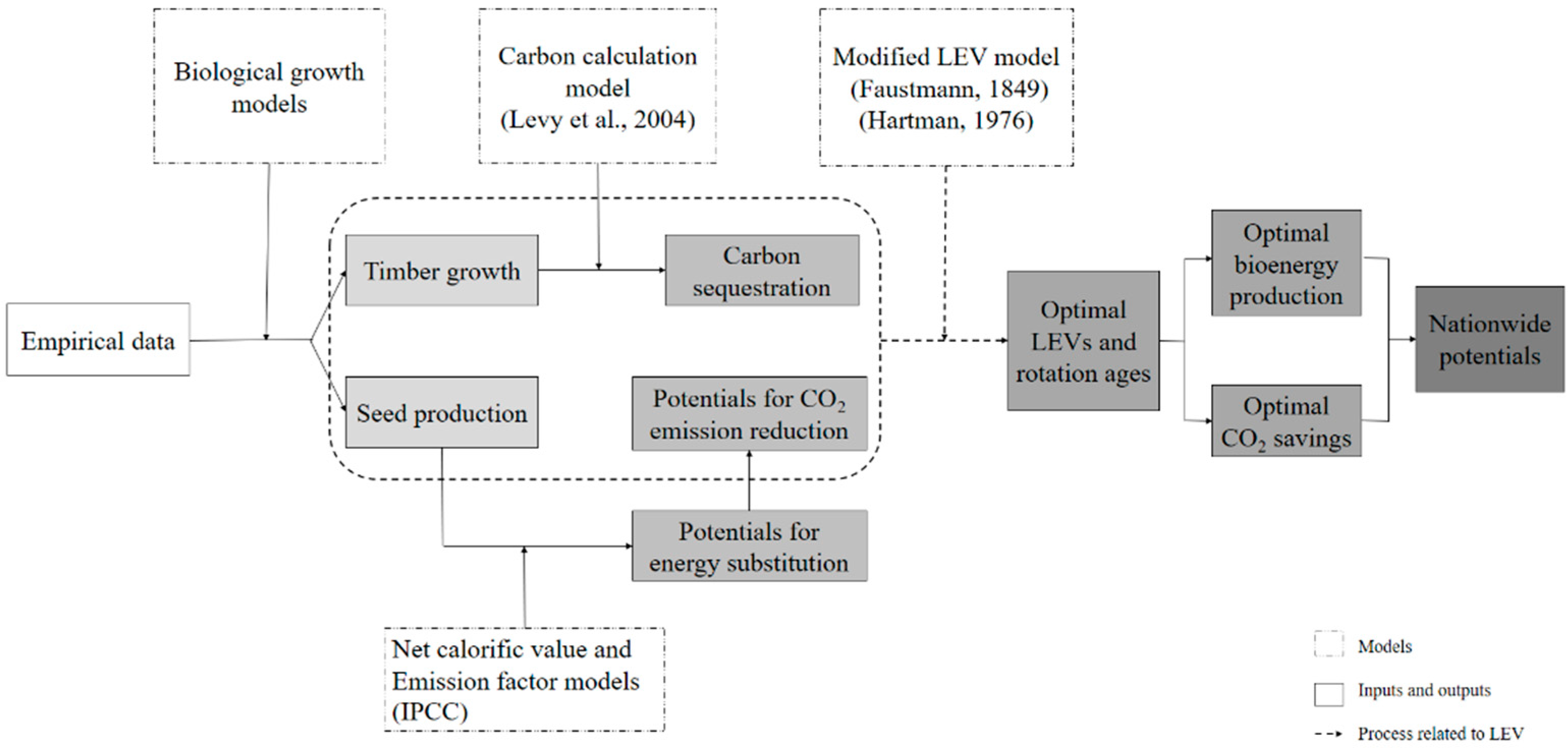
2. Methods and Materials
2.1. Modified LEV Model Considering Values of Seed Production and Carbon Savings
2.2. Biolgical Growth of Timber Estimates; Seed Production and Carbon Sequestration Calculations
2.3. Estimations of Energy Substitution and CO2 Emission Reductions
2.4. Model Upscale to the National Level
2.5. Data Description and Processing
3. Results
3.1. Results of Model Parameter Estimations
3.1.1. Timber Growth
3.1.2. Seed Production
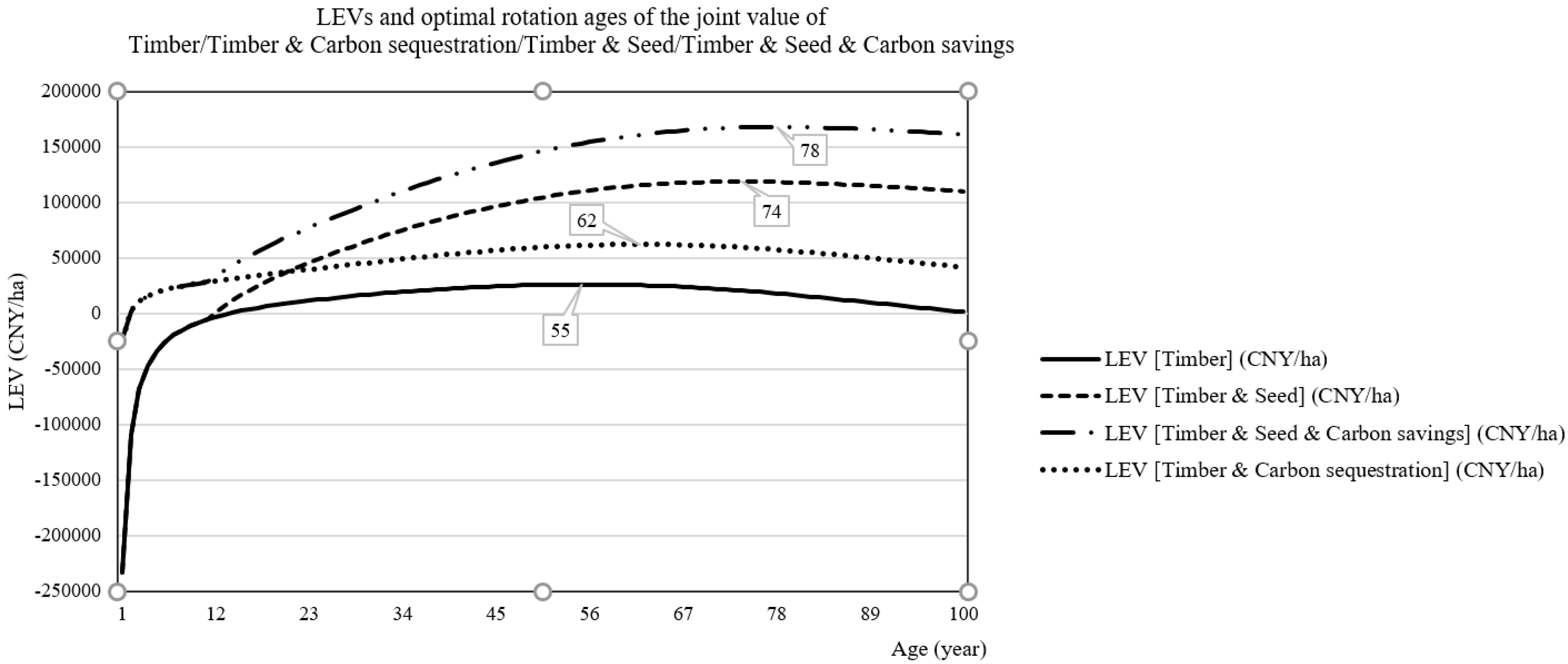
3.2. Results of the Reference Scenario
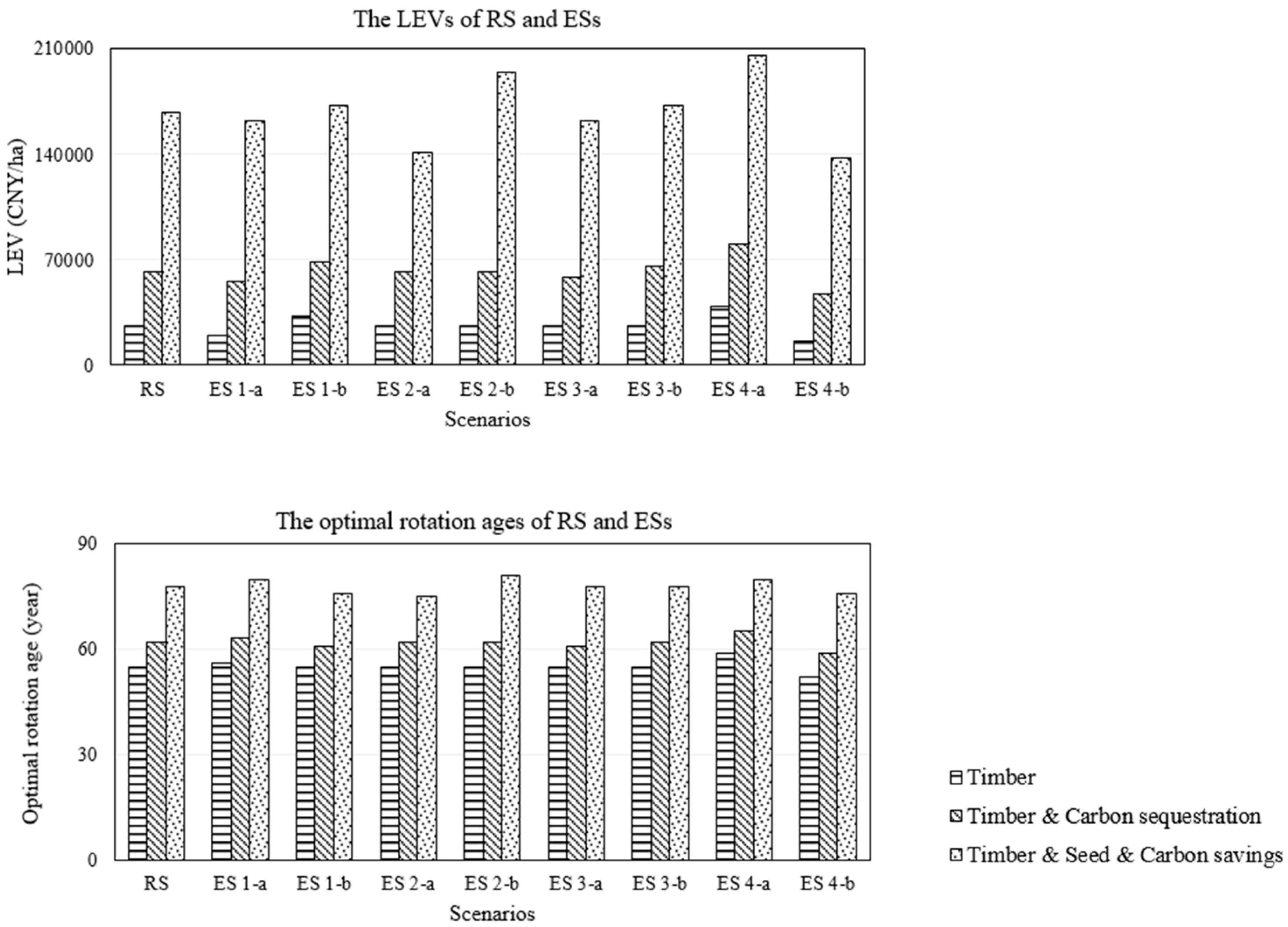
3.3. Results of Sensitivity Analysis
3.3.1. Economic Factors Scenario
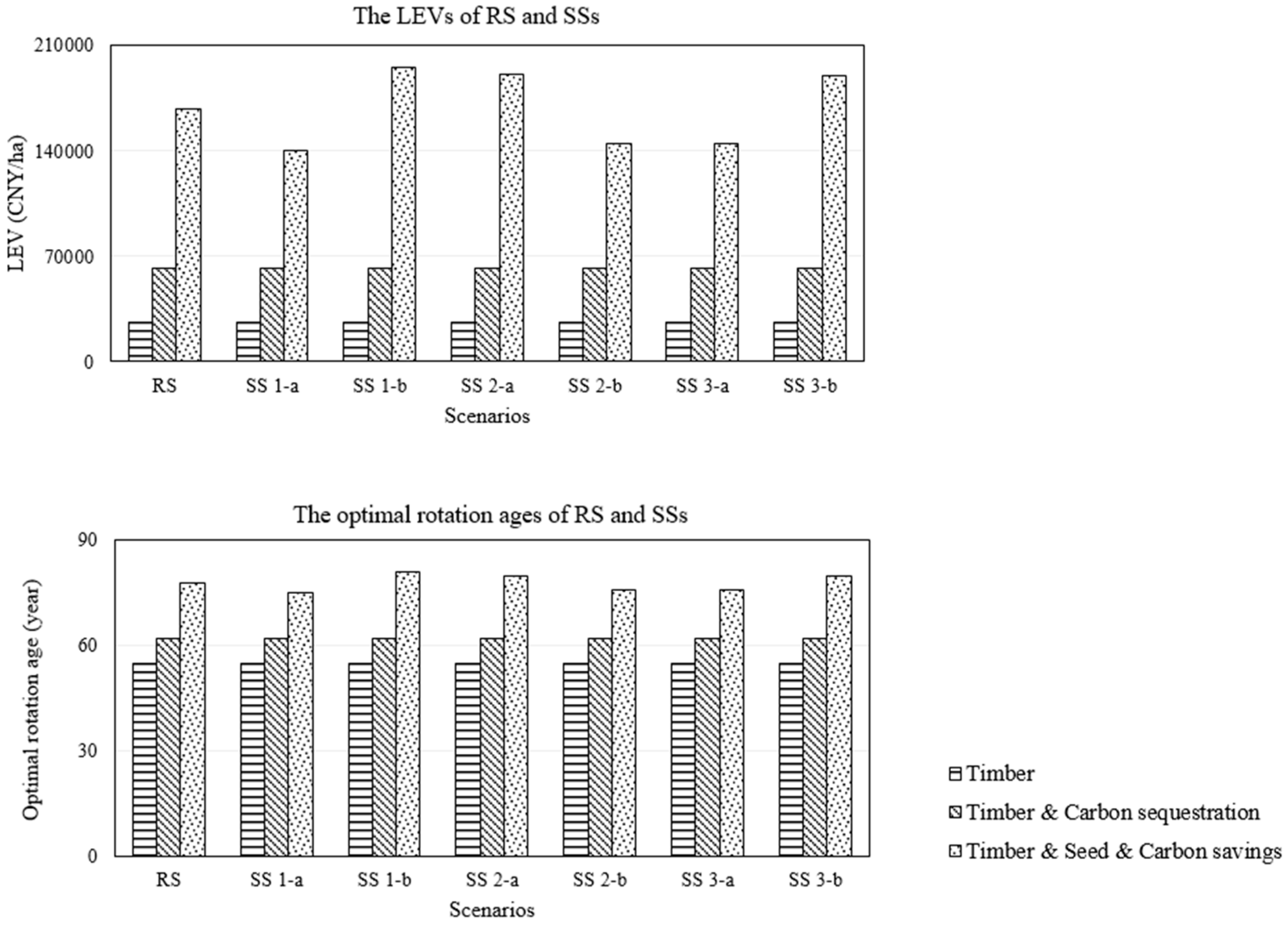
3.3.2. Seed Production Factors Scenario
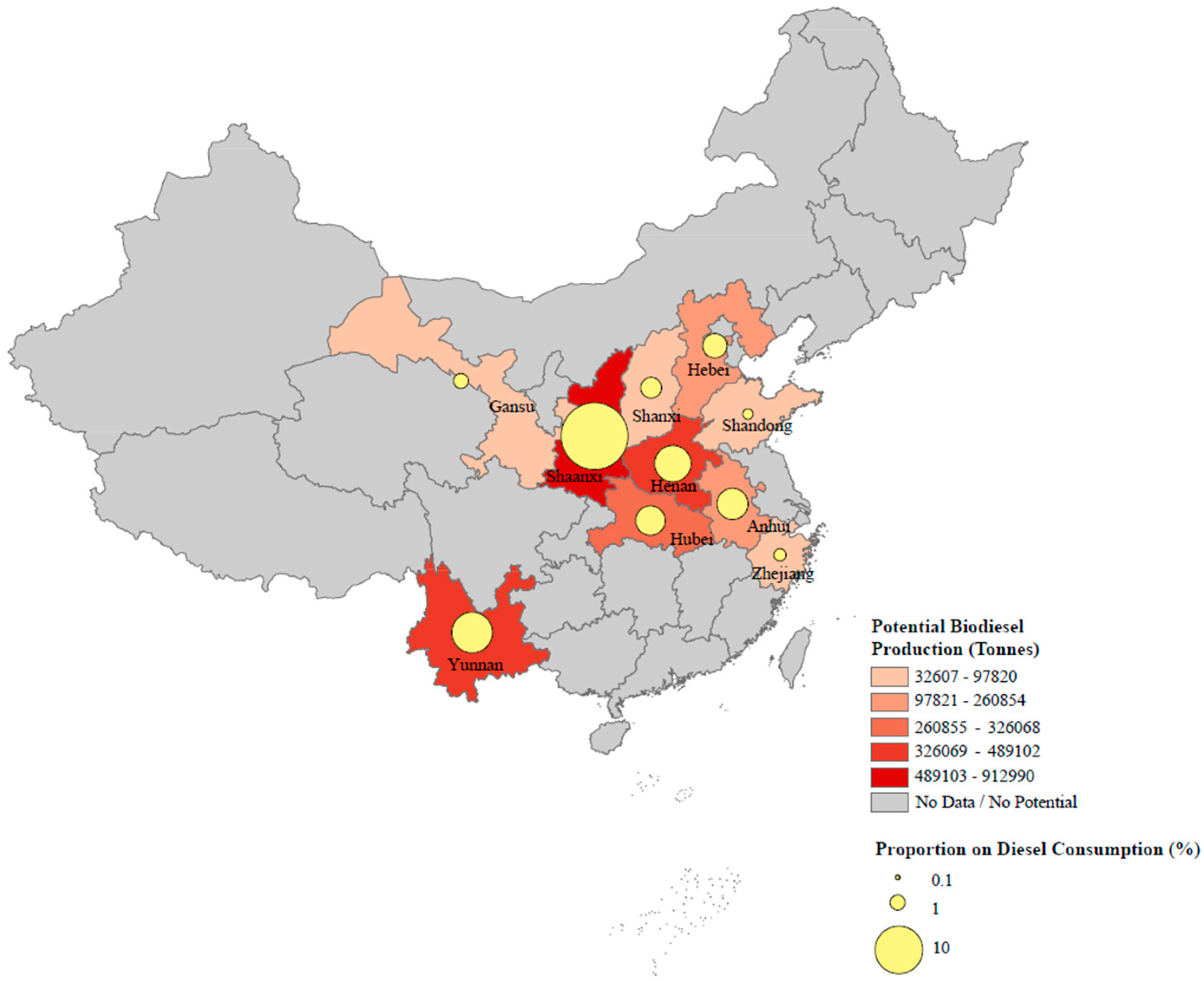
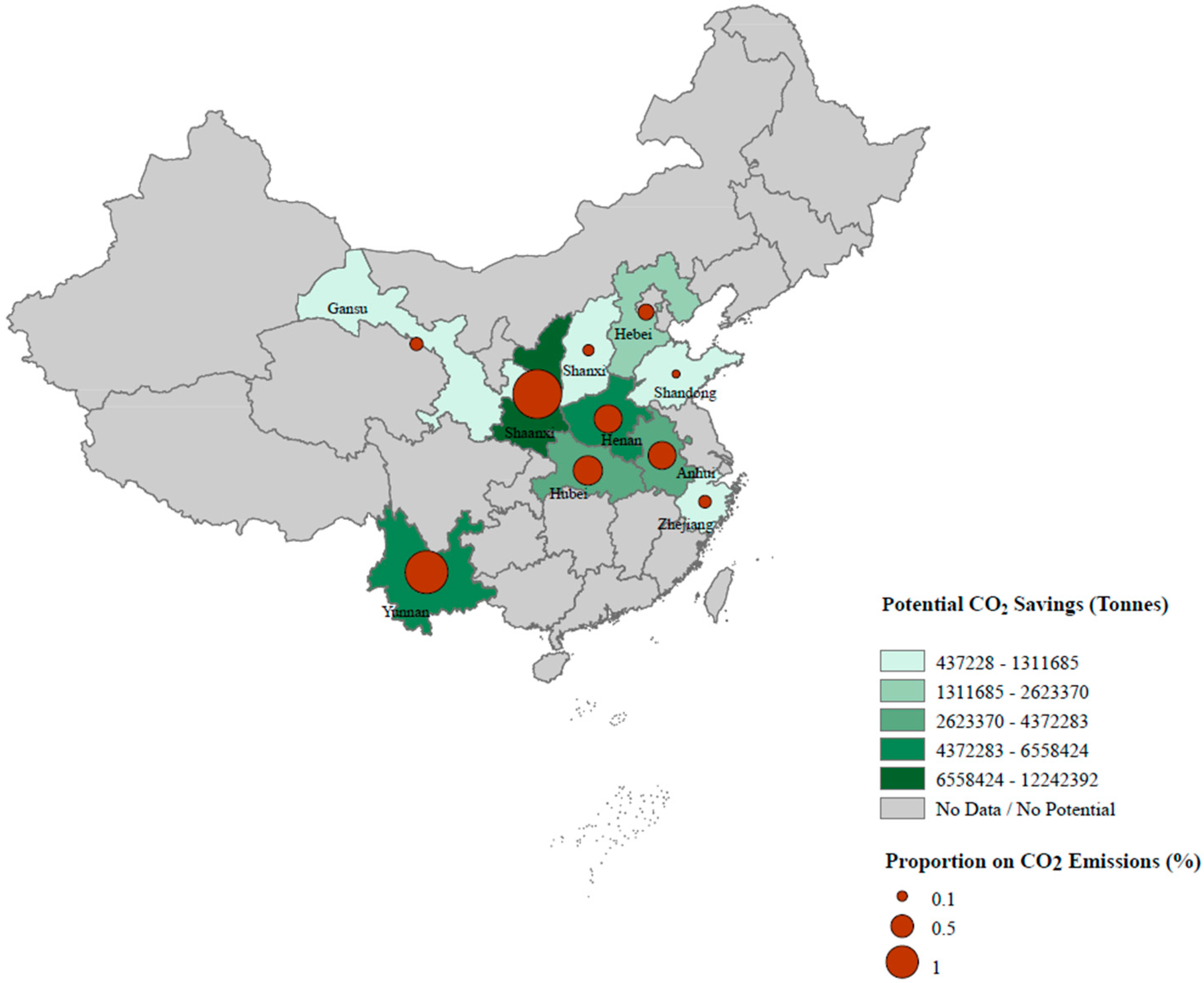
3.4. Upscale of Model Results to the National Level
4. Discussion
4.1. Implication on Climate Change
4.2. Implication on Energy Security
4.3. Implication on Forest Synergy Outputs of Forest Management
4.4. Implication on Sustainable Forest Management
5. Conclusions and Future Directions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A. Coefficient Choices
| Name | Definition | Value |
|---|---|---|
| θ | the density of the tree | 0.685 tonne/m3 |
| BEF | biomass expansion factor | 1.4 |
| μ | carbon content in the dry mass | 0.5 |
| R | root to shoot ratio | 0.31 |
| ω | the molecular mass of CO2 divided by the atomic weight of carbon | 44/12 |
| η | amount of seeds for per kilogram biodiesel production | 2.5 kg |
| n | net calorific value of diesel | 43 |
| λ1 | coefficient to transfer kilogram to gigagram | 106 |
| m | diesel emission factor (kilogram of CO2 per TJ on a diesel net calorific value) | 74,100 |
| λ2 | coefficient to transfer kilogram to tonne | 103 |
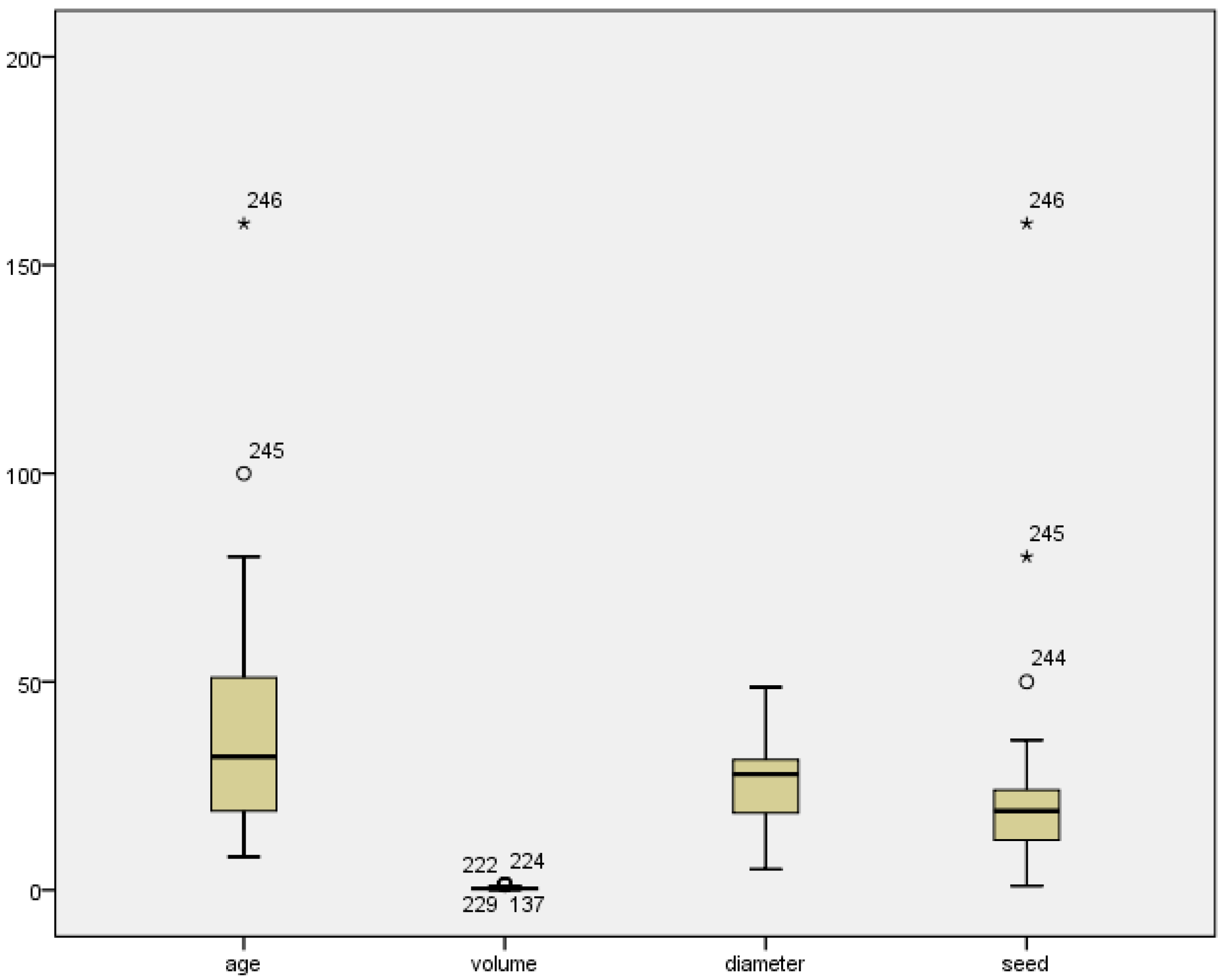
Appendix B. Data Description and Details

Appendix C. Definition of Variables and Parameters
| Name | Definition |
|---|---|
| A | maximum potential growth amount |
| CPa | carbon proportion of time a |
| CPb | carbon proportion of time b |
| CP(t) | carbon proportion at time t |
| Cs(t) | seed harvesting cost changing with time t |
| C0 | establishment cost |
| E(t) | amenity value at time t |
| E1(t) | the amount of carbon sequestration from forest |
| E2(t) | the amount of CO2 emission reduction from energy substitution |
| e | the base of natural logarithm |
| f(T) | timber volume at harvesting time T |
| f(t) | timber volume at time t |
| G | subscript of the Gompertz curve |
| G1 | subscript of estimated timber volume growth based on the Gompertz curve |
| G2 | subscript of estimated seed production based on the Gompertz curve |
| I | the set of 10 provinces that have the plan of cultivating Pistacia chinensis |
| i | an element of set I |
| J | the saved energy from diesel substitution |
| L | subscript of the logistic curve |
| L1 | subscript of estimated timber volume growth based on the logistic curve |
| L2 | subscript of estimated seed production based on the logistic curve |
| NB | potential national biodiesel production from Pistacia chinensis |
| NC | potential national carbon savings from Pistacia chinensis |
| Pc | carbon price |
| Ps | seed price |
| Pt | stumpage price |
| PD | proportion that the potentials of the provincial biodiesel production from Pistacia chinensis on the provincial diesel consumptions |
| PE | proportion that the potentials of the provincial carbon savings from Pistacia chinensis on the provincial CO2 emissions |
| Qi | potential planting areas of Pistacia chinensis of each province |
| r | discounting rate |
| S(t) | seed production at time t |
| T | harvesting time |
| T1 | the time point tree starting to produce seeds |
| t | time |
| Y | the number of years between time b and time a |
| y(t) | collectively referring to the timber volume or seed production of the tree at the time t |
| α | biological constant |
| β | the growth rate |
| ΔCP | annual change of carbon stocks |
Appendix D. Description of Costs
| Activities | Cost per Chinese acre CNY/Chinese acre | Cost per Hectare CNY/ha | Data Source/References |
|---|---|---|---|
| Establishment cost | |||
| Land preparation fee | 10,250 | Guo et al. [61] | |
| Seedlings | 55 | 825 | interview |
| Management and tending fee | 300 | 4500 | interview |
| Labour wages (planting) | 1080 | 16,200 | interview |
| Harvesting cost (seed) | |||
| Labour wages | 330 | 4950 | interview |
Appendix E. The Criteria for Choosing the Magnitude for the Sensitivity Analysis
Appendix F. Annual Biodiesel Production and Total Carbon Savings of RS, ESs and SSs
| Scenarios | RS | ES 1-a | ES 1-b | ES 2-a | ES 2-b | ES 3-a | ES 3-b | ES 4-a | ES 4-b |
|---|---|---|---|---|---|---|---|---|---|
| Carbon savings from diesel substitution (tonne/ha) | 10.39 | 10.57 | 10.21 | 10.11 | 10.65 | 10.39 | 10.39 | 10.57 | 10.21 |
| Carbon savings from forest carbon sequestration (tonne/ha) | 33.33 | 33.66 | 32.96 | 32.75 | 33.80 | 33.33 | 33.33 | 33.66 | 32.96 |
| Total carbon savings (tonne/ha) | 43.72 | 44.22 | 43.16 | 42.86 | 44.45 | 43.72 | 43.72 | 44.22 | 43.16 |
| Biodiesel production (tonne/ha) | 3.26 | 3.32 | 3.20 | 3.17 | 3.34 | 3.26 | 3.26 | 3.32 | 3.20 |
| Scenarios | RS | SS 1-a | SS 1-b | SS 2-a | SS 2-b | SS 3-a | SS 3-b |
|---|---|---|---|---|---|---|---|
| Carbon savings from diesel substitution (tonne/ha) | 10.39 | 9.10 | 11.72 | 10.97 | 9.82 | 9.57 | 11.13 |
| Carbon savings from forest carbon sequestration (tonne/ha) | 33.33 | 32.75 | 33.80 | 33.66 | 32.96 | 32.96 | 33.66 |
| Total carbon savings (tonne/ha) | 43.72 | 41.85 | 45.52 | 44.63 | 42.77 | 42.53 | 44.79 |
| Biodiesel production (tonne/ha) | 3.26 | 2.86 | 3.68 | 3.44 | 3.08 | 3.00 | 3.49 |
References
- United Nations. Paris Agreement. Available online: http://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf (accessed on 18 December 2017).
- Liu, Z. Steps to China’s carbon peak. Nature 2015, 522, 279. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, J. China’s carbon emissions could peak sooner than forecast: Five-year plan advances policy to reduce reliance on coal and expand renewable energy. Nature 2016, 531, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Repo, A.; Tuomi, M.; Liski, J. Indirect carbon dioxide emissions from producing bioenergy from forest harvest residues. GCB Bioenergy 2011, 3, 107–115. [Google Scholar] [CrossRef]
- Routa, J.; Asikainen, A.; Björheden, R.; Laitila, J.; Röser, D. Forest energy procurement: State of the art in Finland and Sweden. Energy Environ. 2013, 2, 602–613. [Google Scholar] [CrossRef]
- Salehian, P.; Karimi, K.; Zilouei, H.; Jeihanipour, A. Improvement of biogas production from pine wood by alkali pretreatment. Fuel 2013, 106, 484–489. [Google Scholar] [CrossRef]
- Chen, L.; Cong, R.-G.; Shu, B.; Mi, Z.-F. A sustainable biogas model in China: The case study of Beijing Deqingyuan biogas project. Renew. Sustain. Energy Rev. 2017, 78, 773–779. [Google Scholar] [CrossRef]
- Itoh, H.; Wada, M.; Honda, Y.; Kuwahara, M.; Watanabe, T. Bioorganosolve pretreatments for simultaneous saccharification and fermentation of beech wood by ethanolysis and white rot fungi. J. Biotechnol. 2003, 103, 273–280. [Google Scholar] [CrossRef]
- Sharma, Y.; Singh, B. Development of biodiesel from karanja, a tree found in rural India. Fuel 2008, 87, 1740–1742. [Google Scholar] [CrossRef]
- Center for Climate and Energy Solutions. China’s Contribution to the Paris Climate Agreement. Available online: https://www.c2es.org/site/assets/uploads/2015/07/chinas-contribution-paris-climate-agreement.pdf (accessed on 18 December 2017).
- State Forestry Administration of the People’s Republic of China. National Forestry Biomass Energy Development Plan; State Forestry Administration of the People’s Republic of China: Beijing, China, 2013. (In Chinese)
- Tang, M.; Zhang, P.; Zhang, L.; Li, M.; Wu, L. A potential bioenergy tree: Pistacia chinensis Bunge. Energy Proc. 2012, 16, 737–746. [Google Scholar] [CrossRef]
- Bjørnstad, E.; Skonhoft, A. Wood fuel or carbon sink? Aspects of forestry in the climate question. Environ. Resour. Econ. 2002, 23, 447–465. [Google Scholar] [CrossRef]
- Olschewski, R.; Benítez, P.C. Optimizing joint production of timber and carbon sequestration of afforestation projects. J. For. Econ. 2010, 16, 1–10. [Google Scholar] [CrossRef]
- Amsalu, D.W.; Jacobsen, J.B.; Lundhede, T.H. Economic assessment of use values of near-natural forestry compared with rotational forestry in Denmark. Eur. J. For. Res. 2014, 133, 611–622. [Google Scholar] [CrossRef]
- Creedy, J.; Wurzbacher, A.D. The economic value of a forested catchment with timber, water and carbon sequestration benefits. Ecol. Econ. 2001, 38, 71–83. [Google Scholar] [CrossRef]
- Faustmann, M. Berechnung des Wertes welchen Walboden sowie noch nicht haubare Holzbestande fur die Waldwirtschaft besitzen. Allg. Forst-Jagdztg. 1849. Republication: Calculation of the value which forest land and immature stands possess for forestry. J. For. Econ. 1995, 1, 7–44. [Google Scholar]
- Hartman, R. The harvesting decision when a standing forest has value. Econ. Inq. 1976, 14, 52–58. [Google Scholar] [CrossRef]
- Fekedulegn, D.; Mac Siúrtáin, M.P.; Colbert, J.J. Parameter Estimation of Nonlinear Models in Forestry. Silva Fenn. 1999, 33, 327–336. [Google Scholar] [CrossRef]
- Levy, P.; Hale, S.; Nicoll, B. Biomass expansion factors and root: Shoot ratios for coniferous tree species in Great Britain. Forestry 2004, 77, 421–430. [Google Scholar] [CrossRef]
- Eggleston, H.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. IPCC Guidelines for National Greenhouse Gas Inventories; The Institute for Global Enviromental Strategies for the IPCC and the Intergovernmental Panel on Climate Change: Hayama, Japan, 2006. [Google Scholar]
- Stainback, G.A.; Alavalapati, J.R. Economic analysis of slash pine forest carbon sequestration in the southern US. J. For. Econ. 2002, 8, 105–117. [Google Scholar]
- Van Kooten, G.C.; Binkley, C.S.; Delcourt, G. Effect of carbon taxes and subsidies on optimal forest rotation age and supply of carbon services. Am. J. Agric. Econ. 1995, 77, 365–374. [Google Scholar] [CrossRef]
- Mutke, S.; Gordo, J.; Gil, L. Variability of Mediterranean Stone pine cone production: Yield loss as response to climate change. Agric. For. Meteorol. 2005, 132, 263–272. [Google Scholar] [CrossRef]
- Kimmins, J. Sustained yield, timber mining, and the concept of eclogical rotation; a British Columbian view. For. Chron. 1974, 50, 27–31. [Google Scholar] [CrossRef]
- Li, X.; Schmid, B.; Wang, F.; Paine, C.T. Net assimilation rate determines the growth rates of 14 species of subtropical forest trees. PLoS ONE 2016, 11, e0150644. [Google Scholar] [CrossRef] [PubMed]
- Rupðys, P.; Petrauskas, E.; Maþeika, J.; Deltuvas, R. The Gompertz type stochastic growth law and a tree diameter distribution. Balt. For. 2007, 13, 197–206. [Google Scholar]
- Franses, P.H. A method to select between Gompertz and logistic trend curves. Technol. Forecast. Soc. 1994, 46, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Goshu, A.T.; Koya, P.R. Derivation of Inflection Points of Nonlinear Regression Curves—Implications to Statistics. Am. J. Theor. Appl. Stat. 2013, 2, 268–272. [Google Scholar] [CrossRef]
- Tjørve, E. Shapes and functions of species—Area curves: A review of possible models. J. Biogeogr. 2003, 30, 827–835. [Google Scholar] [CrossRef]
- Sanquetta, C.R.; Corte, A.P.; da Silva, F. Biomass expansion factor and root-to-shoot ratio for Pinus in Brazil. Carbon Balance Manag. 2011, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. IEA Energy Training and Capacity-Building. Available online: https://www.iea.org/media/training/alumni/CheatSheet.pdf (accessed on 18 December 2017).
- Cong, R.-G.; Caro, D.; Thomsen, M. Is it beneficial to use biogas in the Danish transport sector?—An environmental-economic analysis. J. Clean. Prod. 2017, 165, 1025–1035. [Google Scholar] [CrossRef]
- Shao, H.; Chu, L. Resource evaluation of typical energy plants and possible functional zone planning in China. Biomass Bioenergy 2008, 32, 283–288. [Google Scholar] [CrossRef]
- Zhuang, D.; Jiang, D.; Liu, L.; Huang, Y. Assessment of bioenergy potential on marginal land in China. Renew. Sustain. Energy Rev. 2011, 15, 1050–1056. [Google Scholar] [CrossRef]
- Deng, X.; Yu, Y.; Liu, Y. Temporal and Spatial Variations in Provincial CO2 Emissions in China from 2005 to 2015 and Assessment of a Reduction Plan. Energies 2015, 8, 4549–4571. [Google Scholar] [CrossRef]
- National Bureau of Statistics of the People’s Republic of China. China Energy Statistical Yearbook 2016; China Statistics Press: Beijing, China, 2017. (In Chinese)
- National Bureau of Statistics of the People’s Republic of China. CPI (1978=100). Available online: http://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0902&sj=2015 (accessed on 18 December 2017). (In Chinese)
- Zuo, Y. CPI Rose by 2.0% in 2016. Available online: http://news.xinhuanet.com/local/2017-01/10/c_129439692.htm (accessed on 18 December 2017). (In Chinese).
- State Forestry Administration of the People’s Republic of China. National Forestry Statistical Annual Report 2014. Available online: http://www.forestry.gov.cn/main/304/content-769221.html (accessed on 18 December 2017). (In Chinese)
- Wang, C. Comments on “Plantation development: Economic analysis of forest management in Fujian Province, China”. For. Policy Econ. 2014, 43, 51–52. [Google Scholar] [CrossRef]
- The State Council Information Office of the People’s Republic of China. Xie Zhenhua: A Positive Commitment to Actively Participate in the Governance of Global Climate Change Processes. Available online: http://www.scio.gov.cn/ztk/dtzt/2014/32252/32260/Document/1389330/1389330.htm (accessed on 18 December 2017). (In Chinese)
- Schulze, E.D.; Körner, C.; Law, B.E.; Haberl, H.; Luyssaert, S. Large-scale bioenergy from additional harvest of forest biomass is neither sustainable nor greenhouse gas neutral. GCB Bioenergy 2012, 4, 611–616. [Google Scholar] [CrossRef]
- Gren, M.; Aklilu, A.Z. Policy design for forest carbon sequestration: A review of the literature. For. Policy Econ. 2016, 70, 128–136. [Google Scholar] [CrossRef]
- Cong, R.-G. An optimization model for renewable energy generation and its application in China: A perspective of maximum utilization. Renew. Sustain. Energy Rev. 2013, 17, 94–103. [Google Scholar] [CrossRef]
- Li, G. Genetic resources and development of Pistacia chinensis bunge. Agric. Sci. 2009, 15, 68–70. (In Chinese) [Google Scholar]
- National Bureau of Statistics of the People’s Republic of China. China Statistical Yearbook 2016; China Statistics Press: Beijing, China, 2017.
- Foster, K. The Role of China’s Forests in Mitigating Climate Change. Available online: http://blog.worldagroforestry.org/index.php/2013/07/23/the-role-of-chinas-forests-in-mitigating-climate-change/ (accessed on 18 December 2017).
- Vass, M.M.; Elofsson, K. Is forest carbon sequestration at the expense of bioenergy and forest products cost-efficient in EU climate policy to 2050? J. For. Econ. 2016, 24, 82–105. [Google Scholar] [CrossRef]
- Jin, L.; Yuan, Q. Growth regularity and cost-benefit analysis of Pistacia chinensis in the south of Taihang Mountain. Cent. South For. Inventory Plan. 1995, 14, 17–19. (In Chinese) [Google Scholar]
- Zhang, L.; Zhang, C. Potentials of Forest Bioenergy in China Based on Forest Ecological Security; Zhang, Z., Zhang, R., Zhang, J., Eds.; LISS 2012; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1211–1215. [Google Scholar]
- Kimmins, J. Evaluation of the consequences for future tree productivity of the loss of nutrients in whole-tree harvesting. For. Ecol. Manag. 1976, 1, 169–183. [Google Scholar] [CrossRef]
- Freedman, B. The Ecological Effects of Pollution, Disturbance and Other Stresses. Environmental Ecology; Academic Press Inc.: San Diego, CA, USA, 1995. [Google Scholar]
- Bi, J.; Blanco, J.; Seely, B.; Kimmins, J.; Ding, Y.; Welham, C. Yield decline in Chinese-fir plantations: A simulation investigation with implications for model complexity. Can. J. For. Res. 2007, 37, 1615–1630. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Rodriguez-Fernandez, J. Effect of biodiesel fuels on diesel engine emissions. Prog. Energy Combust. 2008, 34, 198–223. [Google Scholar] [CrossRef]
- Koh, L.P.; Miettinen, J.; Liew, S.C.; Ghazoul, J. Remotely sensed evidence of tropical peatland conversion to oil palm. Proc. Nat. Acad. Sci. USA 2011, 108, 5127–5132. [Google Scholar] [CrossRef] [PubMed]
- Wilcove, D.S.; Koh, L.P. Addressing the threats to biodiversity from oil-palm agriculture. Biodivers. Conserv. 2010, 19, 999–1007. [Google Scholar] [CrossRef]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Towards a Worldwide Wood Economics Spectrum; Dryad Data Repository: Durham, NC, USA, 2009. [Google Scholar]
- Penman, J.; Gytarsky, M.; Hiraishi, T.; Krug, T.; Kruger, D.; Pipatti, R.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. Good Practice Guidance for Land Use, Land-Use Change and Forestry; The Institute for Global Enviromental Strategies for the IPCC and the Intergovernmental Panel on Climate Change: Hayama, Japan, 2003. [Google Scholar]
- Guo, Z.; Zhang, Y.; Deegen, P.; Uibrig, H. Economic analyses of rubber and tea plantations and rubber-tea intercropping in Hainan, China. Agrofor. Syst. 2006, 66, 117–127. [Google Scholar] [CrossRef]
- State Forestry Administration of the People’s Republic of China. National Primary Forestry Industrical Product Sellings Price 2012. Available online: http://zcl.forestry.gov.cn/portal/cylhh/s/3849/content-649018.html (accessed on 18 December 2017). (In Chinese)
- State Forestry Administration of the People’s Republic of China. National Forestry Statistical Annual Report 2013. Available online: http://www.forestry.gov.cn/uploadfile/main/2014-5/file/2014-5-15-bf6af40b14b34db0bda284a82af0d776.pdf (accessed on 18 December 2017). (In Chinese)
| Name | Formula |
|---|---|
| logistic | |
| Gompertz |
| Functions | Parameters Results | p-Value | R-Square | |
|---|---|---|---|---|
| 2.12 | 0.062 | 0.8944 | ||
| 29.34 | 0.013 | |||
| 0.051 | 0.000 | |||
| 3.15 | 0.172 | 0.8961 | ||
| 4.31 | 0.000 | |||
| 0.020 | 0.010 | |||
| Functions | Parameters Results | p-Value | R-Square | |
|---|---|---|---|---|
| 32.58 | 0.000 | 0.9622 | ||
| 7.57 | 0.000 | |||
| 0.071 | 0.000 | |||
| 36.14 | 0.000 | 0.9636 | ||
| 2.72 | 0.000 | |||
| 0.044 | 0.000 | |||
| Scenario Name | Abbreviation | Stumpage Price (Pt) | Seed Price (Ps) | Carbon Price (Pc) | Discounting Rate (r) | A | α | β |
|---|---|---|---|---|---|---|---|---|
| Reference Scenario | RS 1 | 425 CNY/m3 | 1.5 CNY/kg | 45 CNY/tonne | 3% | 36.14 | 2.72 | 0.044 |
| Economic factors scenario—Pt decreasing by 10% | ES 1-a | 382.5 CNY/m3 | ||||||
| Economic factors scenario—Pt increasing by 10% | ES 1-b | 467.5 CNY/m3 | ||||||
| Economic factors scenario—Ps decreasing by 10% | ES 2-a | 1.35 CNY/kg | ||||||
| Economic factors scenario—Ps increasing by 10% | ES 2-b | 1.65 CNY/kg | ||||||
| Economic factors scenario—Pc decreasing by 10% | ES 3-a | 40.5 CNY/tonne | ||||||
| Economic factors scenario—Pc increasing by 10% | ES 3-b | 49.5 CNY/tonne | ||||||
| Economic factors scenario—r decreasing by 10% | ES 4-a | 2.7% | ||||||
| Economic factors scenario—r increasing by 10% | ES 4-b | 3.3% | ||||||
| Seed production factors scenario—A decreasing by 10% | SS 1-a | 32.53 | ||||||
| Seed production factors scenario—A increasing by 10% | SS 1-b | 39.75 | ||||||
| Seed production factors scenario—α decreasing by 10% | SS 2-a | 2.45 | ||||||
| Seed production factors scenario—α increasing by 10% | SS 2-b | 2.99 | ||||||
| Seed production factors scenario—β decreasing by 10% | SS 3-a | 0.04 | ||||||
| Seed production factors scenario—β increasing by 10% | SS 3-b | 0.048 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Cong, R.-G.; Hasler, B. Sustainable Management of Oleaginous Trees as a Source for Renewable Energy Supply and Climate Change Mitigation: A Case Study in China. Energies 2018, 11, 1123. https://doi.org/10.3390/en11051123
Zhang J, Cong R-G, Hasler B. Sustainable Management of Oleaginous Trees as a Source for Renewable Energy Supply and Climate Change Mitigation: A Case Study in China. Energies. 2018; 11(5):1123. https://doi.org/10.3390/en11051123
Chicago/Turabian StyleZhang, Jin, Rong-Gang Cong, and Berit Hasler. 2018. "Sustainable Management of Oleaginous Trees as a Source for Renewable Energy Supply and Climate Change Mitigation: A Case Study in China" Energies 11, no. 5: 1123. https://doi.org/10.3390/en11051123




