Computational Fluid Dynamics Approach for Performance Prediction in a Zinc–Air Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Model of Zn–Air Fuel Cell
2.2. Comparison of Simulation and Experiment
2.3. Simulation Results of Mass Fraction
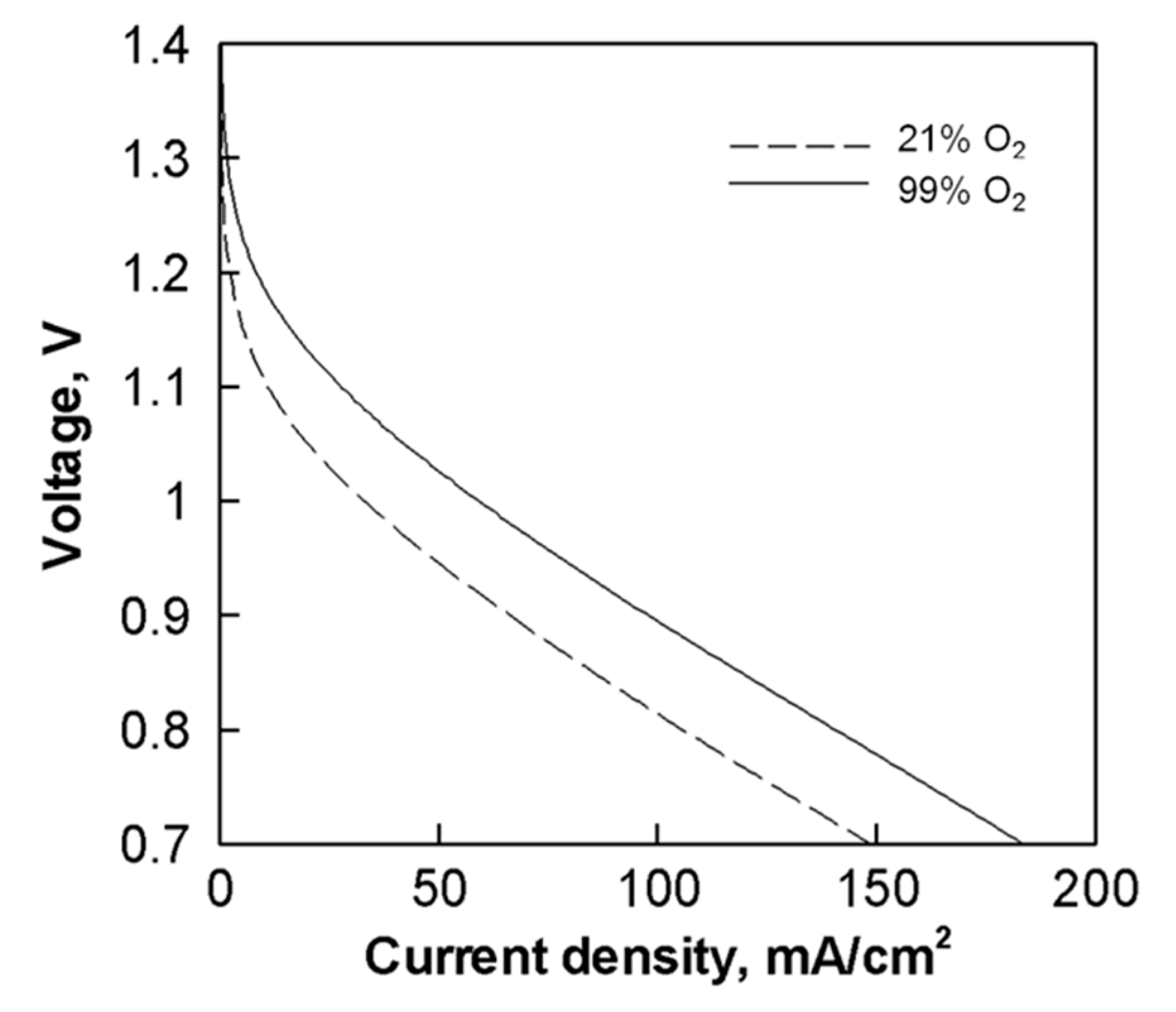
2.4. Effect of Oxidant Concentration
2.5. Effect of Electrolyte Concentration
3. Methods
3.1. Theoretical Model
3.2. Experimental Apparatus
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Nomenclature | |
| Mass diffusivity (m2·s−1) | |
| Faraday constant, 96,487 C·mol−1 | |
| Gas velocity vector (m·s−1) | |
| Pressure (Pa) | |
| Mass fraction of species | |
| Diffusion flux | |
| Source term in species equation | |
| Molecular weight (kg·kmol−1) | |
| Transfer current densities (Am−3) | |
| Universal gas constant, 8.314 J·mol−1·K−1 | |
| Temperature (K) | |
| Current density vector | |
| Greek letters | |
| Density (kg·m−3) | |
| Porosity | |
| Stress tensor (N·m−2) | |
| Permeability (m2) | |
| Transfer coefficients | |
| Conductivities | |
| Electric potentials | |
| Electrode overpotential | |
| Superscripts | |
| Bruggemann coefficient | |
| Concentration parameters | |
| Subscripts | |
| i | Species of anode, cathode, electrolyte, and air flow channel |
| j | Source term of species (O2, Zn, or ZnO) |
| Acronyms | |
| a | Anode |
| c | Cathode |
| F | Fluid phase |
| S | Solid phase |
| Abbreviations | |
| eff | Effective |
| ref | Reference |
References
- Rahman, M.A.; Wang, X.; Wen, C. High energy density metal-air batteries: A review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Panchal, S. Impact of Vehicle Charge and Discharge Cycles on the Thermal Characteristics of Lithium-ion Batteries. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2014. [Google Scholar]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Sathiyanarayanan, K.; Eom, S.W.; Yun, M.S. Novel alloys to improve the electrochemical behavior of zinc anodes for zinc/air battery. J. Power Sour. 2006, 160, 1436–1441. [Google Scholar] [CrossRef]
- Zhu, W.H.; Poole, B.A.; Cahela, D.R.; Tatarchuk, B.J. New structures of thin air cathodes for zinc–air batteries. J. Appl. Electrochem. 2003, 33, 29–36. [Google Scholar] [CrossRef]
- Zhu, A.L.; Wilkinson, D.P.; Zhang, X.; Xing, Y.; Rozhin, A.G.; Kulinich, S.A. Zinc regeneration in rechargeable zinc-air fuel cells: A review. J. Energy Storage 2016, 8, 35–50. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Cheiky, M.C.; Danczyk, L.G.; Wehrey, M.C. Rechargeable zinc-air batteries for electric vehicle applications. SAE Tech. Pap. 1990, 901516. [Google Scholar] [CrossRef]
- Cheiky, M.C.; Danczyk, L.G.; Scheffler, R.L. Zinc-air powered electric vehicle systems integration issues. SAE Tech. Pap. 1991, 910249. [Google Scholar] [CrossRef]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cycle life: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Choi, K.W.; Bennion, D.N.; Newman, J. Engineering analysis of shape change in zinc secondary electrodes I theoretical. J. Electrochem. Soc. 1976, 123, 1616–1627. [Google Scholar] [CrossRef]
- Sunu, W.G.; Bennion, D.N. Transient and failure analyses of the porous zinc electrode I theoretical. J. Electrochem. Soc. 1980, 127, 2007–2015. [Google Scholar] [CrossRef]
- Isaacson, M.J.; McLarnon, F.R.; Cairns, E.J. Current density and ZnO precipitation-dissolution distributions in Zn-ZnO porous electrodes and their effect on material redistribution: A two-dimensional mathematical model. J. Electrochem. Soc. 1990, 137, 2014–2021. [Google Scholar] [CrossRef]
- Mao, Z.; White, R.E. Mathematical modeling of a primary zinc/air battery. J. Electrochem. Soc. 1992, 139, 1105–1113. [Google Scholar] [CrossRef]
- Deiss, E.; Holzer, F.; Haas, O. Modeling of an electrically rechargeable alkaline Zn–air battery. Electrochim. Acta 2002, 47, 3995–4010. [Google Scholar] [CrossRef]
- Jung, C.Y.; Kim, T.H.; Kim, W.J.; Yi, S.C. Computational analysis of the zinc utilization in the primary zinc-air batteries. Energy 2016, 102, 694–704. [Google Scholar] [CrossRef]
- Stamm, J.; Varzi, A.; Latz, A.; Horstmann, B. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sour. 2017, 360, 136–149. [Google Scholar] [CrossRef] [Green Version]
- Schröder, D.; Krewer, U. Model based quantification of air-composition impact on secondary zinc air batteries. Electrochim. Acta 2014, 117, 541–553. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Transient electrochemical heat transfer modeling and experimental validation of a large sized LiFePO4/graphite battery. Int. J. Heat Mass Transf. 2017, 109, 1239–1251. [Google Scholar] [CrossRef]
- Um, S.; Wang, C.Y.; Chen, K.S. Computational fluid dynamics modeling of proton exchange membrane fuel cells. J. Electrochem. Soc. 2000, 147, 4485–4493. [Google Scholar] [CrossRef]
- Giridharan, M.G.; Krishnan, A. An implicit numerical model for electrophoretic systems, Micro-Electro-Mechanical Systems (MEMS). In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Anaheim, CA, USA, 15–20 November 1998. [Google Scholar]
- Coltrin, M.E.; Kee, R.J.; Rupley, F.M. Surface chemkin: A general formalism and software for analyzing heterogeneous chemical kinetics at a gas-surface interface. Int. J. Chem. Kinet. 1991, 23, 1111–1128. [Google Scholar] [CrossRef]
- CFD-ACE(U)® V2004 User’s Manual; ESI-CFD Inc.: Huntsville, AL, USA, 2004; Available online: www.cfdrc.com (accessed on 18 August 2018).
- Song, H.; Xu, X.Z.; Li, F. Numerical simulation of discharge process and failure mechanisms of zinc electrode. Acta Phys. Chim. Sin. 2013, 29, 1961–1974. [Google Scholar]
- Xu, M.; Ivey, D.G.; Xie, Z.; Qu, W. Rechargeable Zn-air batteries: progress in electrolyte development and cell configuration advancement. J. Power Sour. 2015, 283, 358–371. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lin, H.H.; Lai, G.J. Numerical prediction of the effect of catalyst layer Nafion loading on the performance of PEM fuel cells. J. Power Sour. 2007, 164, 730–741. [Google Scholar] [CrossRef]
- Siu, S.; Evans, J.W. Flow and transport due to natural convection in a galvanic cell. J. Electrochem. Soc. 1997, 144, 2705–2711. [Google Scholar] [CrossRef]
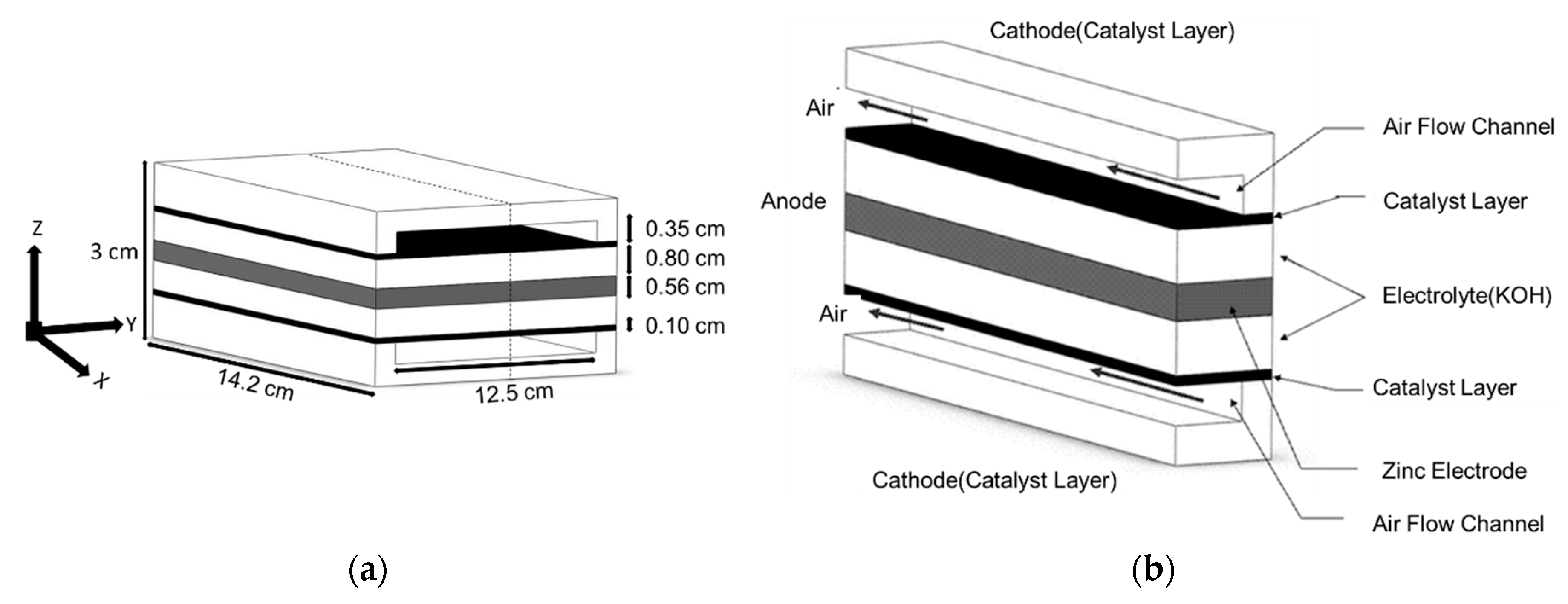

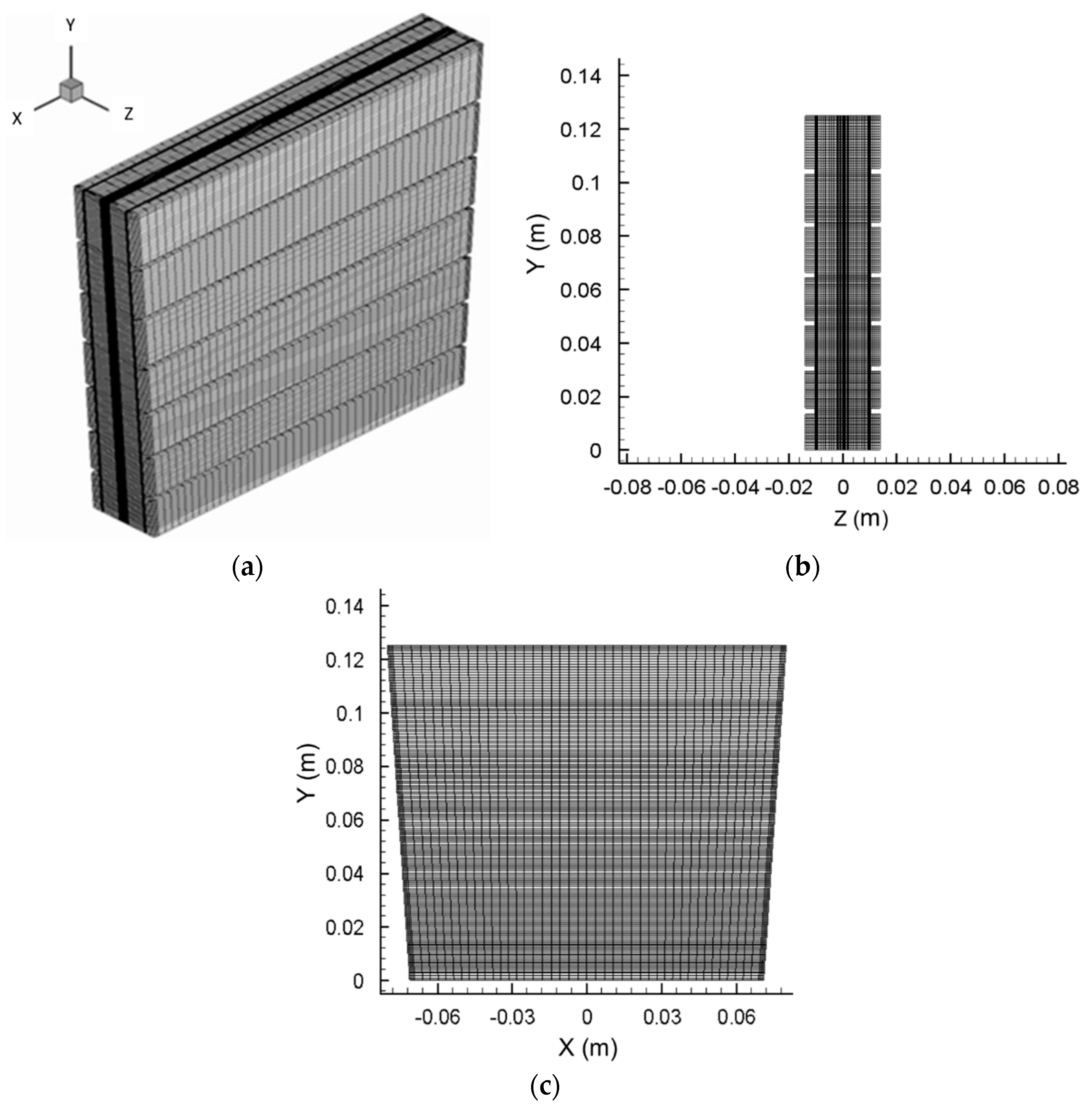





| Parameter | Symbol | Value | Unit | References |
|---|---|---|---|---|
| Gas channel length | 14.2–16 | cm | # | |
| Gas channel height | 1.6 | cm | # | |
| Gas channel width | 0.35 | cm | # | |
| Zn plate length | 14.2–16 | cm | # | |
| Zn plate height | 12.5 | cm | # | |
| Zn plate width | 0.56 | cm | # | |
| Catalyst layer thickness | 0.1 | cm | # | |
| Catalyst layer porosity | 0.4 | [27] | ||
| Zn plate porosity | 0.4 | [27] | ||
| Bruggemann coefficient | 1.5 | [27] | ||
| Transfer coefficients for anode | 0.5 | [27] | ||
| Transfer coefficients for cathode | 0.5 | [27] | ||
| Reference transfer current density at anode | 1 × 107 | Am−3 | [27] | |
| Reference transfer current density at cathode | 300 | Am−3 | [27] | |
| Anode concentration parameter | 1 | [27] | ||
| Cathode concentration parameter | 1 | [27] | ||
| Electronic conductivity of catalyst layer | 5.96 × 107 | Ω−1 m−1 | [27] | |
| Electronic conductivity of Zn | 1.66 × 107 | Ω−1 m−1 | [27] | |
| Cell operational temperature | T | 300 | K | [27] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.D.; Sangeetha, T.; Cheng, W.-F.; Lin, C.; Chen, P.-T. Computational Fluid Dynamics Approach for Performance Prediction in a Zinc–Air Fuel Cell. Energies 2018, 11, 2185. https://doi.org/10.3390/en11092185
Huang KD, Sangeetha T, Cheng W-F, Lin C, Chen P-T. Computational Fluid Dynamics Approach for Performance Prediction in a Zinc–Air Fuel Cell. Energies. 2018; 11(9):2185. https://doi.org/10.3390/en11092185
Chicago/Turabian StyleHuang, K. David, Thangavel Sangeetha, Wu-Fu Cheng, Chunyo Lin, and Po-Tuan Chen. 2018. "Computational Fluid Dynamics Approach for Performance Prediction in a Zinc–Air Fuel Cell" Energies 11, no. 9: 2185. https://doi.org/10.3390/en11092185





