Design, Construction, and Testing of a Gasifier-Specific Solid Oxide Fuel Cell System
Abstract
:1. Introduction
2. Selection of the Design of SOFC System
2.1. SOFC Materials
2.2. Carbon Suppression Technique
2.3. SOFC Temperature Control Strategy
2.4. Thermal Management
3. Description of the SOFC System
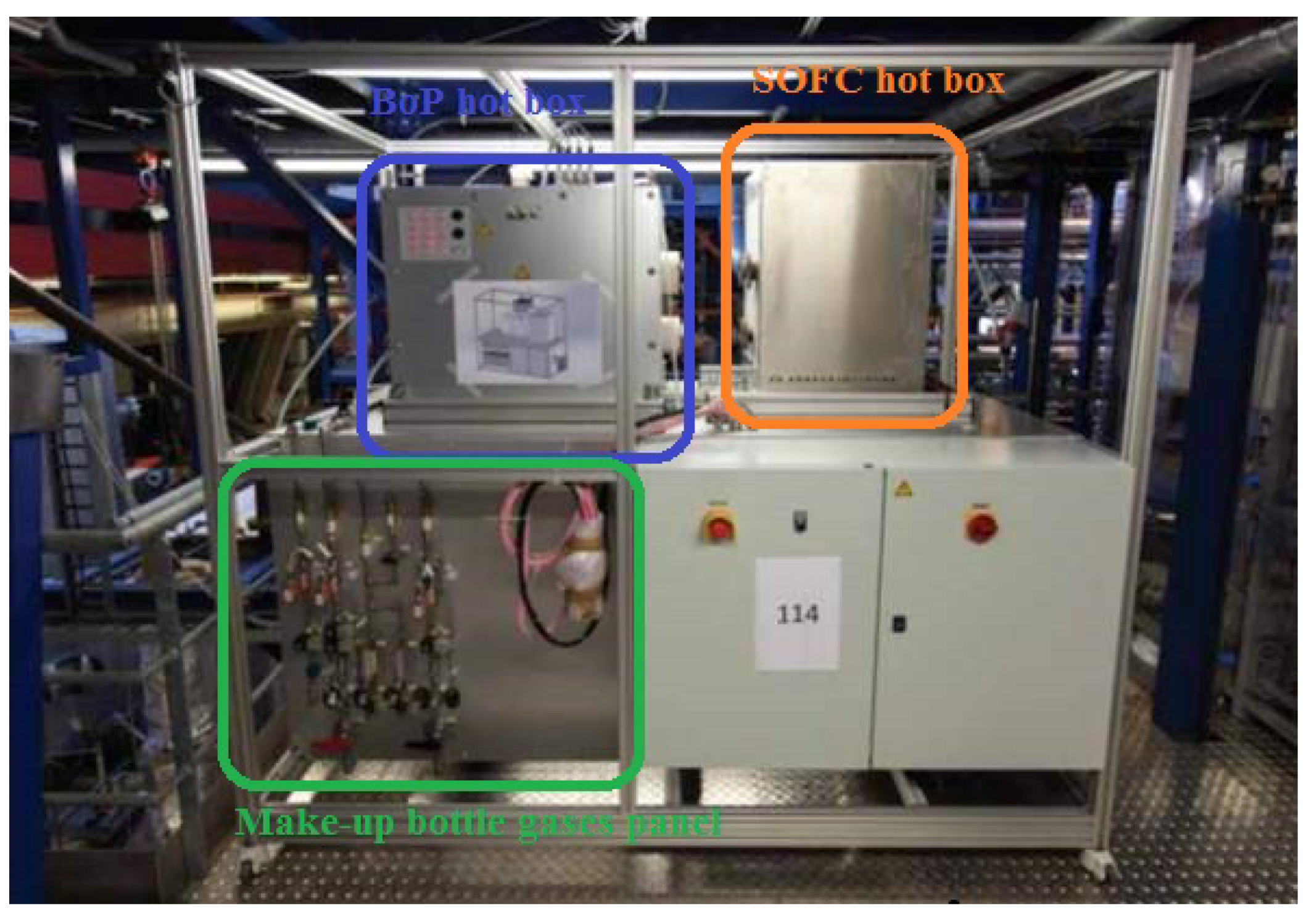
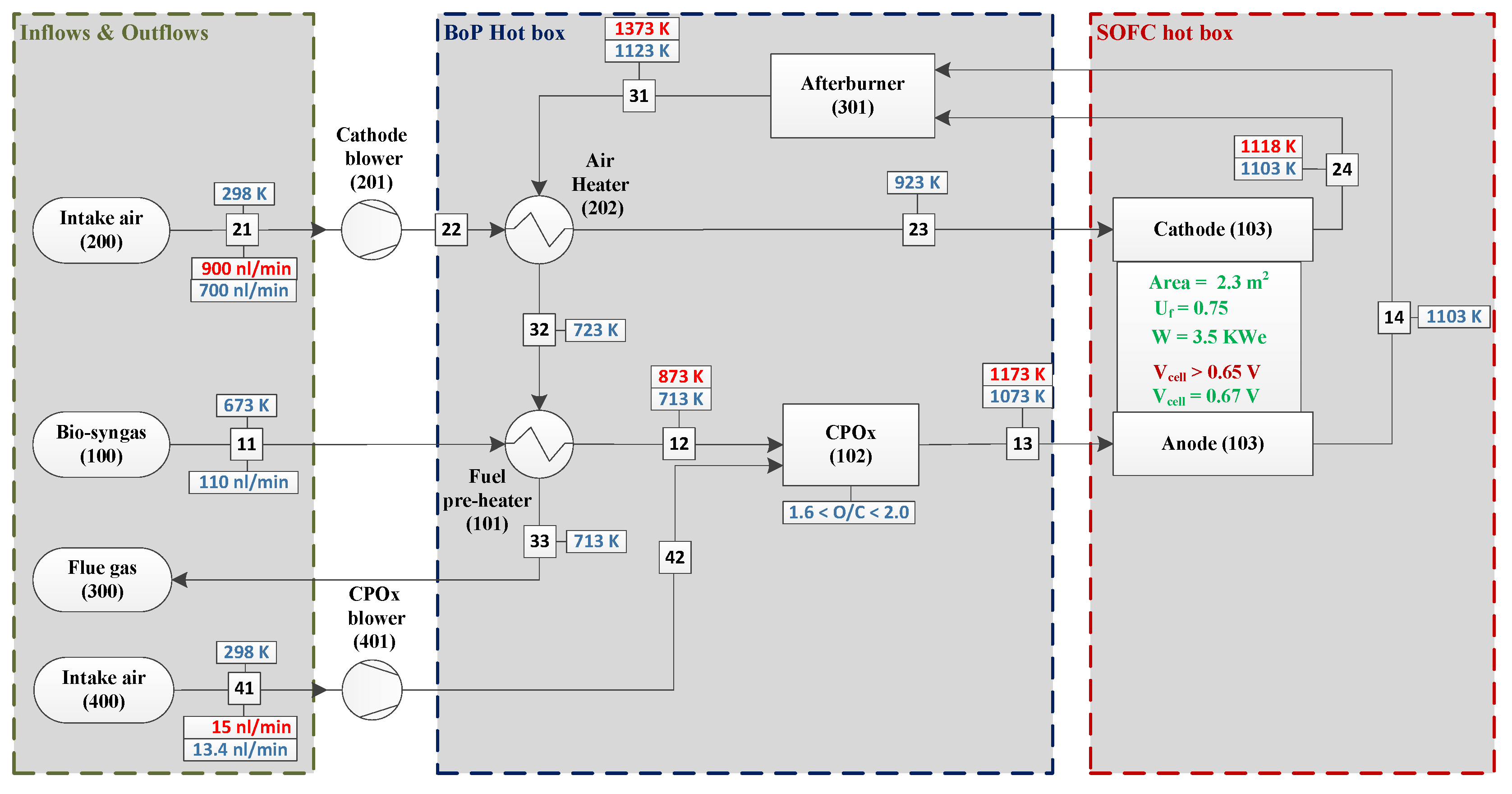
3.1. System Construction Design
3.2. Safe Operation Parameters
4. System Modelling
5. Test Runs with the SOFC System
- Constant SOFC fuel utilization of 0.75 0.01,
- Constant O/C ratio of 1.6 0.002 in the CPOx unit,
- Constant outlet temperature of 1073 10 K at the SOFC cathode.
6. Results and Discussion
6.1. Thermodynamic Comparison for Different Syngas Supply Conditions and SOFC Temperatures
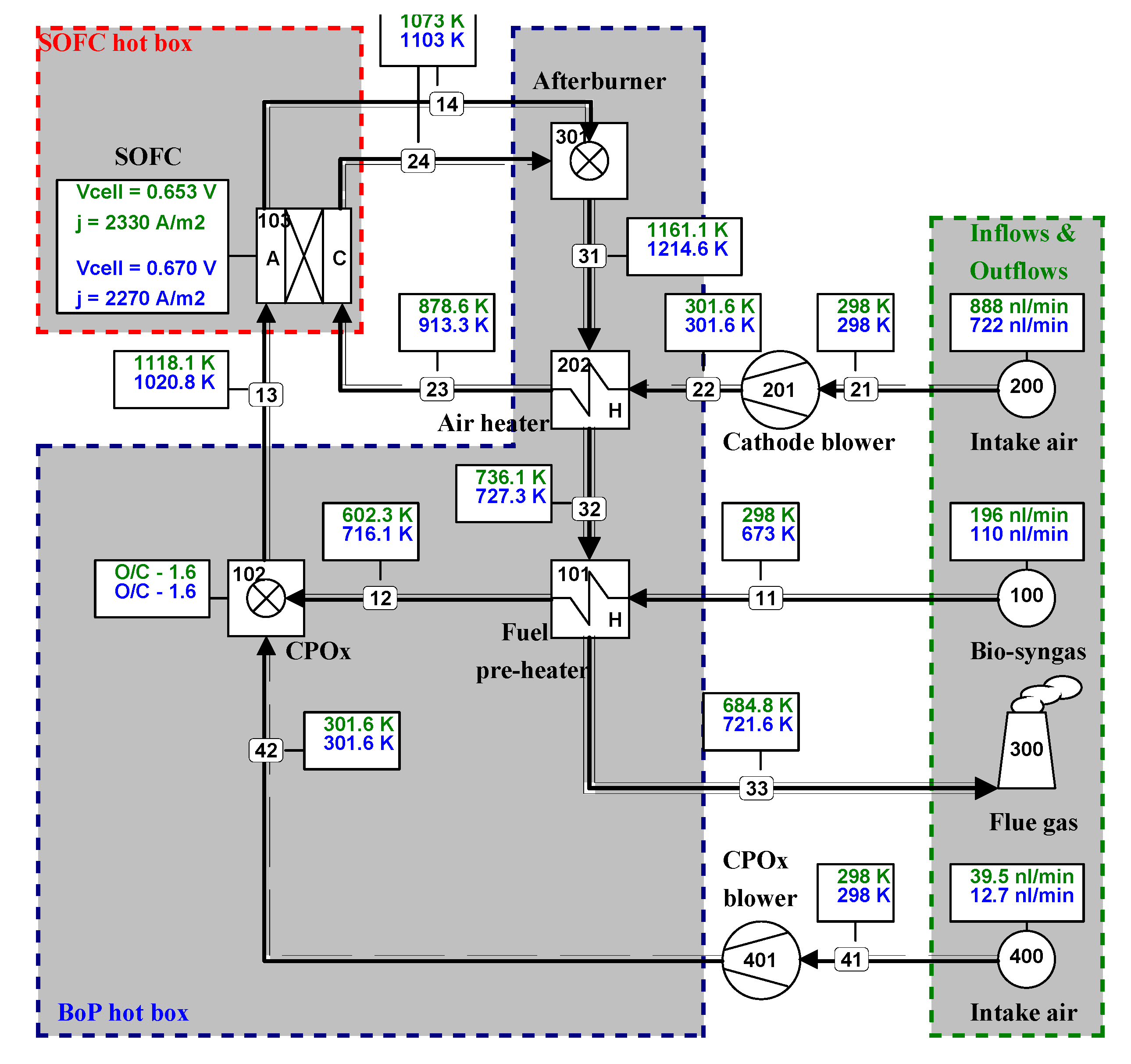
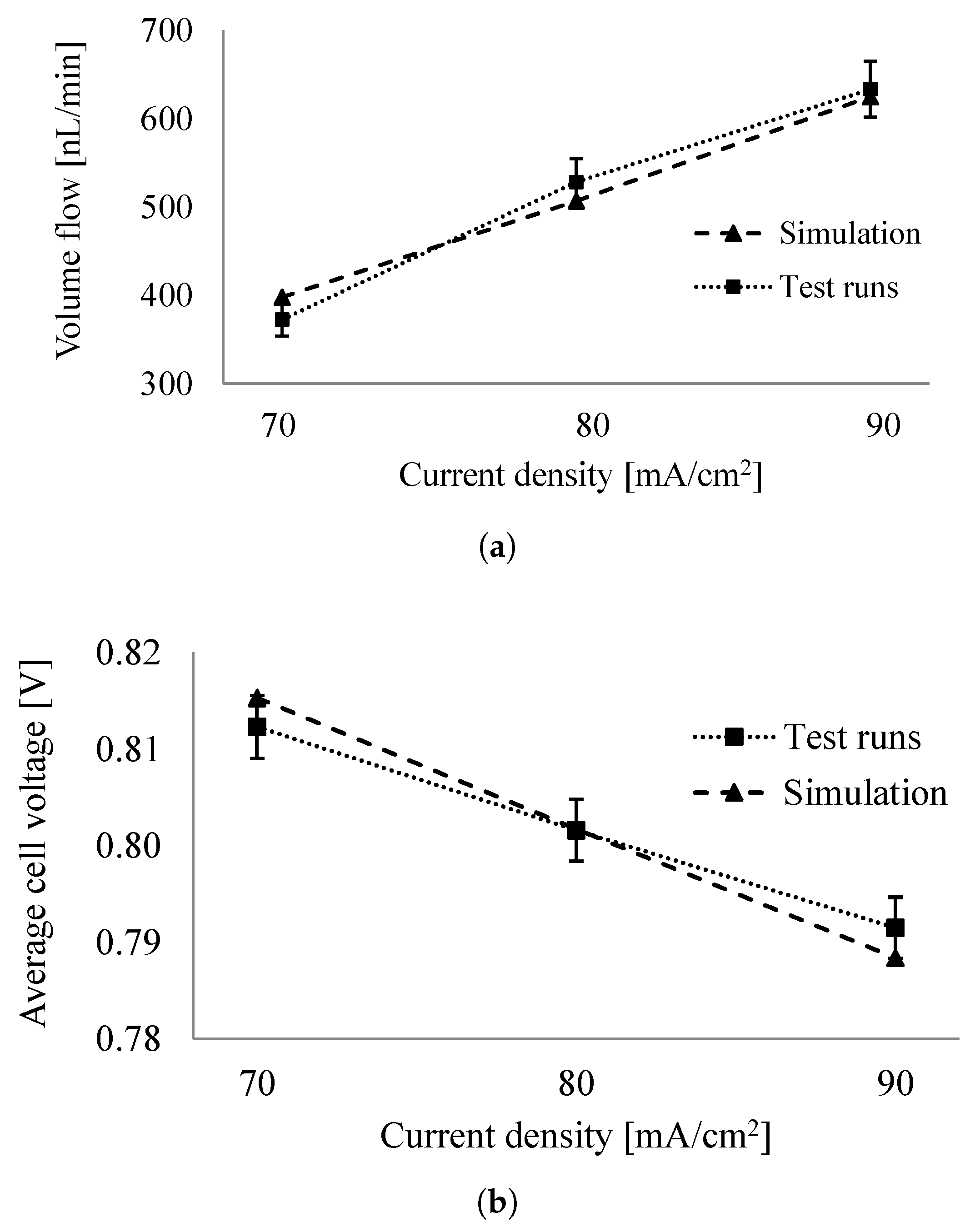
6.2. Comparison of System Simulation with Test Runs
6.3. System Efficiency
7. Future Considerations for Improving the Design
8. Summary
- System development: this is the first study to describe the design, development, and testing of an SOFC system to be integrated with a gasifier. The gasifier considered in this study is a plasma reactor with the capacity to process 8.84 kW of human waste (before pre-drying) [22,48]. The SOFC system was designed based on discussions between TU Delft and Sunfire GmbH.
- Calculated results exhibited good agreement with experimentally recorded values under different operating conditions. This clearly demonstrates the advantage of a rigorous thermodynamic model of new fuel cell power systems when they are being designed and built.
- The validated model clearly indicates where the thermodynamic losses are occurring and provides indications on how to minimize these losses in such a system, resulting in improved designs in the future.
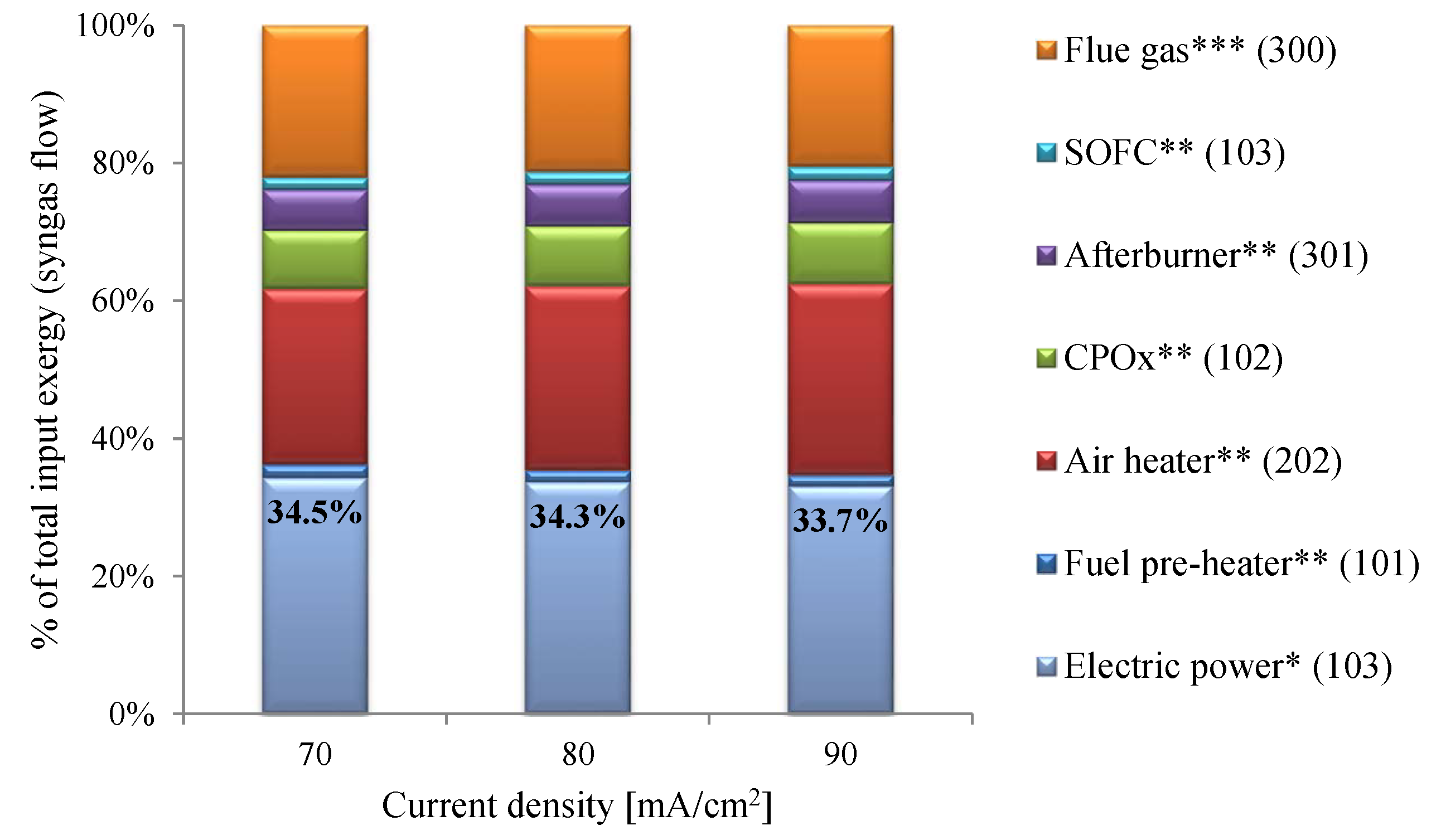
- System efficiencies of 33.7–34.5% were estimated. The CPOx unit and heat exchangers, especially the air heater, were identified as the major contributors to reductions in efficiency.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eurostat. The Key to European Statistics. 2017. Available online: http://ec.europa.eu/eurostat/web/energy/data/database (accessed on 22 April 2017).
- Guercio, A.; Bini, R. 15—Biomass-fired Organic Rankine Cycle combined heat and power systems. In Organic Rankine Cycle (ORC) Power Systems; Macchi, E., Astolfi, M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 527–567. [Google Scholar] [CrossRef]
- Hurskainen, M.; Vainikka, P. 7—Technology options for large-scale solid-fuel combustion. In Fuel Flexible Energy Generation; Oakey, J., Ed.; Woodhead Publishing: Boston, MA, USA, 2016; pp. 177–199. [Google Scholar] [CrossRef]
- Chacartegui, R.; Sanchez, D.; de Escalona, J.M.; Monje, B.; Sanchez, T. On the effects of running existing combined cycle power plants on syngas fuel. Fuel Process. Technol. 2012, 103, 97–109. [Google Scholar] [CrossRef]
- Asadullah, M. Barriers of commercial power generation using biomass gasification gas: A review. Renew. Sustain. Energy Rev. 2014, 29, 201–215. [Google Scholar] [CrossRef]
- Santhanam, S.; Schilt, C.; Turker, B.; Woudstra, T.; Aravind, P. Thermodynamic modeling and evaluation of high efficiency heat pipe integrated biomass Gasifier-Solid Oxide Fuel Cells-Gas Turbine systems. Energy 2016, 109, 751–764. [Google Scholar] [CrossRef]
- Bellomare, F.; Rokni, M. Integration of a municipal solid waste gasification plant with solid oxide fuel cell and gas turbine. Renew. Energy 2013, 55, 490–500. [Google Scholar] [CrossRef]
- Singh, J. Management of the agricultural biomass on decentralized basis for producing sustainable power in India. J. Clean. Prod. 2017, 142, 3985–4000. [Google Scholar] [CrossRef]
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Aziz, A.R.A.; Sulaiman, S.A. Study of syngas combustion parameters effect on internal combustion engine. Asian J. Sci. Res. 2013, 6, 187–196. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Ottaviano, A. Part load operation of SOFC/GT hybrid systems: Stationary analysis. Int. J. Hydrogen Energy 2012, 37, 16140–16150. [Google Scholar] [CrossRef]
- Andersson, D.; Aberg, E.; Yuan, J.; Sunden, B.; Eborn, J. Dynamic Modeling of a Solid Oxide Fuel Cell System in Modelica. In Proceedings of the ASME 2010 8th International Conference on Fuel Cell Science, Engineering and Technology, Brooklyn, NY, USA, 14–16 June 2010; American Society of Mechanical Engineers: New York, NY, USA, 2010; pp. 65–72. [Google Scholar] [Green Version]
- Rokni, M. Performance comparison on repowering of a steam power plant with gas turbines and solid oxide fuel cells. Energies 2016, 9, 399. [Google Scholar] [CrossRef] [Green Version]
- Ugartemendia, J.; Ostolaza, J.X.; Zubia, I. Operating point optimization of a hydrogen fueled hybrid solid oxide fuel cell-steam turbine (SOFC-ST) plant. Energies 2013, 6, 5046–5068. [Google Scholar] [CrossRef]
- Chung, T.D.; Hong, W.T.; Chyou, Y.P.; Yu, D.D.; Lin, K.F.; Lee, C.H. Efficiency analyses of solid oxide fuel cell power plant systems. Appl. Therm. Eng. 2008, 28, 933–941. [Google Scholar] [CrossRef]
- Chitsaz, A.; Mahmoudi, S.M.S.; Rosen, M.A. Greenhouse gas emission and exergy analyses of an integrated trigeneration system driven by a solid oxide fuel cell. Appl. Therm. Eng. 2015, 86, 81–90. [Google Scholar] [CrossRef]
- Stamatis, A.; Vinni, C.; Bakalis, D.; Tzorbatzoglou, F.; Tsiakaras, P. Exergy analysis of an intermediate temperature solid oxide fuel cell-gas turbine hybrid system fed with ethanol. Energies 2012, 5, 4268–4287. [Google Scholar] [CrossRef]
- Xu, H.; Dang, Z.; Bai, B.F. Analysis of a 1 kW residential combined heating and power system based on solid oxide fuel cell. Appl. Therm. Eng. 2013, 50, 1101–1110. [Google Scholar] [CrossRef]
- Somekawa, T.; Nakamura, K.; Kushi, T.; Kume, T.; Fujita, K.; Yakabe, H. Examination of a high-efficiency solid oxide fuel cell system that reuses exhaust gas. Appl. Therm. Eng. 2017, 114, 1387–1392. [Google Scholar] [CrossRef]
- Huerta, G.V.; Álvarez Jordán, J.; Dragon, M.; Leites, K.; Kabelac, S. Exergy analysis of the diesel pre-reforming solid oxide fuel cell system with anode off-gas recycling in the SchIBZ project. Part I: Modeling and validation. Int. J. Hydrogen Energy, 2018. [Google Scholar] [CrossRef]
- D’Andrea, G.; Gandiglio, M.; Lanzini, A.; Santarelli, M. Dynamic model with experimental validation of a biogas-fed SOFC plant. Energy Convers. Manag. 2017, 135, 21–34. [Google Scholar] [CrossRef]
- Liu, M.; Woudstra, T.; Promes, E.; Restrepo, S.; Aravind, P. System development and self-sustainability analysis for upgrading human waste to power. Energy 2014, 68, 377–384. [Google Scholar] [CrossRef]
- Din, Z.U.; Zainal, Z. The fate of SOFC anodes under biomass producer gas contaminants. Renew. Sustain. Energy Rev. 2016. [Google Scholar] [CrossRef]
- Cassidy, M.; Connor, P.; Irvine, J.; Savaniu, C. 5—Anodes. In High-Temperature Solid Oxide Fuel Cells for the 21st Century (Second Edition), 2nd ed.; Kendall, K., Kendall, M., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 133–160. [Google Scholar] [CrossRef]
- Kuramoto, K.; Hosokai, S.; Matsuoka, K.; Ishiyama, T.; Kishimoto, H.; Yamaji, K. Degradation behaviors of SOFC due to chemical interaction between Ni-YSZ anode and trace gaseous impurities in coal syngas. Fuel Process. Technol. 2017, 160, 8–18. [Google Scholar] [CrossRef]
- Liu, M.; van der Kleij, A.; Verkooijen, A.; Aravind, P. An experimental study of the interaction between tar and SOFCs with Ni/GDC anodes. Appl. Energy 2013, 108, 149–157. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.P.; He, H.Q.; Chen, X.; Ma, J.; Song, X.C. A comparative study of H2S poisoning on electrode behavior of Ni/YSZ and Ni/GDC anodes of solid oxide fuel cells. Int. J. Hydrogen Energy 2010, 35, 12359–12368. [Google Scholar] [CrossRef]
- GmbH, S. Power Core—Efficient Electricity Generator. 2017. Available online: http://www.sunfire.de/en/products-technology/power-core (accessed on 6 October 2017).
- Delft, T. Cycle-Tempo 5.0. 2015. Available online: http://www.asimptote.nl/software/cycle-tempo/ (accessed on 22 September 2017).
- De Groot, A. Advanced Energy Analysis of High Temperature Fuel Cell Systems. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2004. [Google Scholar]
- Miedema, J.A. CYCLE: A general computer code for thermodynamic cycle computations. Studies of cogeneration in district heating systems. In NASA STI/Recon Technical Report N; NASA: Washington, DC, USA, 1981; Volume 82. [Google Scholar]
- Suwanwarangkul, R.; Croiset, E.; Entchev, E.; Charojrochkul, S.; Pritzker, M.; Fowler, M.; Douglas, P.; Chewathanakup, S.; Mahaudom, H. Experimental and modeling study of solid oxide fuel cell operating with syngas fuel. J. Power Sources 2006, 161, 308–322. [Google Scholar] [CrossRef]
- Baldinelli, A.; Cinti, G.; Desideri, U.; Fantozzi, F. Biomass integrated gasifier-fuel cells: Experimental investigation on wood syngas tars impact on NiYSZ-anode Solid Oxide Fuel Cells. Energy Convers. Manag. 2016, 128, 361–370. [Google Scholar] [CrossRef]
- Fernandes, A.; Woudstra, T.; van Wijk, A.; Verhoef, L.; Aravind, P. Fuel cell electric vehicle as a power plant and SOFC as a natural gas reformer: An exergy analysis of different system designs. Appl. Energy 2016, 173, 13–28. [Google Scholar] [CrossRef]
- Lee, Y.D.; Ahn, K.Y.; Morosuk, T.; Tsatsaronis, G. Exergetic and exergoeconomic evaluation of an SOFC-Engine hybrid power generation system. Energy 2018, 145, 810–822. [Google Scholar] [CrossRef]
- Hosseinpour, J.; Chitsaz, A.; Eisavi, B.; Yari, M. Investigation on performance of an integrated SOFC-Goswami system using wood gasification. Energy 2018, 148, 614–628. [Google Scholar] [CrossRef]
- Peters, R.; Blum, L.; Deja, R.; Hoven, I.; Tiedemann, W.; Küpper, S.; Stolten, D. Operation experience with a 20 kW SOFC system. Fuel Cells 2014, 14, 489–499. [Google Scholar] [CrossRef]
- Bloom Energy Corporation. Bloom Energy Server ES5—250 kW. Available online: https://www.bloomenergy.com/ (accessed on 8 July 2018).
- Noponen, M.; Hottinen, T.; Sandstrom, C. WFC20 Biogas Unit Operation. In Proceedings of the 9th European Solid Oxide Fuel Cell Forum, Lucerne, Switzerland, 29 June–2 July 2010; pp. 2–90. [Google Scholar]
- Halinen, M.; Rautanen, M.; Saarinen, J.; Pennanen, J.; Pohjoranta, A.; Kiviaho, J.; Pastula, M.; Nuttall, B.; Rankin, C.; Borglum, B. Performance of a 10 kW SOFC demonstration unit. ECS Trans. 2011, 35, 113–120. [Google Scholar]
- Halinen, M.; Pennanen, J.; Himanen, O.; Kiviaho, J.; Silvennoinen, P.; Backman, J.; Salminen, P.; Pohjoisranta, A. Sofc Power 2007–2012 Project—The Finnish SOFC System Research Flagship. J. Fuel Cell Technol. 2013, 13, 32–39. [Google Scholar]
- Payne, R.; Love, J.; Kah, M. Generating electricity at 60% electrical efficiency from 1–2 kWe SOFC products. ECS Trans. 2009, 25, 231–239. [Google Scholar]
- Suzuki, M.; Takuwa, Y.; Inoue, S.; Higaki, K. Durability verification of residential SOFC CHP system. ECS Trans. 2013, 57, 309–314. [Google Scholar] [CrossRef]
- Yoshida, H.; Seyama, T.; Sobue, T.; Yamashita, S. Development of residential SOFC CHP system with flatten tubular segmented-in-series cells stack. ECS Trans. 2011, 35, 97–103. [Google Scholar]
- Mai, A.; Iwanschitz, J.; Schuler, A.; Denzler, R.; Nerlich, V.; Schuler, A. Hexis and the SOFC System Galileo 1000 N—Past, Present, Future. In Proceedings of the 11th European SOFC SOE Forum A, Lucerne, Switzerland, 1–4 July 2014; pp. 3–11. [Google Scholar]
- Bucheli, O.; Bertoldi, M.; Modena, S.; Ravagni, A. Development and manufacturing of SOFC-based products at SOFCpower SpA. ECS Trans. 2013, 57, 81–88. [Google Scholar] [CrossRef]
- Pfeifer, T.; Chakradeo, A.; Ahire, N.; Baade, J.; Barthel, M.; Dosch, C.; Näke, R.; Hartmann, M. Development of a SOFC Power Generator for the Indian Market. Fuel Cells 2017, 17, 550–561. [Google Scholar] [CrossRef] [Green Version]
- Sturm, G.S.J.; Muñoz, A.N.; Aravind, P.V.; Stefanidis, G.D. Microwave-Driven Plasma Gasification for Biomass Waste Treatment at Miniature Scale. IEEE Trans. Plasma Sci. 2016, 44, 670–678. [Google Scholar] [CrossRef]


| Item | Id | Parameters | ||||
|---|---|---|---|---|---|---|
| Syngas | (100) | 15.6% H2, 18.3% CO, 6.1% CO2, 60% N2 (vol. basis) | ||||
| 298 K | ||||||
| SOFC | (103) | Req = 1.1 cm2 Area = 2300 cm2 | ||||
| T14,24 = 1073 K, Uf = 0.75 | ||||||
| Intake air | (200; 400) | 79% N2, 21% O2 (vol. basis) | ||||
| 101.3 kPa and 298 K | ||||||
| Air heater | (202) | 13.41 kg/s; 55.87 W/K; 1.45 | ||||
| Fuel pre-heater | (101) | 1.4 kg/s; 3.44 W/K; 0.8 | ||||
| Blowers | (201; 401) | ; | ||||
| All components | - | |||||
| Measurement Type | Measurement Device | Accuracy |
|---|---|---|
| Temperature | Thermocouples type N | 1.1 K or 0.4% of Rd 0.3% terminal (PLC) |
| Cell voltage | In-situ wiring | 0.3% signal analog input (PLC) |
| CPOx air flow | Mass flow controller | 0.5% of Rd 0.1 of Fs (16 nL/min) |
| H flow | Mass flow controller | 0.5% of Rd 0.1 of Fs (20 nL/min) |
| N flow | Mass flow controller | 0.5% of Rd 0.1 of Fs (20 nL/min) |
| CO flow | Mass flow controller | 0.8% of Rd 0.2 of Fs (5 nL/min) |
| CO flow | Mass flow controller | 1% of Rd 1% of Fs (1 nL/min) |
| Cathode air | Flow meter | 2% of Rd 0.3 signal analog input (PLC) |
| Current | Electronic load | 0.2% of Fs (160 A) |
| Simulation Considering Design Conditions | |||||||
|---|---|---|---|---|---|---|---|
| Pipe number | |||||||
| 11 | 13 | 14 | 23 | 24 | 31 | 41 | |
| mol % H2 | 26.95 | 21.70 | 5.57 | ||||
| mol % CO | 22.00 | 18.55 | 4.54 | ||||
| mol % CO2 | 7.01 | 8.01 | 22.05 | 3.90 | |||
| mol % N2 | 37.85 | 43.09 | 43.04 | 79.0 | 81.04 | 76.06 | 79.0 |
| mol % O2 | 21.0 | 18.96 | 15.57 | 21.0 | |||
| mol % H2O | 6.19 | 8.60 | 24.81 | 4.46 | |||
| Mole flow (mol/s) | 0.082 | 0.089 | 0.089 | 0.537 | 0.523 | 0.608 | 0.009 |
| Vol. flow (nL/min) | 110.0 | 119.9 | 119.9 | 721.8 | 703.3 | 817.2 | 12.7 |
| Exergy (kW) | 10,382 | 10,118 | 3984 | 4773 | 6958 | 10,287 | ~0 |
| Auxiliaries consumption = 0.092 kW | System electric efficiency = 32.8% | ||||||
| Simulation Considering Experimental Conditions | |||||||
| Pipe number | |||||||
| 11 | 13 | 14 | 23 | 24 | 31 | 41 | |
| mol % H2 | 15.60 | 8.71 | 2.23 | ||||
| mol % CO | 18.36 | 13.21 | 3.25 | ||||
| mol % CO2 | 6.10 | 7.84 | 17.80 | 4.38 | |||
| mol % N2 | 60.0 | 65.51 | 65.50 | 79.0 | 80.70 | 77.99 | 79.0 |
| mol % O2 | 21.0 | 19.30 | 14.82 | 21.0 | |||
| mol % H2O | 4.74 | 11.22 | 2.80 | ||||
| Mole flow (mol/s) | 0.146 | 0.169 | 0.169 | 0.661 | 0.647 | 0.811 | 0.029 |
| Vol. flow (nL/min) | 195.8 | 227.0 | 227.0 | 888.0 | 869.2 | 1090 | 39.5 |
| Exergy (kW) | 12,545 | 11,946 | 5166 | 5381 | 8129 | 12,606 | ~0 |
| Auxiliaries consumption = 0.116 kW | System electric efficiency = 27.0% | ||||||
| Apparatus/Pipe | Parameter | Units | Simulation | Test Runs |
|---|---|---|---|---|
| 11 | Syngas flow | nL/min | 75.6 | 75.7 0.54 |
| 21 | Cathode air flow | nL/min | 631.8 | 633.2 18 |
| 41 | CPOx air flow | nL/min | 15.3 | 15.3 0.09 |
| 103 | Cell voltage, | V | 0.789 | 0.791 0.0023 |
| 103 | SOFC power, | kW | 1.632 | 1.631 0.013 |
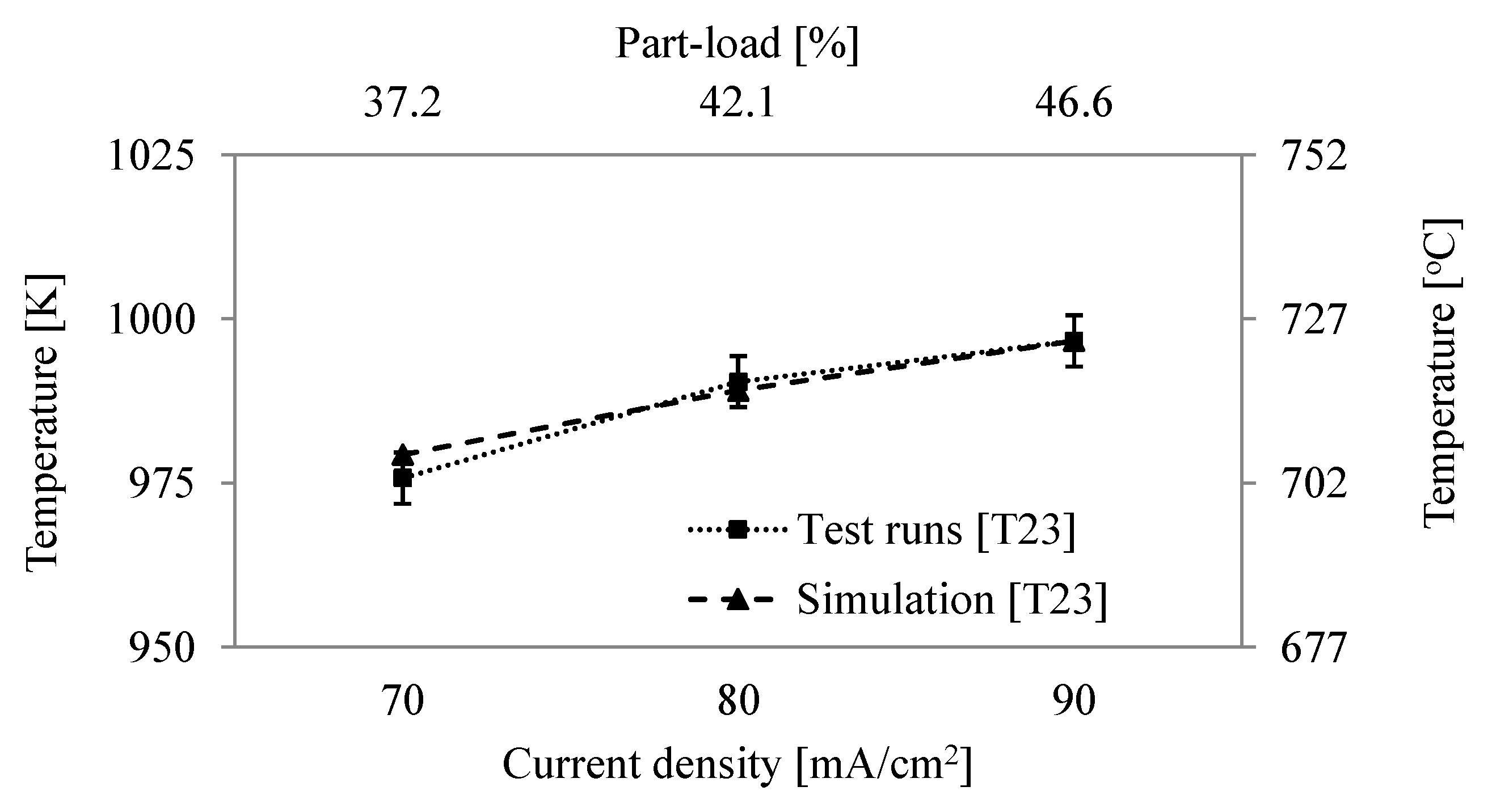
| 13 | Temperature after CPOx, | K | 1049.9 | 1065.4 5.5 |
| 23 | Cathode inlet temp., | K | 996.7 | 996.5 5.0 |
| 24 | Temperature cathode outlet, | K | 1073 | 1073 5.6 |
| 31 | Temp. after afterburner, | K | 1126.1 | 1127.8 6.0 |
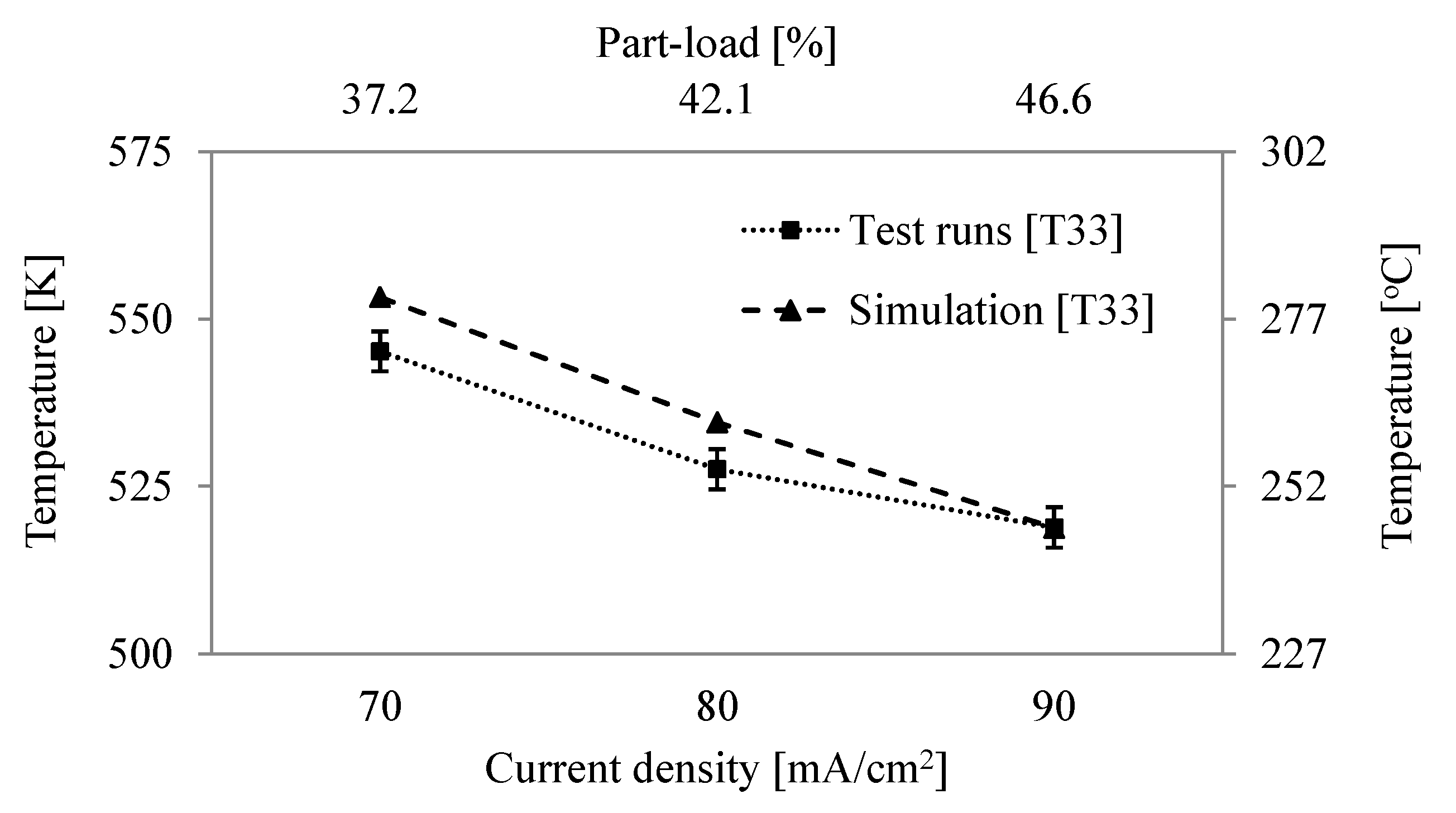
| 33 | Temp. exhaust flue gas, | K | 516.9 | 518.9 1.8 |
| System Manufacturer | Output Power (kW) | (%) | Fuel Processing | Ref. |
|---|---|---|---|---|
| Bloom energy | 250 AC | >53 | - | [38] |
| Wärtsilä | 24 DC | 47 | SR/AOGR | [39] |
| FZJ | 20 DC | 41 | External SR | [37] |
| VTT(2010) | 7 AC | 43 | SR/AOGR | [40] |
| VTT(2011) | 8 AC | 49 | SR/AOGR | [41] |
| CFCL | 1.5 AC | 60 | SR/WR | [42] |
| ENE-farm | 0.7 AC | 41–47 | SR/WR | [43,44] |
| Hexis | 1 AC | 35 | CPOx | [45] |
| SOFCpower | 1 AC | 32 | CPOx | [46] |
| IKTS | 1.26 AC | 39 | CPOx | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.; Brabandt, J.; Posdziech, O.; Saadabadi, A.; Recalde, M.; Fan, L.; Promes, E.O.; Liu, M.; Woudstra, T.; Aravind, P.V. Design, Construction, and Testing of a Gasifier-Specific Solid Oxide Fuel Cell System. Energies 2018, 11, 1985. https://doi.org/10.3390/en11081985
Fernandes A, Brabandt J, Posdziech O, Saadabadi A, Recalde M, Fan L, Promes EO, Liu M, Woudstra T, Aravind PV. Design, Construction, and Testing of a Gasifier-Specific Solid Oxide Fuel Cell System. Energies. 2018; 11(8):1985. https://doi.org/10.3390/en11081985
Chicago/Turabian StyleFernandes, Alvaro, Joerg Brabandt, Oliver Posdziech, Ali Saadabadi, Mayra Recalde, Liyuan Fan, Eva O. Promes, Ming Liu, Theo Woudstra, and Purushothaman Vellayan Aravind. 2018. "Design, Construction, and Testing of a Gasifier-Specific Solid Oxide Fuel Cell System" Energies 11, no. 8: 1985. https://doi.org/10.3390/en11081985
APA StyleFernandes, A., Brabandt, J., Posdziech, O., Saadabadi, A., Recalde, M., Fan, L., Promes, E. O., Liu, M., Woudstra, T., & Aravind, P. V. (2018). Design, Construction, and Testing of a Gasifier-Specific Solid Oxide Fuel Cell System. Energies, 11(8), 1985. https://doi.org/10.3390/en11081985




