Dice-Like Nanostructure of a CuS@PbS Composite for High-Performance Supercapacitor Electrode Applications
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of CuS, PbS and CuS@PbS Electrodes
2.3. Materials Characterization
2.4. Electrochemical Measurements
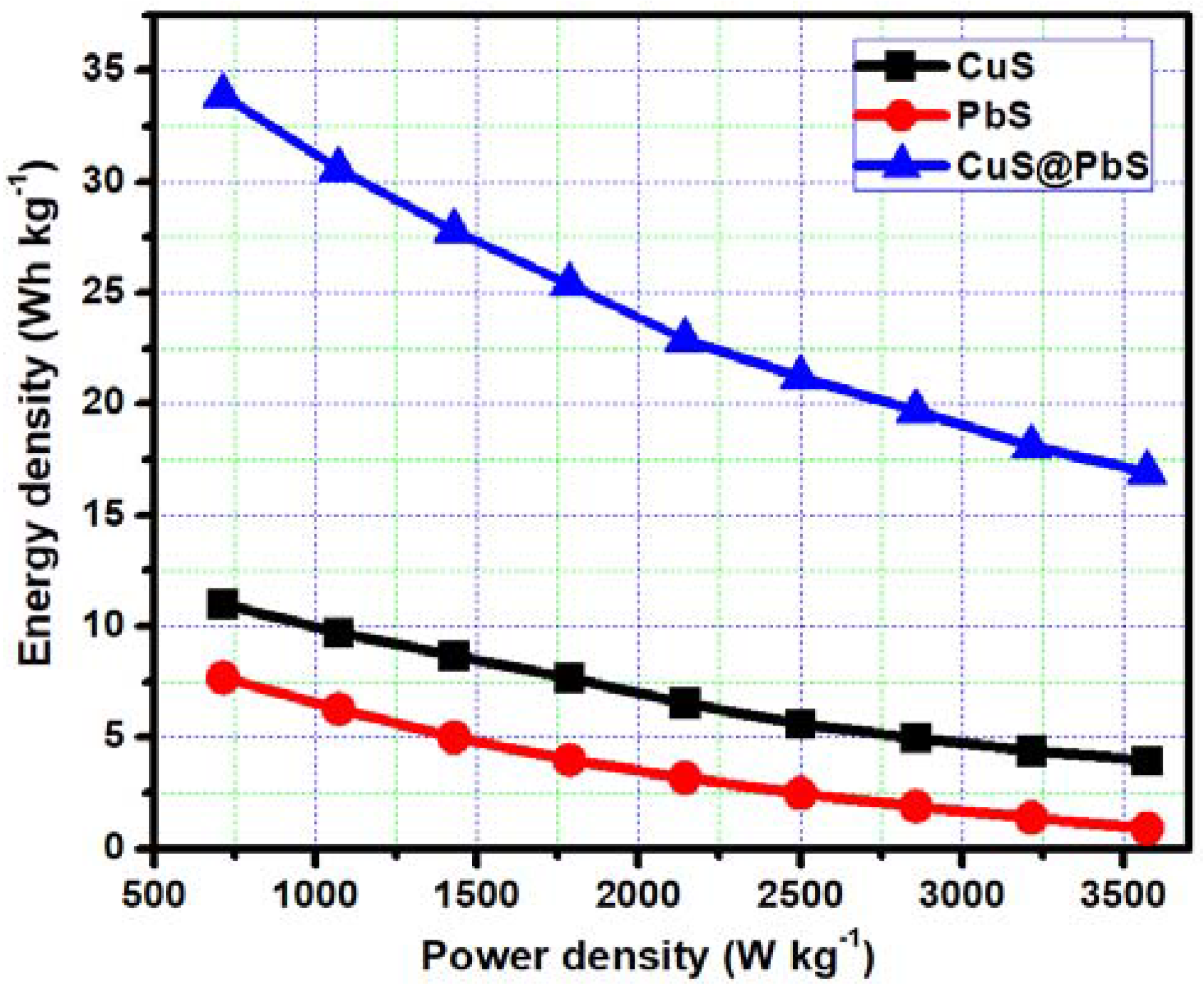
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly Flexible and All-Solid-State Paper like Polymer Supercapacitors. Nano Lett. 2010, 10, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, X.F.; Chen, H.T.; Wang, Z.R.; Chen, D.; Cheng, Y.B.; Zhou, C.W.; Shen, G.Z. Hierarchical silicon nanowires-carbon textiles matrix as a binder-free anode for high-performance advanced lithium-ion batteries. Sci. Rep. 2013, 3, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Manickam, M.S. Electrochemical synthesis of polyaniline cross-linked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 7456–7464. [Google Scholar] [CrossRef]
- Maryam, J.B.; Manickam, M. Tuning the Redox Properties of the Nanostructured CoMoO4Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar]
- Rashmirekha, S.; Barsha, D.; Chinmaya, K.S.; Kali, S.; Tondepu, S.; Gamini, S.; Manickam, M. Influence of Synthesis Temperature on the Growth and Surface Morphology of Co3O4 Nanocubes for Supercapacitor Applications. Nanomaterials 2017, 7, 356. [Google Scholar]
- Jiang, Z.; Lu, W.; Li, Z.; Ho, K.H.; Li, X.; Jiao, X.; Chen, J.D. Synthesis of amorphous cobalt sulfide polyhedral nanocages for high performance supercapacitors. Mater. Chem. A 2014, 2, 8603–8606. [Google Scholar] [CrossRef]
- Wei, C.; Cheng, C.; Zhao, J.; Wang, Y.; Cheng, Y.; Xu, Y.; Du, W.; Pang, H. NiS Hollow Spheres for High-Performance Supercapacitors and Non-Enzymatic Glucose Sensors. Chem. Asian J. 2015, 10, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Saranya, M.; Kollu, P.; Raghupathy, B.P.C.; Jeong, S.K.; Grace, A.N. Solvothermal synthesis of Zinc sulfide decorated Graphene (ZnS/G) nanocomposites for novel Supercapacitor electrodes. Electrochim. Acta 2015, 178, 647–657. [Google Scholar] [CrossRef]
- Chauhan, H.; Singh, M.K.; Hashmi, S.A.; Deka, S. Synthesis of surfactant-free SnS nanorods by a solvothermal route with better electrochemical properties towards supercapacitor applications. RSC Adv. 2015, 5, 17228–17235. [Google Scholar] [CrossRef]
- Justin Raj, C.; Kim, B.C.; Cho, W.-J.; Lee, W.-G.; Seo, Y.; Yu, K.-H. Electrochemical capacitor behavior of copper sulfide (CuS) nanoplatelets. J. Alloy. Compd. 2014, 586, 191–196. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Ia, Y.-L.; Xing, K.; Liu, Y.-M. Acetylene black incorporated layered copper sulfide nanosheets for high-performance supercapacitor. J. Alloy. Compd. 2015, 641, 119–126. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Liu, Y.; Liu, Y.-M. Synthesis of reduced graphene oxide wrapped-copper sulfide hollow spheres as electrode material for supercapacitor. J. Hydrogen Energy 2015, 40, 10158–10167. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Lou, X.W. General Solution Growth of Mesoporous NiCo2O4 Nanosheets on Various Conductive Substrates as High-Performance Electrodes for Supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Xia, B.; Zhou, L.; Lou, X. Arrays of ultrafine CuS nanoneedles supported on a CNT backbone for application in supercapacitors. J. Mater. Chem. 2012, 22, 7851–7855. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Chen, Y.-C.; Lin, Y.-G. Synthesis of copper sulfide nanowire arrays for high-performance supercapacitors. Electrochim. Acta 2014, 139, 401–407. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Mu, J.; Sun, K.; Lei, Z. Controllable synthesis of CuS with hierarchical structures via a surfactant-free method for high-performance supercapacitors. Mater. Lett. 2014, 122, 25–28. [Google Scholar] [CrossRef]
- Tian, J.; Shen, T.; Liu, X.; Fei, C.; Lv, L.; Cao, G. Enhanced Performance of PbS-quantum-dot-sensitized Solar Cells via Optimizing Precursor Solution and Electrolytes. Sci. Rep. 2016, 6, 23094. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Wang, J.; Shen, Q.; Li, Y.; Zhong, X. Surface engineering of PbS quantum dot sensitized solar cells with a conversion efficiency exceeding 7%. J. Mater. Chem. A 2016, 4, 7214–7221. [Google Scholar] [CrossRef]
- Kim, G.H.; García de Arquer, F.P.; Yoon, Y.J.; Lan, X.; Liu, M.; Voznyy, O.; Jagadamma, L.K.; Abbas, A.S.; Yang, Z.; Fan, F.; et al. High-Efficiency Colloidal Quantum Dot Photovoltaics via Robust Self-Assembled Monolayers. Nano Lett. 2015, 15, 7691–7696. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Wang, X.; Chen, D.; Chen, X.; Li, D.; Shen, G. Three-Dimensional Structural Engineering for Energy-Storage Devices: From Microscope to Macroscope. ChemElectroChem 2014, 1, 975–1002. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Designing 3D Highly Ordered Nanoporous CuO Electrodes for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Buller, S.; Strunk, J. Nanostructure in energy conversion. J. Energy Chem. 2016, 25, 171–190. [Google Scholar] [CrossRef]
- Rao, S.S.; Durga, I.K.; Kundakarla, N.; Punnoose, D.; Gopi, C.V.V.M.; Reddy, A.E.; Jagadeesh, M.; Kim, H.J. A hydrothermal reaction combined with a post anion-exchange reaction of hierarchically nanostructured NiCo2S4 for high-performance QDSSCs and supercapacitors. New J. Chem. 2017, 41, 10037–10047. [Google Scholar] [CrossRef]
- Rao, S.S.; Punnoose, D.; Bae, J.H.; Durga, I.K.; Varma, C.V.T.; Naresh, B.; Subramanian, A.; Raman, V.; Kim, H.J. Preparation and electrochemical performances of NiS with PEDOT:PSS chrysanthemum petal like nanostructure for high performance supercapacitors. Electrochim. Acta 2017, 254, 269–279. [Google Scholar]
- Durga, I.K.; Rao, S.S.; Reddy, A.E.; Gopi, C.V.V.M.; Kim, H.J. Achieving copper sulfide leaf like nanostructure electrode for high performance supercapacitor and quantum-dot sensitized solar cells. Appl. Surface Sci. 2018, 435, 666–675. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Pavan Kumar, CH.S.S.; Punnoose, D.; Kim, S.K.; Gopi, C.V.V.M.; Rao, S.S. Facile chemical bath deposition of CuS nano peas like structure as a high efficient counter electrode for quantum-dot sensitized solar cells. J. Electroanal. Chem. 2015, 739, 20–27. [Google Scholar] [CrossRef]
- Rao, S.S.; Durga, I.K.; Varma, C.V.T.; Punnoose, D.; Cheol, L.J.; Kim, H.J. The synthesis and characterization of lead sulfide with cube-like structure as a counter electrode in the presence of urea using a hydrothermal method. New J. Chem. 2015, 39, 7379–7388. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Li, H.; Wang, L.; Qin, X.; Asiri, A.; Al-Youbi, A.; Sun, X. Biomolecule-Assisted, Environmentally Friendly, One-Pot Synthesis of CuS/Reduced Graphene Oxide Nanocomposites with Enhanced Photocatalytic Performance. Langmuir 2012, 28, 12893–12900. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; Niitsoo, O.; Itzhaik, Y.; Cahen, D.; Hodes, G. Copper sulphide as a light absorber in wet-chemical synthesized extremely thin absorber (ETA) solar cells, Energy Environ. Energy Environ. Sci. 2009, 2, 220–223. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Chen, P.; Liao, H.; Cheng, S. In situ preparation of CuS cathode with unique stability and high rate performance for lithium ion batteries. Electrochim. Acta 2012, 80, 264–268. [Google Scholar] [CrossRef]
- Wei, W.; Mi, L.; Gao, Y.; Zheng, Z.; Chen, W.; Guan, X. Partial ion-exchange of nickel-sulfide-derived electrodes for high performance supercapacitors. Chem. Mater. 2014, 26, 3418–3426. [Google Scholar] [CrossRef]
- Prusty, B.; Adhikary, M.C.; Das, C.K. Synthesis of NiS anchored graphenenanocomposites: High performance supercapacitor electrode material. Int. J. Curr. Res. 2014, 6, 7448–7452. [Google Scholar]
- Xia, H.; Zhu, D.; Luo, Z.; Yu, Y.; Shi, X.; Yuan, G.; Xie, J. Hierarchically Structured Co3O4@Pt@MnO2 Nanowire Arrays for High-Performance Supercapacitors. Sci. Rep. 2013, 3, 2978. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Q.; Peng, C. Facile Synthesis of Core-shell Structured CuS@PANI Microspheres and Electrochemical Capacitance Investigations. Polym. Polym. Compos. 2017, 25, 483–488. [Google Scholar]
- Beka, L.G.; Li, X.; Liu, W. Nickel Cobalt Sulfide core/shell structure on 3D Graphene for supercapacitor application. Sci. Rep. 2017, 7, 2105. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Bu, Y.G.; Xiong, W.L. Construction of Complex CoS Hollow Structures with Enhanced Electrochemical Properties for Hybrid Supercapacitors. Chem 2016, 1, 102–113. [Google Scholar]
- Nazish, P.; Sajid, A.A.; Hatem, R.A.; Moo, H.C. Facile Synthesis of SnS2 Nanostructures with Different Morphologies for High-Performance Supercapacitor Applications. ACS Omega 2018, 3, 1581–1588. [Google Scholar]








| Material | Electrolyte | Current Density | Specific Capacitance | Ref. |
|---|---|---|---|---|
| CuS@PbS composite | 3 M KOH | 2.85 A g−1 | 1004.42 F g−1 | This study |
| NiS nanoparticles | 2 M KOH | 0.5 A g−1 | 516 F g−1 | [33] |
| rGO-NiS | - | 2.5 A g−1 | 109.37 F g−1 | [34] |
| Co3O4@Pt@MnO2 NA | 1.0 M Na2SO4 | 1 A g−1 | 539 F g−1 | [35] |
| CuS@PANI | 0.1 M Li2SO4 | 0.5 A g−1 | 308.1 F g−1 | [36] |
| Graphene/NiCo2S4/CoxNi(3−x)S2 | - | 10 mA cm−2 | 15.6 F/cm2 | [37] |
| CoS-NP/CoS-NS DSNB | 2 M KOH | 1 A g−1 | 980 F g−1 | [38] |
| ZnS/G-60 | 6 M KOH | 5 mV s−1 | 197.1 F g−1 | [9] |
| FL-SnS2 | 2 M KOH | 1 A g−1 | 431.82 F g−1 | [39] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanaka Durga, I.; Srinivasa Rao, S.; Ahn, J.-W.; Park, T.-Y.; Jin-Soo, B.; Ho, C.-I.; Prabakar, K.; Kim, H.-J. Dice-Like Nanostructure of a CuS@PbS Composite for High-Performance Supercapacitor Electrode Applications. Energies 2018, 11, 1624. https://doi.org/10.3390/en11071624
Kanaka Durga I, Srinivasa Rao S, Ahn J-W, Park T-Y, Jin-Soo B, Ho C-I, Prabakar K, Kim H-J. Dice-Like Nanostructure of a CuS@PbS Composite for High-Performance Supercapacitor Electrode Applications. Energies. 2018; 11(7):1624. https://doi.org/10.3390/en11071624
Chicago/Turabian StyleKanaka Durga, Ikkurthi, S. Srinivasa Rao, Jin-Woo Ahn, Tae-Yong Park, Bak Jin-Soo, Cho-In Ho, K. Prabakar, and Hee-Je Kim. 2018. "Dice-Like Nanostructure of a CuS@PbS Composite for High-Performance Supercapacitor Electrode Applications" Energies 11, no. 7: 1624. https://doi.org/10.3390/en11071624
APA StyleKanaka Durga, I., Srinivasa Rao, S., Ahn, J.-W., Park, T.-Y., Jin-Soo, B., Ho, C.-I., Prabakar, K., & Kim, H.-J. (2018). Dice-Like Nanostructure of a CuS@PbS Composite for High-Performance Supercapacitor Electrode Applications. Energies, 11(7), 1624. https://doi.org/10.3390/en11071624







