Fundamental Material Properties of the 2LiBH4-MgH2 Reactive Hydride Composite for Hydrogen Storage: (II) Kinetic Properties
Abstract
:1. Introduction
2. Experimental Details
2.1 Sample Preparation
2.2 Experimental Methods
2.2.1. Differential Scanning Calorimetry
2.2.2. Kinetic Studies
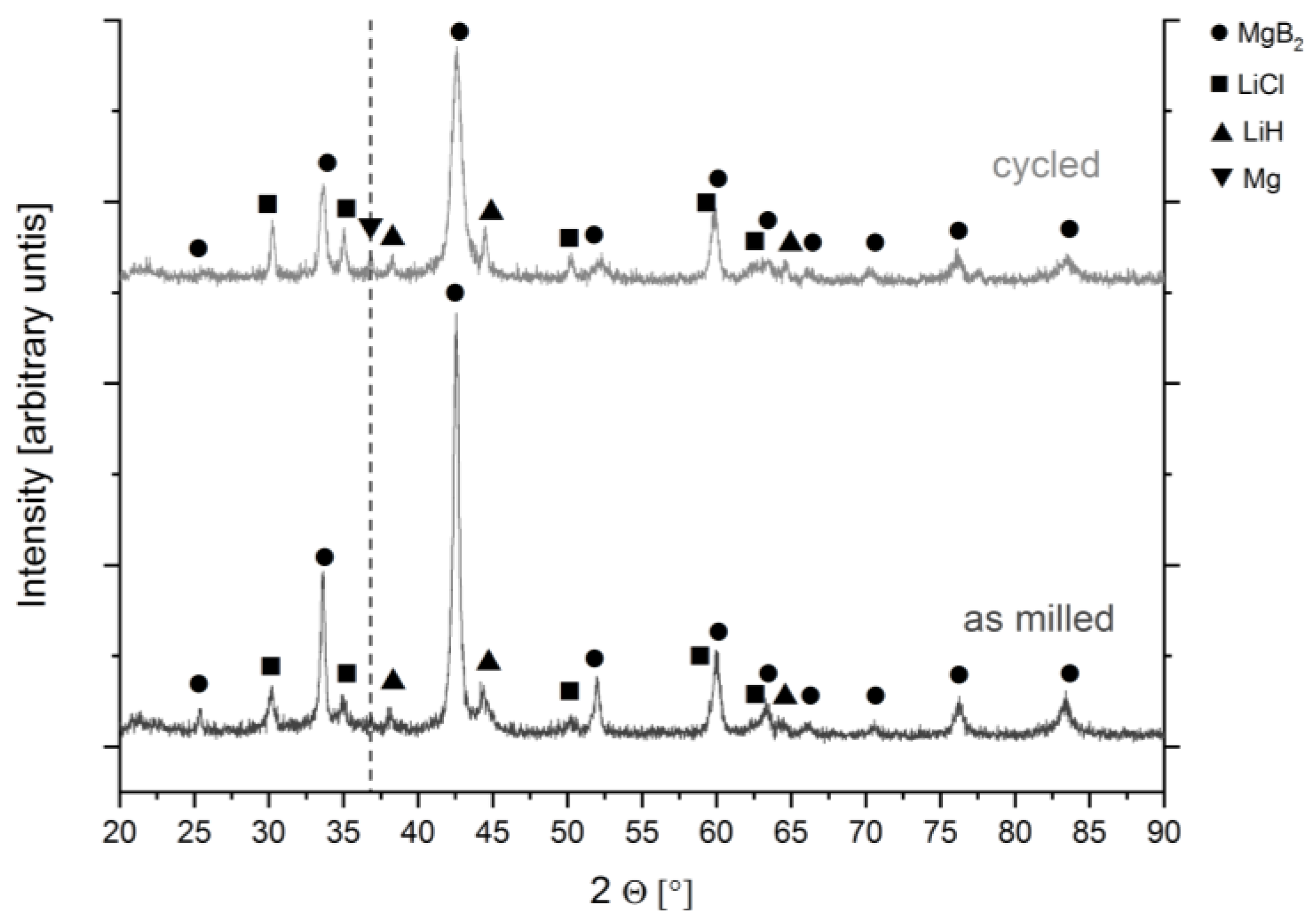
2.2.3. Crystalline Phase Detection: X-ray Diffraction (XRD)
2.2.4. Phase Equilibrium Compositions: Thermodynamic Calculations
3. Results
3.1. Kinetic Properties
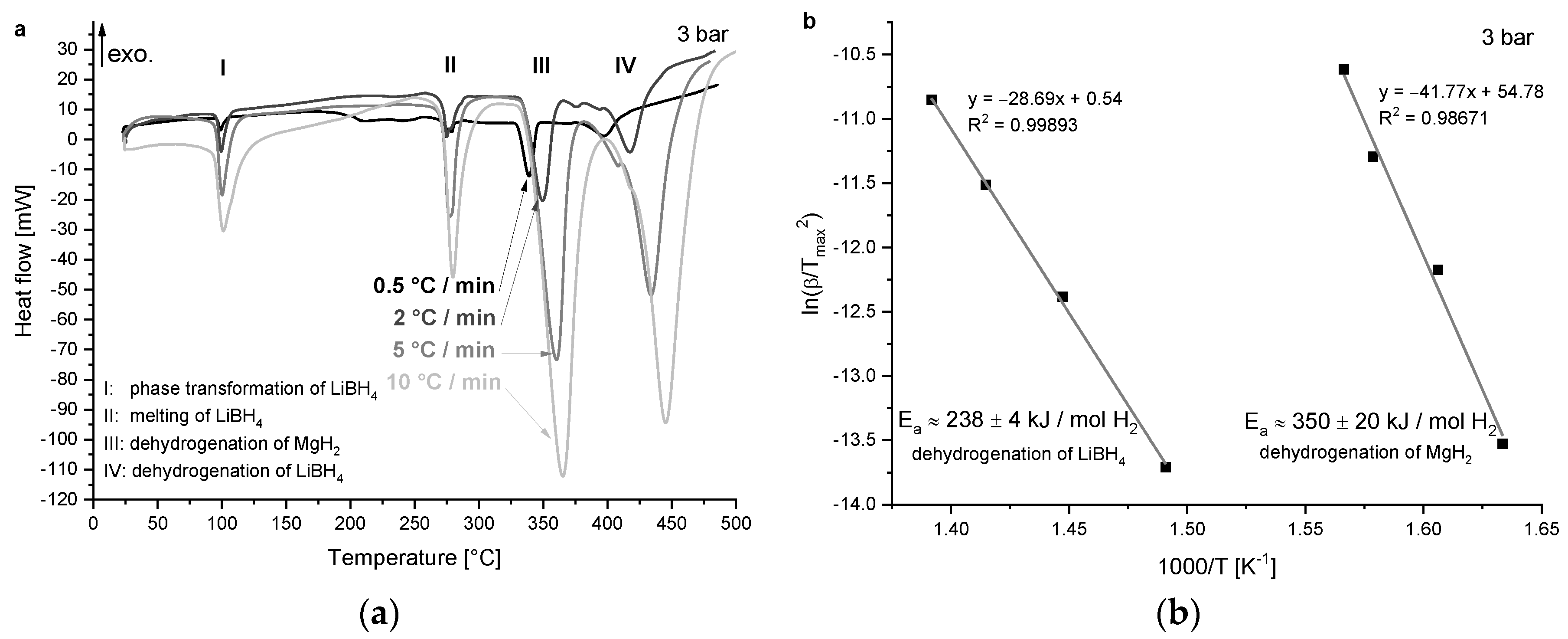
3.1.1. Activation Energy
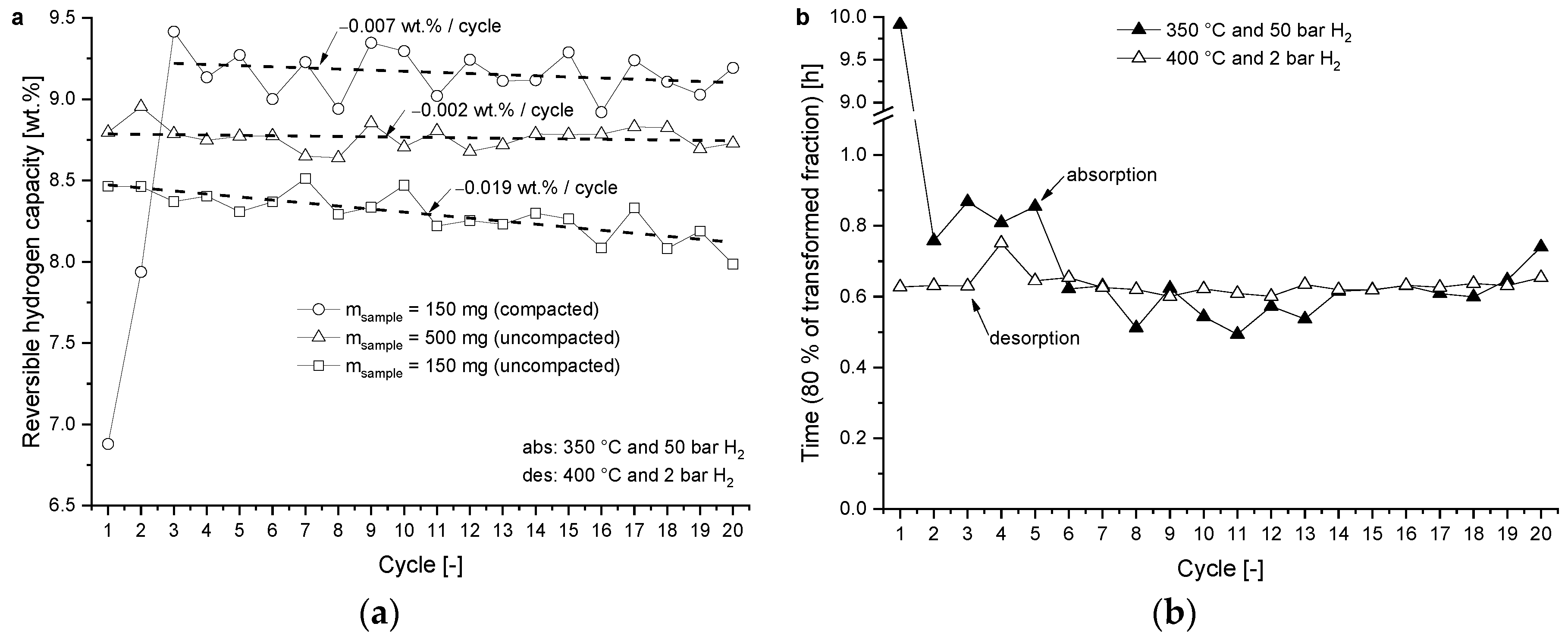
3.1.2. Cycling Behaviour
4. Discussion
4.1. Kinetic Behaviour
4.2. Reversible Hydrogen Capacity
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Description |
| A | Arrhenius factor |
| Ea | Activation energy |
| f | Function |
| g(α) | Reaction model function |
| k | Rate constant |
| p | Pressure |
| R | Gas constant |
| t | Time |
| T | Temperature |
| α | Transformed fraction |
| β | Heating rate |
References
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. Handbook of Chemistry and Physics; CRC Press: London, UK, 1994. [Google Scholar]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Nanomaterials for Solid State Hydrogen Storage; Springer: New York, NY, USA, 2009. [Google Scholar]
- Jepsen, J.; Bellosta von Colbe, J.M.; Klassen, T.; Dornheim, M. Economic potential of complex hydrides compared to conventional hydrogen storage systems. Int. J. Hydrogen Energy 2012, 37, 4204–4214. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C. Metallo Borohydrides. III. Lithium Borohydride. J. Am. Chem. Soc. 1940, 62, 3429–3435. [Google Scholar] [CrossRef]
- Varin, R.A.; Li, S.; Wronski, Z.; Morozova, O.; Khomenko, T. The effect of sequential and continuous high-energy impact mode on the mechano-chemical synthesis of nanostructured complex hydride Mg2FeH6. J. Alloys Compd. 2005, 390, 282–296. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Martin, M.; Gommel, C.; Borkhart, C.; Fromm, E. Absorption and desorption kinetics of hydrogen storage alloys. J. Alloys Compd. 1996, 238, 193–201. [Google Scholar] [CrossRef]
- Konstanchuk, Y.G.; Ivanov, E.; Boldyrev, V. Interaction of alloys and intermetallic compounds obtained by mechanochemical methods with hydrogen. Russ. Chem. Rev. 1998, 67, 69–79. [Google Scholar] [CrossRef]
- Fernández, J.F.; Sánchez, C.R. Rate determining step in the absorption and desorption of hydrogen by magnesium. J. Alloys Compd. 2002, 340, 189–198. [Google Scholar] [CrossRef]
- Puszkiel, J.A. Preparation, Study and Optimization of Complex Hydrides for Hydrogen Storage; Universidad Nacional de Cuyo: Ciudad de Mendoza, Argentina, 2012. [Google Scholar]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic Effect of Nanoparticle 3d-Transition Metals on Hydrogen Storage Properties in Magnesium Hydride MgH2 Prepared by Mechanical Milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm=Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Huot, J.; Ravnsbæk, D.B.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T.R. Mechanochemical synthesis of hydrogen storage materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Jepsen, J.; Milanese, C.; Puszkiel, J.; Girella, A.; Schiavo, B.; Lozano, G.A.; Capurso, G.; Colbe, J.M.B.; Marini, A.; Kabelac, S.; et al. Fundamental material properties of the 2LiBH4-MgH2 reactive hydride composite for hydrogen storage (I) Thermodynamic and heat transfer properties. Energies 2018, 11, 1081. [Google Scholar] [CrossRef]
- Bogdanović, B.; Bohmhammel, K.; Christ, B.; Reiser, A.; Schlichte, K.; Vehlen, R.; Wolf, U. Thermodynamic investigation of the magnesium–hydrogen system. J. Alloys Compd. 1999, 282, 84–92. [Google Scholar] [CrossRef]
- Dornheim, M.; Doppiu, S.; Barkhordarian, G.; Boesenberg, U.; Klassen, T.; Gutfleisch, O.; Bormann, R. Hydrogen storage in magnesium-based hydrides and hydride composites. Scr. Mater. 2007, 56, 841–846. [Google Scholar] [CrossRef]
- Westerwaal, R.J.; Haije, W.G. Evaluation Solid-State Hydrogen Storage Systems; ECN Publication: Petten, The Netherlands, 2008. [Google Scholar]
- Barkhordarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible Storage of Hydrogen in Destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Puszkiel, J.; Garroni, S.; Milanese, C.; Gennari, F.; Klassen, T.; Dornheim, M.; Pistidda, C. Tetrahydroborates: Development and Potential as Hydrogen Storage Medium. Inorganics 2017, 5, 74. [Google Scholar] [CrossRef]
- Jepsen, J. Technical and Economic Evaluation of Hydrogen Storage Systems Based on Light Metal Hydrides; HZG Report 2014-2; Helmut-Schmidt-University: Hamburg, Germany, 2014. [Google Scholar]
- Jepsen, J.; Milanese, C.; Girella, A.; Lozano, G.A.; Pistidda, C.; Bellosta von Colbe, J.M.; Marini, A.; Klassen, T.; Dornheim, M. Compaction pressure influence on material properties and sorption behaviour of LiBH4–MgH2 composite. Int. J. Hydrogen Energy 2013, 38, 8357–8366. [Google Scholar] [CrossRef]
- HSC Chemistry, version 9.4.1; Software for Various Kinds of Chemical Reactions and Equilibria Calculations; Outotec: Espoo, Finland, 2018.
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Bösenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Jensen, T.R.; Cerenius, Y.; Gutfleisch, O.; Klassen, T.; et al. Hydrogen sorption properties of MgH2-LiBH4 composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Bösenberg, U.; Kim, J.W.; Gosslar, D.; Eigen, N.; Jensen, T.R.; von Colbe, J.M.B.; Zhou, Y.; Dahms, M.; Kim, D.H.; Günther, R.; et al. Role of additives in LiBH4-MgH2 reactive hydride composites for sorption kinetics. Acta Mater. 2010, 58, 3381–3389. [Google Scholar] [CrossRef]
- Fedneva, E.M.; Alpatova, V.L.; Mikheeva, V.I. Thermal stability of lithium trtrahydroborate. Russ. J. Inorg. Chem. 9 1964, 6, 826–827. [Google Scholar]
- Nakagawa, T.; Ichikawa, T.; Hanada, N.; Kojima, Y.; Fujii, H. Thermal analysis on the Li–Mg–B–H systems. J. Alloys Compd. 2007, 446 (Suppl. C), 306–309. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-state kinetic models: Basics and mathematical fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef] [PubMed]
- Puszkiel, J.A.; Castro Riglos, M.V.; Ramallo-Lopez, J.M.; Mizrahi, M.; Karimi, F.; Santoru, A.; Hoell, A.; Gennari, F.C.; Larochette, P.A.; Pistidda, C.; et al. A novel catalytic route for hydrogenation-dehydrogenation of 2LiH + MgB2 via in situ formed core-shell LixTiO2 nanoparticles. J. Mater. Chem. A 2017, 5, 12922–12933. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S.; Meisner, G.P.; Balogh, M.P.; Vajo, J.J. Phase Boundaries and Reversibility of LiBH4/MgH2 Hydrogen Storage Material. J. Phys. Chem. C 2007, 111, 12881–12885. [Google Scholar] [CrossRef]
- Bösenberg, U. LiBH4-MgH2 Composites for Hydrogen Storage; Technische Universität Hamburg-Harburg: Hamburg, Germany, 2009. [Google Scholar]
- Rudman, P.S. Hydriding and dehydriding kinetics. J. Less Common Metals 1983, 89, 93–110. [Google Scholar] [CrossRef]
- Lozano, G.A.; Ranong, C.N.; Bellosta von Colbe, J.M.; Bormann, R.; Fieg, G.; Hapke, J.; Dornheim, M. Empirical kinetic model of sodium alanate reacting system (I). Hydrogen absorption. Int. J. Hydrogen Energy 2010, 35, 6763–6772. [Google Scholar] [CrossRef]
- Marty, P.; Fourmigue, J.F.; Rango, P.D.; Fruchart, D.; Charbonnier, J. Numerical simulation of heat and mass transfer during the absorption of hydrogen in a magnesium hydride. Energy Convers. Manag. 2006, 47, 3632–3643. [Google Scholar] [CrossRef]
- Patah, A.; Takasaki, A.; Szmyd, J.S. Influence of multiple oxide (Cr2O3/Nb2O5) addition on the sorption kinetics of MgH2. Int. J. Hydrogen Energy 2009, 34, 3032–3037. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloys Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Milanese, C.; Girella, A.; Garroni, S.; Bruni, G.; Berbenni, V.; Matteazzi, P.; Marini, A. Synergetic effect of C (graphite) and Nb2O5 on the H2 sorption properties of the Mg–MgH2 system. Int. J. Hydrogen Energy 2010, 35, 9027–9037. [Google Scholar] [CrossRef]
- Pighin, S.A.; Capurso, G.; Lo Russo, S.; Peretti, H.A. Hydrogen sorption kinetics of magnesium hydride enhanced by the addition of Zr8Ni21 alloy. J. Alloys Compd. 2012, 530, 111–115. [Google Scholar] [CrossRef]
- Brown, M.E. The Prout-Tompkins rate equation in solid-state kinetics. Thermochim. Acta 1997, 300, 93–106. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Ma, L.P.; Kang, X.D.; Wang, P.J.; Wang, P.; Cheng, H.M. In situ formation and rapid decomposition of Ti(BH4)3 by mechanical milling LiBH4 with TiF3. Appl. Phys. Lett. 2009, 94, 044104. [Google Scholar] [CrossRef]
- Callini, E.; Szilagyi, P.A.; Paskevicius, M.; Stadie, N.P.; Rehault, J.; Buckley, C.E.; Borgschulte, A.; Zuttel, A. Stabilization of volatile Ti(BH4)3 by nano-confinement in a metal-organic framework. Chem. Sci. 2016, 7, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-B.; Shim, J.-H.; Cho, Y.W.; Oh, K.H. Pressure-enhanced dehydrogenation reaction of the LiBH4-YH3 composite. Chem. Commun. 2011, 47, 9831–9833. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-B.; Shim, J.-H.; Park, S.-H.; Choi, I.-S.; Oh, K.H.; Cho, Y.W. Dehydrogenation Reaction Pathway of the LiBH4–MgH2 Composite under Various Pressure Conditions. J. Phys. Chem. C 2015, 119, 9714–9720. [Google Scholar] [CrossRef]
- Kim, K.-B.; Shim, J.-H.; Oh, K.H.; Cho, Y.W. Role of Early-Stage Atmosphere in the Dehydrogenation Reaction of the LiBH4–YH3 Composite. J. Phys. Chem. C 2013, 117, 8028–8031. [Google Scholar] [CrossRef]
- Kang, X.; Wang, K.; Zhong, Y.; Yang, B.; Wang, P. A novel three-step method for preparation of a TiB2-promoted LiBH4-MgH2 composite for reversible hydrogen storage. Phys. Chem. Chem. Phys. 2013, 15, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Castro Riglos, M.; Santoru, A.; Hoell, A.; Raghuwanshi, V.; Milanese, C.; Bergemann, N.; Pistidda, C.; Nolis, P.; Baró, M.; et al. In situ formation of TiB2 nanoparticles for enhanced dehydrogenation/hydrogenation reaction kinetics of LiBH4-MgH2 as a reversible solid-state hydrogen storage system. J. Phys. Chem. C 2018. under review, (Manuscript jp-2018-02258a). [Google Scholar]
- Bellosta von Colbe, J.M.; Bogdanovic, B.; Felderhoff, M.; Pommerin, A.; Schüth, F. Recording of hydrogen evolution—A way for controlling the doping process of sodium alanate by ball milling. J. Alloys Compd. 2004, 370, 104–109. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Wu, F.; Fang, F.; Sun, D.; Guo, Z.; Huang, Z.; Yu, X. Graphene-wrapped reversible reaction for advanced hydrogen storage. Nano Energy 2016, 26, 488–495. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Fang, F.; Sun, D.; Li, X.; Guo, Z.; Yu, X. Oxygen-free Layer-by-Layer Assembly of Lithiated Composites on Graphene for Advanced Hydrogen Storage. Adv. Sci. 2017, 4, 1600257. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Tan, Y.; Chen, X.; Sun, D.; Guo, Z.; Liu, H.; Ouyang, L.; Zhu, M.; Yu, X. Monodisperse Magnesium Hydride Nanoparticles Uniformly Self-Assembled on Graphene. Adv. Mater. 2015, 27, 5981–5988. [Google Scholar] [CrossRef] [PubMed]



| Species | Starting Composition | After Milling (Milling at 25 °C and 1 bar Ar) | Hydrogenation at 350 °C and 50 bar of H2 | Dehydrogenation at 400 °C and 2 bar of H2 |
|---|---|---|---|---|
| 2LiH+MgB2+0.05TiCl3 | ||||
| LiH (S) | 66 | 60 | - | 55 |
| MgB2 (S) | 32 | 30 | - | 37 |
| TiCl3 (S) | 2 | - | - | - |
| LiBH4 (S) | - | - | 62 | - |
| MgH2 (S) | - | 2 | 31 | - |
| TiB2 (S) | - | 2 | 2 | 2 |
| Ti (S) | - | - | - | - |
| TiH2 (S) | - | - | - | - |
| B (S) | - | - | - | - |
| LiCl (S) | 5 | 5 | 5 | |
| Mg (S) | - | - | - | 1 |
| B2H6 (g) | - | - | - | - |
| HCl (g) | - | - | - | - |
| H2 (g) | - | 1 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jepsen, J.; Milanese, C.; Puszkiel, J.; Girella, A.; Schiavo, B.; Lozano, G.A.; Capurso, G.; Bellosta von Colbe, J.M.; Marini, A.; Kabelac, S.; et al. Fundamental Material Properties of the 2LiBH4-MgH2 Reactive Hydride Composite for Hydrogen Storage: (II) Kinetic Properties. Energies 2018, 11, 1170. https://doi.org/10.3390/en11051170
Jepsen J, Milanese C, Puszkiel J, Girella A, Schiavo B, Lozano GA, Capurso G, Bellosta von Colbe JM, Marini A, Kabelac S, et al. Fundamental Material Properties of the 2LiBH4-MgH2 Reactive Hydride Composite for Hydrogen Storage: (II) Kinetic Properties. Energies. 2018; 11(5):1170. https://doi.org/10.3390/en11051170
Chicago/Turabian StyleJepsen, Julian, Chiara Milanese, Julián Puszkiel, Alessandro Girella, Benedetto Schiavo, Gustavo A. Lozano, Giovanni Capurso, José M. Bellosta von Colbe, Amedeo Marini, Stephan Kabelac, and et al. 2018. "Fundamental Material Properties of the 2LiBH4-MgH2 Reactive Hydride Composite for Hydrogen Storage: (II) Kinetic Properties" Energies 11, no. 5: 1170. https://doi.org/10.3390/en11051170










