Engineering Study of a Pilot Scale Process Plant for Microalgae-Oil Production Utilizing Municipal Wastewater and Flue Gases: Fukushima Pilot Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology Framework
2.2. Process Simulation
2.2.1. Cultivation Pond Section
2.2.2. Harvesting and Dehydration
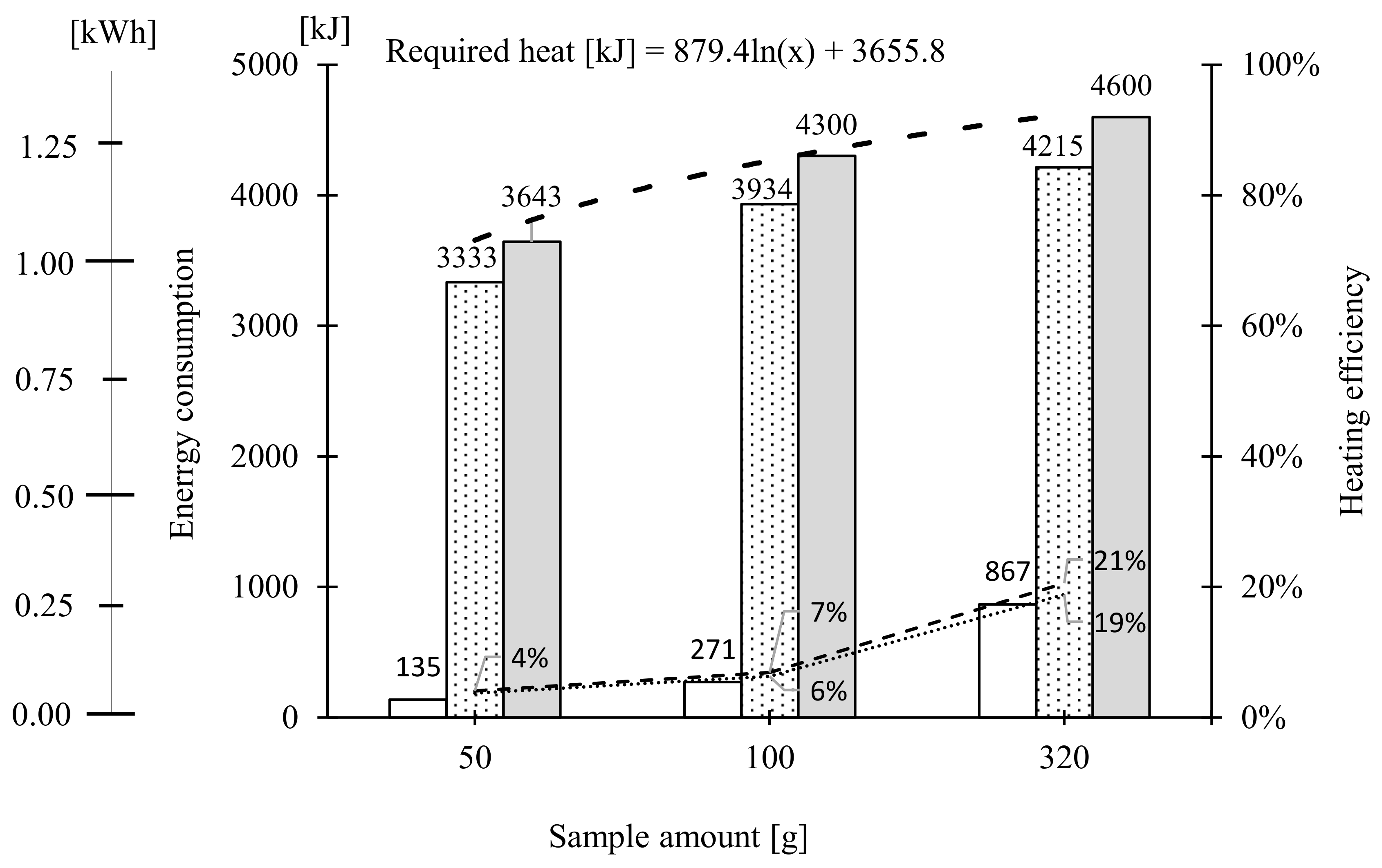
2.2.3. Extraction
3. Results and Discussion
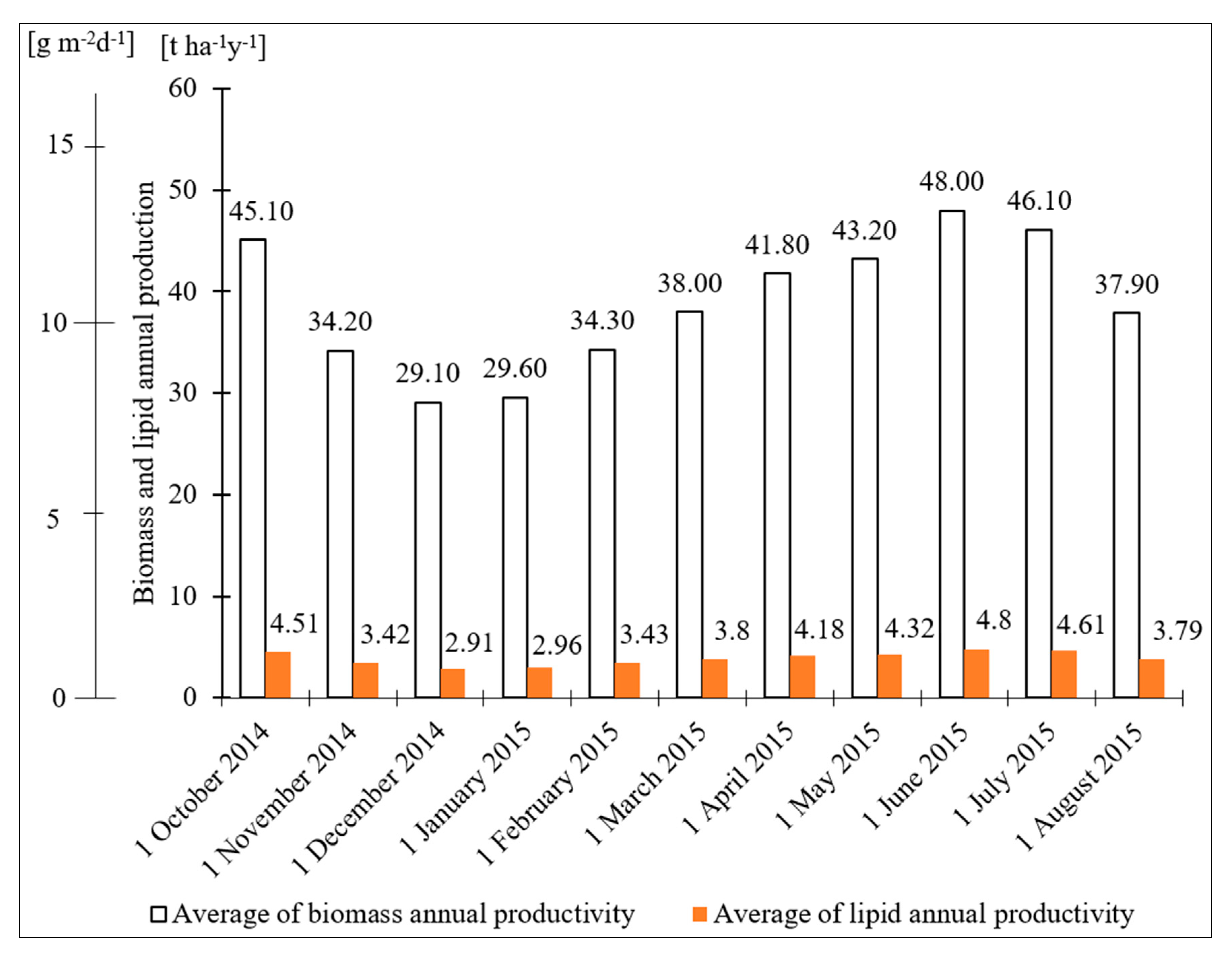
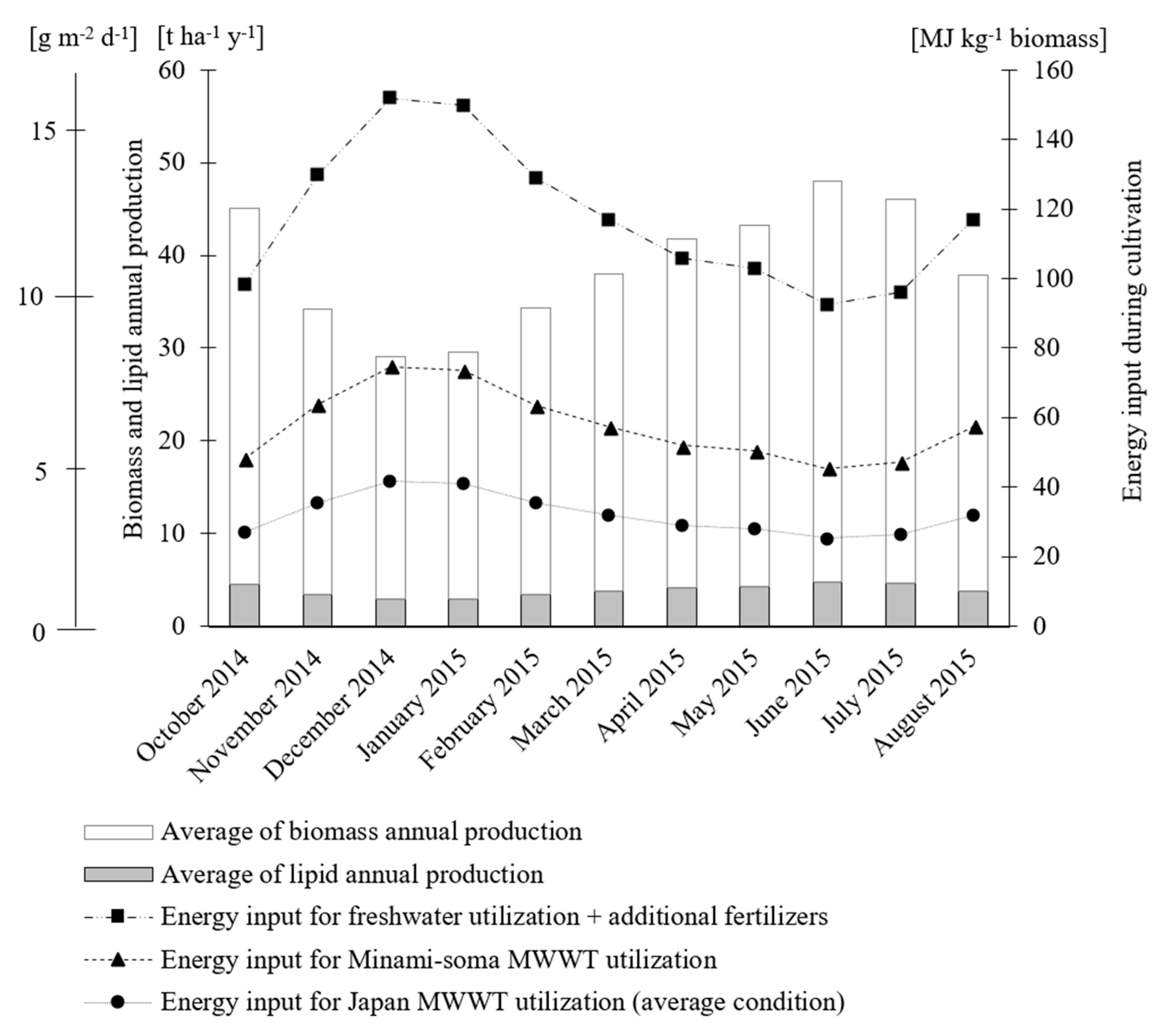
3.1. Microalgae Biomass Productivity
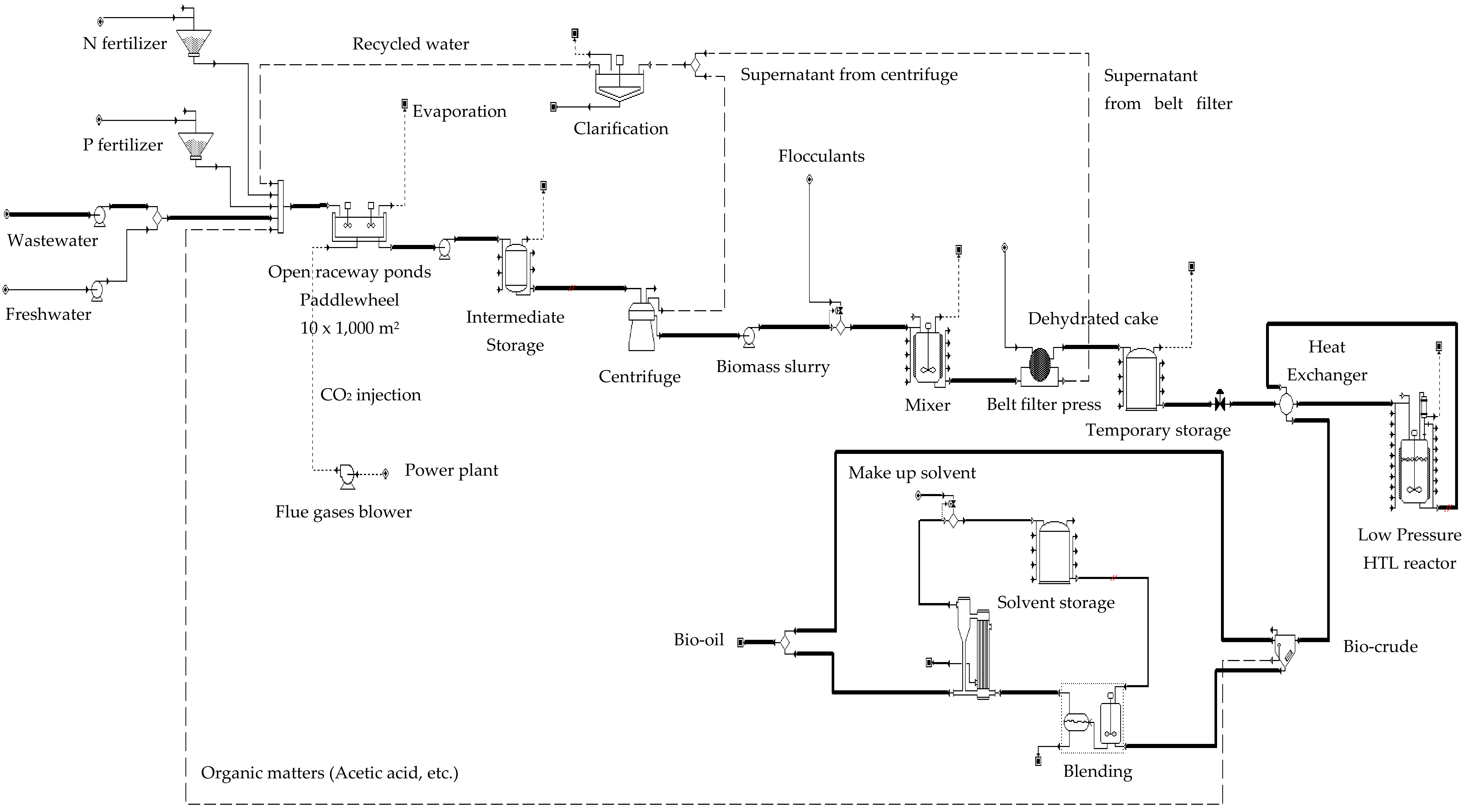
3.2. Integrated Process Plant System
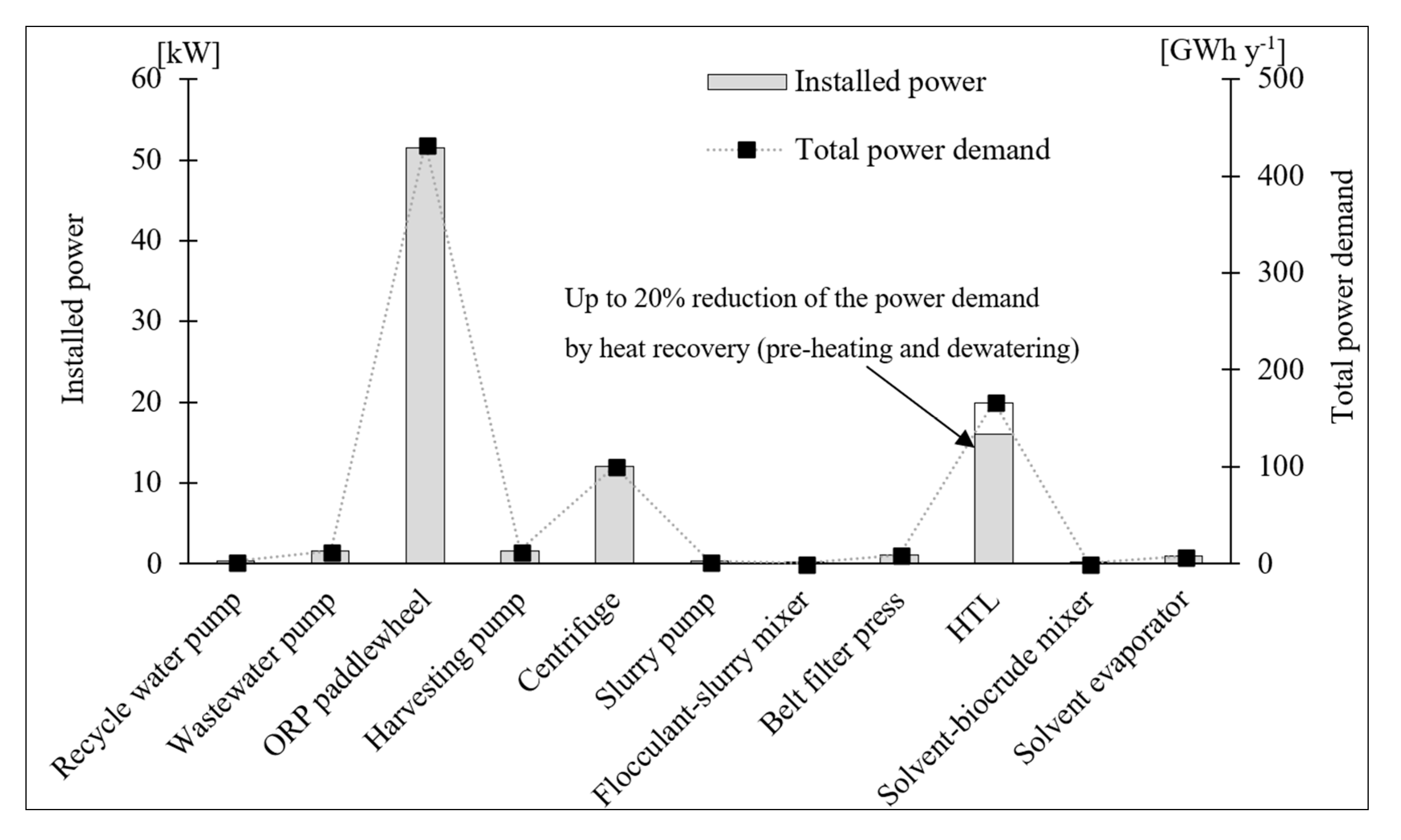
3.3. Material and Energy Balance
3.4. GHG Emission from Cultivation Stage
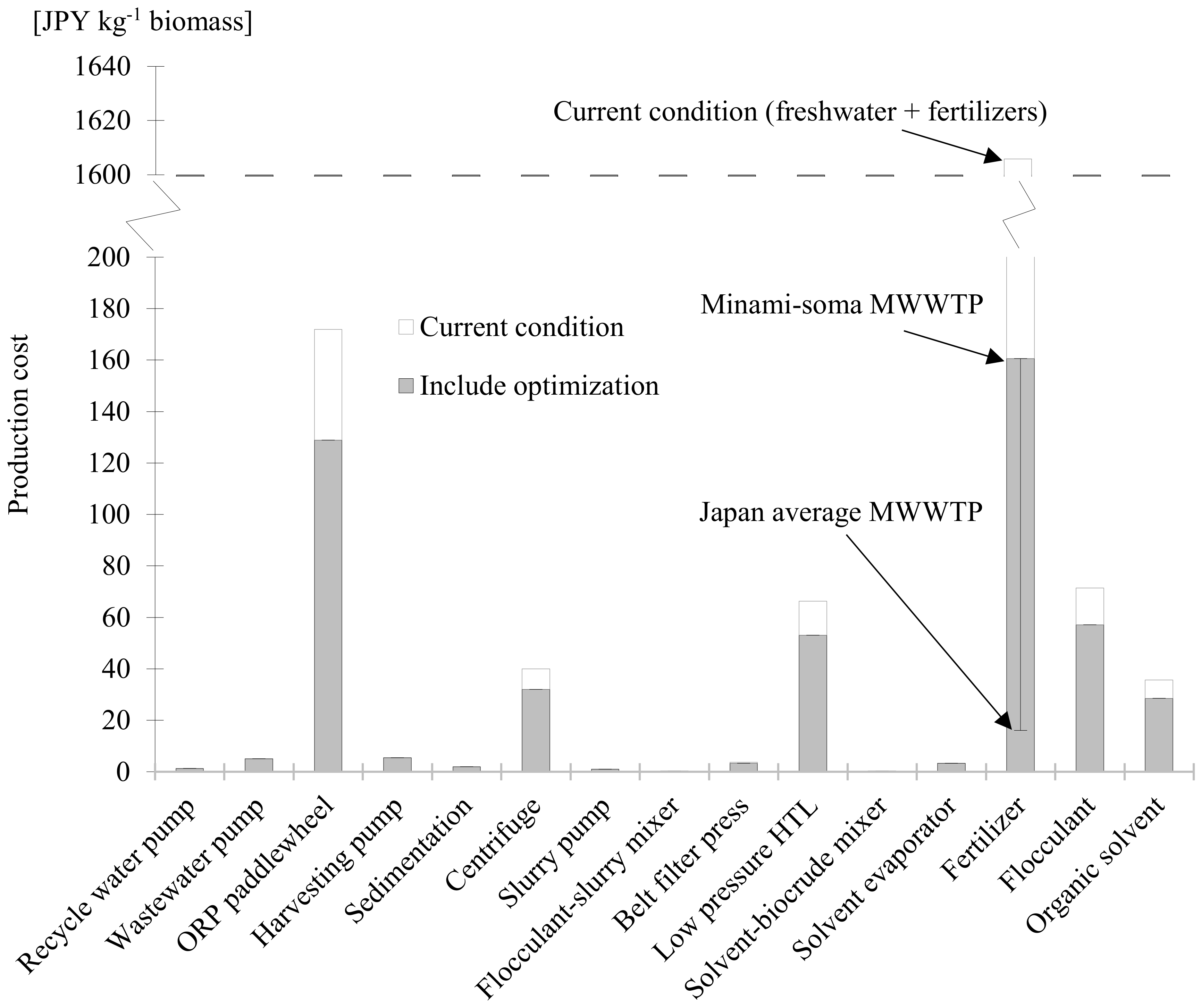
3.5. Economic Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.; Markham, J.; Kinchin, C.; Grundl, N.; Tan, E.C.D. Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing through Dewatering for Downstream Conversion; National Renewable Energy Laboratory: Golden, CO, USA, 2016. Available online: http://www.nrel.gov/docs/fy16osti/64772.pdf (accessed on 1 March 2016).
- Sasongko, N.A.; Noguchi, R.; Ahamed, T. Environmental load assessment for an integrated design of microalgae system of palm oil mill in Indonesia. Energy 2018. [Google Scholar] [CrossRef]
- Sasongko, N.A.; Noguchi, R.; Ahamed, T.; Takaigawa, T. Introduction of Integrated Energy Plantation Model for Microalgae-Using Palm Oil Mill Effluent (POME). J. Jpn. Inst. Energy 2015, 94, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Devi, M.P.; Subhash, G.V.; Mohan, S.V. Heterotrophic cultivation of mixed microalgae for lipid accumulation and wastewater treatment during sequential growth and starvation phases: Effect of nutrient supplementation. Renew. Energy 2012, 43, 276–283. [Google Scholar] [CrossRef]
- Ebrahimian, A.; Kariminia, H.-R.; Vosoughi, M. Lipid production in mixotrophic cultivation of Chlorella vulgaris in a mixture of primary and secondary municipal wastewater. Renew. Energy 2014, 71, 502–508. [Google Scholar] [CrossRef]
- Lam, M.K.; Yusoff, M.I.; Uemura, Y.; Lim, J.W.; Khoo, C.G.; Lee, K.T.; Ong, H.C. Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renew. Energy 2017, 103, 197–207. [Google Scholar] [CrossRef]
- Craggs, R.J.; Lundquist, T.; Benemann, J. Wastewater Treatment Pond Algal Production for Biofuel. Sci. Algal Fuels 2012, 425–445. [Google Scholar] [CrossRef]
- Craggs, R.J.; Sutherland, D.; Campbell, H. Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J. Appl. Phycol. 2012, 24, 329–337. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. Enhancing microalgal photosynthesis and productivity in wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2015, 184, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, A.; Craggs, R.J.; Farid, M.M. Wastewater treatment high rate algal ponds (WWT HRAP) for low-cost biofuel production. Bioresour. Technol. 2015, 184, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Andersson, V.; Viklund, S.B.; Hackl, R.; Karlsson, M.; Berntsson, T. Algae-based biofuel production as part of an industrial cluster. Biomass Bioenergy 2014, 71, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, S.; Mientus, M.; Frey, D.; Amini-Hajibashi, A.; Ozturk, S.; Shaikh, F.; Sengupta, D.; El-Halwagi, M. A review of biodiesel production from microalgae. Clean Technol. Environ. Policy 2017, 19, 637–668. [Google Scholar] [CrossRef]
- Okada, M. Regulations of Wastewater Discharge in Japan. WEPA Workshop, Vientiane, Lao PDR. Available online: http://wepa-db.net/3rd/en/meeting/20160125/pdf/WS1_KeynoteSpeech_ProfOkada160125.pdf (accessed on 25 January 2016).
- Coons, J.E.; Kalb, D.M.; Dale, T.; Marrone, B.L. Getting to low-cost algal biofuels: A monograph on conventional and cutting-edge harvesting and extraction technologies. Algal Res. 2014, 6, 250–270. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A review of the harvesting of microalgae for biofuel production. Rev. Environ. Sci. Biotechnol. 2013, 12, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, D. Flocculation Based Harvesting Processes for Microalgae Biomass Production. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2013. Available online: www.vliz.be/imisdocs/publications/254002.pdf (accessed on 5 January 2016).
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M.L. Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef]
- Smayda, T.J. The suspension and sinking of phytoplankton in the sea. Oceanogr. Mar. Biol. Ann. Rev. 1970, 8, 353–414. [Google Scholar]
- Van Ierland, E.T.; Peperzak, L. Separation of marine seston and density determination of marine diatoms by density gradient centrifugation. J. Plankton Res. 1984, 6, 29–44. [Google Scholar] [CrossRef]
- Kromkamp, J.; Walsby, A.E. A computer model of buoyancy and vertical migration in cyanobacteria. J. Plankton Res. 1990, 12, 161–183. [Google Scholar] [CrossRef]
- Visser, P.M.; Passarge, J.; Mur, L.R. Modelling vertical migration of the cyanobacterium Microcystis. Hydrobiologia 1997, 349, 99–109. [Google Scholar] [CrossRef]
- Hu, W. Dry Weight and Cell Density of Individual Algal and Cyanobacterial Cells for Algae Research and Development. Master’s Thesis, The University of Missouri-Columbia, Columbia, MO, USA, 2014. Available online: https://mospace.umsystem.edu/xmlui/bitstream/handle/10355/46477/research.pdf?sequence=1 (accessed on 12 January 2017).
- Roux, J.M.; Lamotte, H.; Achard, J.L. An Overview of Microalgae Lipid Extraction in a Biorefinery Framework. Energy Procedia 2017, 112, 680–688. [Google Scholar] [CrossRef]
- Biller, P.; Sharma, B.K.; Kunwar, B.; Ross, A.B. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 2015, 159, 197–205. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Demura, M.; Yoshida, M.; Yokoyama, A.; Ito, J.; Kobayashi, H.; Kayano, S.; Tamagawa, Y.; Watanobe, M.; Date, N.; Osaka, M.; et al. Biomass productivity of native algal communities in Minamisoma city, Fukushima Prefecture, Japan. Algal Res. 2018, 29, 22–35. [Google Scholar] [CrossRef]
- National Astronomical Observatory of Japan Calendar of Fukushima Prefecture, Japan. Available online: http://eco.mtk.nao.ac.jp/koyomi/dni/dni07.html (accessed on 15 June 2018).
- Shimamura, R.; Watanabe, S.; Sakakura, Y.; Shiho, M.; Kaya, K.; Watanabe, M.M. Development of Botryococcus seed culture system for future mass culture. Procedia Environ. Sci. 2012, 15, 80–89. [Google Scholar] [CrossRef]
- Ptacnik, R.; Andersen, T.; Tamminen, T. Performance of the Redfield Ratio and a Family of Nutrient Limitation Indicators as Thresholds for Phytoplankton N vs. P Limitation. Ecosystems 2010, 13, 1201–1214. [Google Scholar] [CrossRef] [Green Version]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Scenario Analysis of Nutrient Removal from Municipal Wastewater by Microalgal Biofilms. Water 2012, 4, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Japan Sewage Works Association. Design Standard for Municipal Wastewater Treatment Plants, 2nd ed.; Japan Sewage Works Association: Tokyo, Japan, 2013; Available online: http://gcus.jp/wp/wp-content/uploads/2014/06/735735ded23d4d28db9cc4f879e8da24.pdf (accessed on 17 January 2016).
- Wang, T.; Yabar, H.; Higano, Y. Perspective assessment of algae-based biofuel production using recycled nutrient sources: The case of Japan. Bioresour. Technol. 2013, 128, 688–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redfield, A.C. The Biological Control of Chemical Factors in the Environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Geider, R.J.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Tohoku Electric Power Group. Environmental Action Report. 2016. Available online: http://www.tohoku-epco.co.jp/enviro/tea2016/pdf/2016_all_e.pdf (accessed on 25 December 2016).
- Posten, C.; Schaubb, G. Microalgae and terrestrial biomass as source for fuels—A process view. J. Biotechnol. 2009, 142, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Large-Scale Production of Algal Biomass: Raceway Ponds. Algae Biotechnol. 2016, 21–40. [Google Scholar] [CrossRef]
- Chen, W.-T.; Zhang, Y.; Zhang, J.; Yu, G.; Schideman, L.C.; Zhang, P.; Minarick, M. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil. Bioresour. Technol. 2014, 152, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Davis, R.; Zhu, Y.; Kinchin, C.; Anderson, D.; Hallen, R.; Elliott, D.; Schmidt, A.; Albrecht, K.; Hart, T.; et al. Process Design and Economics for the Conversion of Algal Biomass to Hydrocarbons: Whole Algae Hydrothermal Liquefaction and Upgrading; U.S. Department of Energy: Richland, WA, USA, 2014. Available online: http://www.pnnl.gov/main/publications/external/technical_reports/PNNL-23227.pdf (accessed on 3 January 2016).
- Vardon, D.R. Hydrothermal Liquefaction for Energy Recovery from High-Moisture Waste Biomass. Master’s Thesis, The University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2012. Available online: https://www.ideals.illinois.edu/bitstream/handle/2142/34327/Vardon_Derek.pdf?sequence=1 (accessed on 2 January 2015).
- De Bhowmick, G.; Koduru, L.; Sen, R. Metabolic pathway engineering towards enhancing microalgal lipid biosynthesis for biofuel application—A review. Renew. Sustain. Energy Rev. 2015, 50, 1239–1253. [Google Scholar] [CrossRef]
- Weyer, K.M.; Bush, D.R.; Darzins, A.; Willson, B.D. Theoretical Maximum Algal Oil Production. Bioenergy Res. 2010, 3, 204–213. [Google Scholar] [CrossRef]
- Rogers, J.N.; Rosenberg, J.N.; Guzman, B.J.; Oh, V.H.; Mimbela, L.E.; Ghassemi, A.; Betenbaugh, M.J.; Oyler, G.A.; Donohue, M.D. A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res. 2014, 4, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wiley, P.E.; Campbell, E.J.; McKuin, B. Production of Biodiesel and Biogas from Algae: A Review of Process Train Options. Water Environ. Res. 2011, 83, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef] [Green Version]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Zhang, X. Microalgae Removal of CO2 from Flue Gas; IEA Clean Coal Centre: London, UK, 2015; Available online: https://www.usea.org/sites/default/files/042015_Microalgae%20removal%20of%20CO2%20from%20flue%20gas_ccc250.pdf (accessed on 8 January 2016).
- Iasimone, F.; de Felice, V.; Panico, A.; Pirozzi, F. Experimental study for the reduction of CO2 emissions in wastewater treatment plant using microalgal cultivation. J. CO2 Util. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.-M.; Ling, T.C.; Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2017, 1–15. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Lammers, P.J.; van Voorhies, W. Feasibility of algal systems for sustainable wastewater treatment. Renew. Energy 2015, 82, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Collet, P.; Lardon, L.; Helias, A.; Bricout, S.; Lombaert-Valot, I.; Perrier, B.; Lepine, O.; Steyer, J.-P.; Bernard, O. Biodiesel from microalgae—Life cycle assessment and recommendations for potential improvements. Renew. Energy 2014, 71, 525–533. [Google Scholar] [CrossRef]
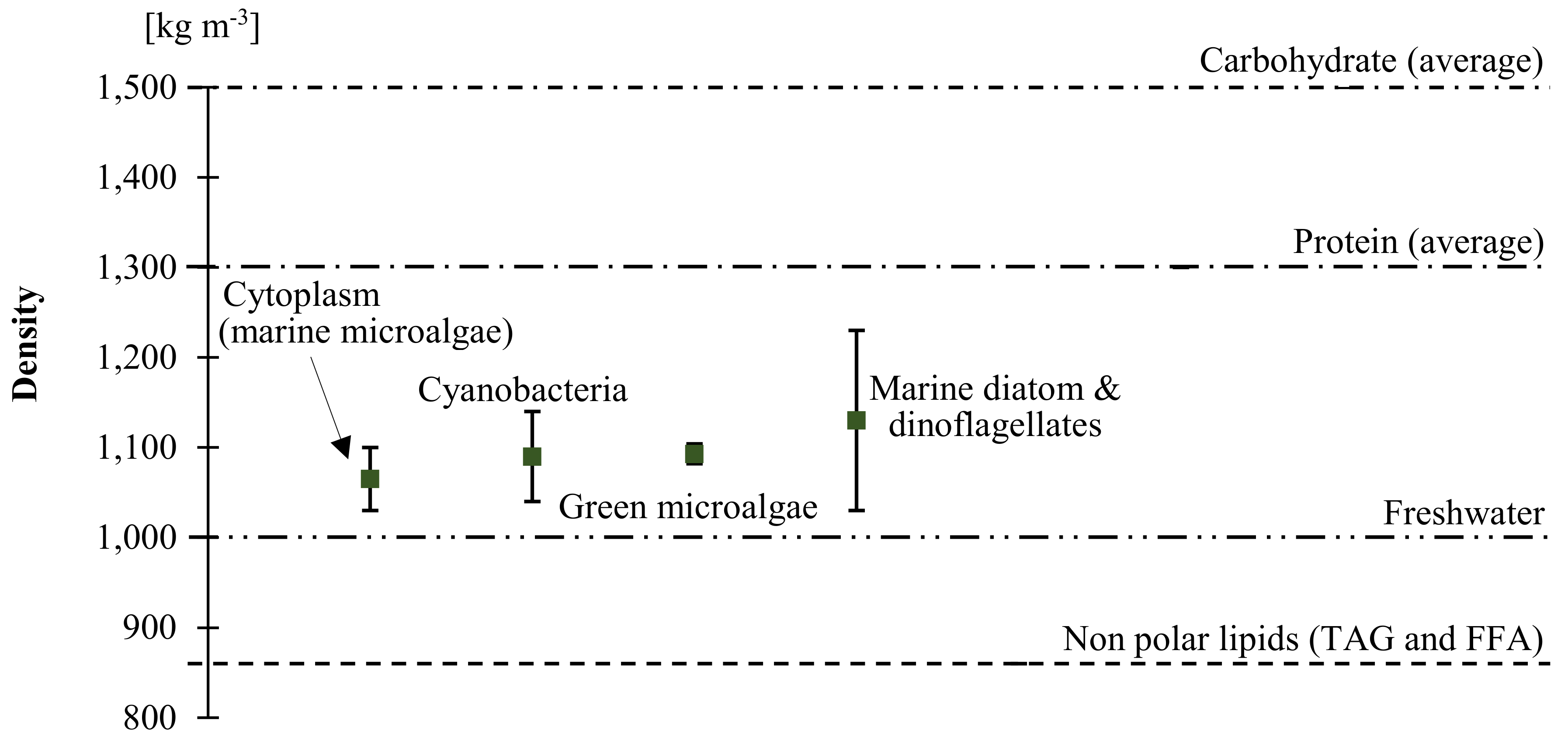

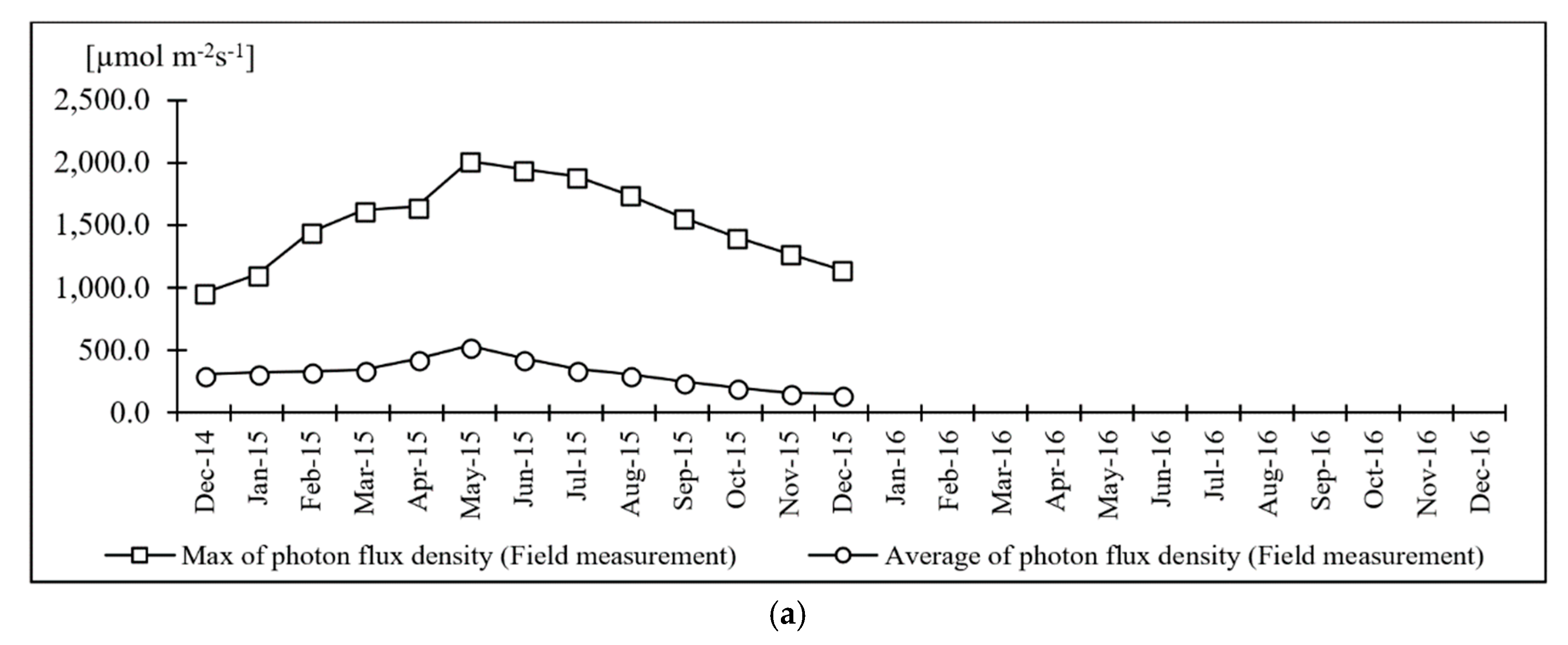
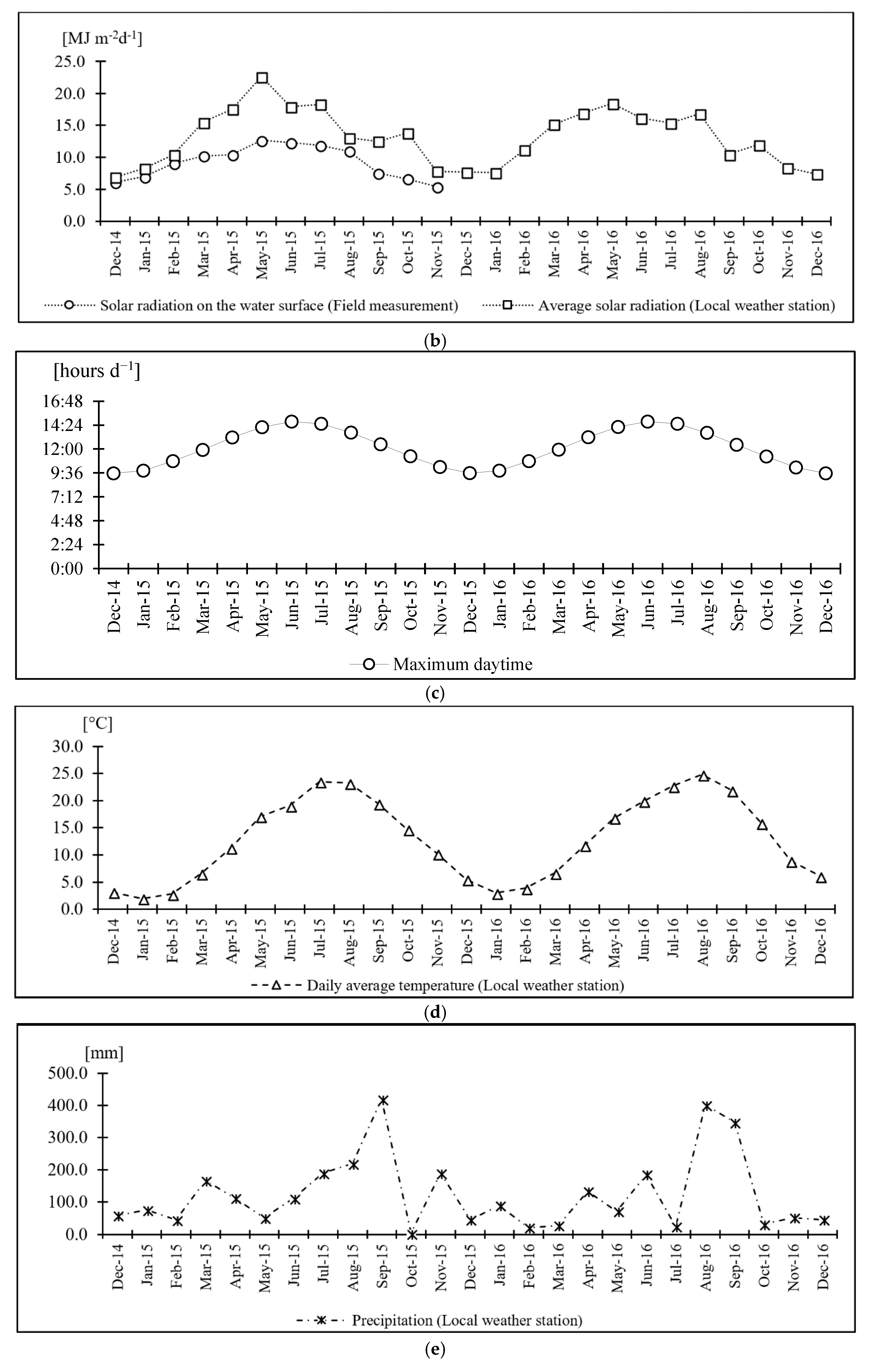
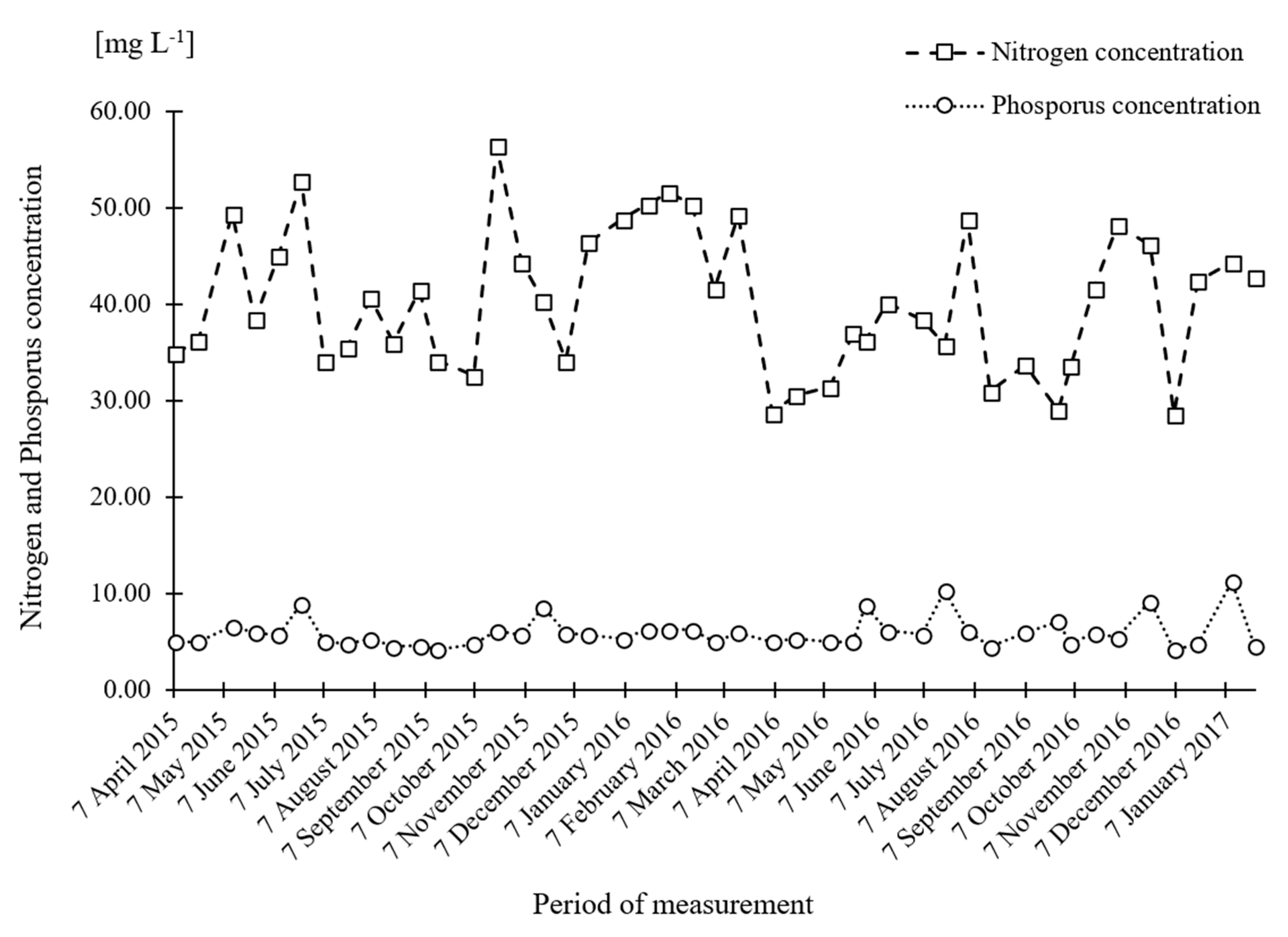



| Medium K Reagent List | Concentration of Selected Nutrients | Medium K [mg L−1] | Unit Cost [JPY g−1] | Cultivation Cost [JPY ha−1 7d−1] | Total [JPY ha−1y−1] |
|---|---|---|---|---|---|
| Commercially supplied fertilizer liquid (Megasoru 2 gou ®) | N: 0.11 (TN 11.0%, NO3−N 9.50%, NH4−N 1.50%) P: 0.09 × 0.44 (P2O5:142.00 g mol−1, 9.00%) | 55.00 | 0.564 | 93,060 | 4,852,414 |
| Potassium nitrate (KNO3) | 101.10 g mol−1, N:0.14 | 25.00 | 3.330 | 249,750 | 13,022,679 |
| Ammonium nitrate (NH4NO3) | 80.00 g mol−1, N:0.35 | 9.50 | 3.780 | 107,730 | 5,617,350 |
| Magnesium sulfate heptahydrate (MgSO4·7H2O) | 246.47 g mol−1 | 7.00 | 1.890 | 39,690 | 2,069,550 |
| Magnesium sulfate hexahydrate (MgSO4·6H2O) | 228.46 g mol−1 | 57.15 | 4.770 | 817,817 | 42,643,289 |
| Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) | 164.10 g mol−1 N:0.17 | 58.00 | 3.600 | 626,400 | 32,662,286 |
| EDTA Fe (C10H13FeN2O8) | 345.07 g mol−1 | 2.00 | 1.150 | 5865 | 305,818 |
| TOTAL COST | 1,940,312 | 101,173,385 |
| Parameter | (1) Tolerable Limit for MWWTP in Japan * [mg L−1] | (2) Minami-Soma (Primary MWWTP) 2015/2016 [mg L−1] | (3) Supernatant from Dehydration Process [mg L−1] | |
|---|---|---|---|---|
| 2015 | 2016 | |||
| TOC | - | - | - | - |
| COD | 160.0 | 137.0 | 139.0 | 10.40 |
| BOD | 160.0 | 217.0 | 214.0 | - |
| VFA (volatile fatty acid as acetate) | 30.0 | - | - | - |
| TN | 120.0 | 42.7 | 37.4 | 0.72 |
| Nitrate + Nitrite N NO3− | 0.2 | - | - | 0.36 |
| TP | 16.0 | 5.8 | 6.3 | 0.03 |
| PO43− | 10.0 | - | - | 0.04 |
| TSS | 200.0 | 211.0 | 207.0 | 290.00 |
| VSS | 320.0 | - | - | 85.00 |
| pH | 7.3 | 7.3 | 7.2 | 10.60 |
| Unit | Installation Price [JPY] | Pressure Design [MPa] | Temp Design [°C] | Power Requirement [kW] | Estimated Volume Rate | Estimated Biomass Produced [kg h−1] |
|---|---|---|---|---|---|---|
| Pump system in (centrifugal pump) | 2,078,000 | 0.35 | 25 | 1.53 | 12.00 m3 h−1 | – |
| ORP—Paddlewheel system | 8,474,400 | 0.10 | 25 | 51.56 | 300.00 m3 × 10 unit | 5.7–7.5 |
| Delivery pump system (centrifugal pump) | 2,140,000 | 0.35 | 25 | 1.64 | 12.00 m3 h−1 | 5.7–7.5 |
| Centrifugation (disk stack) | 20,000,000 | 0.10 | 27 | 12.00 | 11.95 m3 h−1 | 5.6–7.4 |
| Slurry pump system (centrifugal pump) | 867,000 | 0.35 | 25 | 0.03 | 0.25 m3 h−1 | 5.6–7.4 |
| Dehydration (Belt filter press) | 6,000,000 | 0.08 | 25 | 1.10 | 0.93 m2 | 5.1–7.1 |
| Low pressure HTL reactor | 40,000,000 | 20.00 | 250 | 16.58 | 23.00 L (estimated size) | 0.51–0.67 L h−1 (bio-crude) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasongko, N.A.; Noguchi, R.; Ito, J.; Demura, M.; Ichikawa, S.; Nakajima, M.; Watanabe, M.M. Engineering Study of a Pilot Scale Process Plant for Microalgae-Oil Production Utilizing Municipal Wastewater and Flue Gases: Fukushima Pilot Plant. Energies 2018, 11, 1693. https://doi.org/10.3390/en11071693
Sasongko NA, Noguchi R, Ito J, Demura M, Ichikawa S, Nakajima M, Watanabe MM. Engineering Study of a Pilot Scale Process Plant for Microalgae-Oil Production Utilizing Municipal Wastewater and Flue Gases: Fukushima Pilot Plant. Energies. 2018; 11(7):1693. https://doi.org/10.3390/en11071693
Chicago/Turabian StyleSasongko, Nugroho Adi, Ryozo Noguchi, Junko Ito, Mikihide Demura, Sosaku Ichikawa, Mitsutoshi Nakajima, and Makoto M. Watanabe. 2018. "Engineering Study of a Pilot Scale Process Plant for Microalgae-Oil Production Utilizing Municipal Wastewater and Flue Gases: Fukushima Pilot Plant" Energies 11, no. 7: 1693. https://doi.org/10.3390/en11071693




