Studying the Effectiveness of Polyacrylamide (PAM) Application in Hydrocarbon Reservoirs at Different Operational Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Solution Preparation
2.2. Ageing and Viscosity Measurement of PAM
2.3. Measurement of Extent of Hydrolysis of PAM
3. Results and Discussion
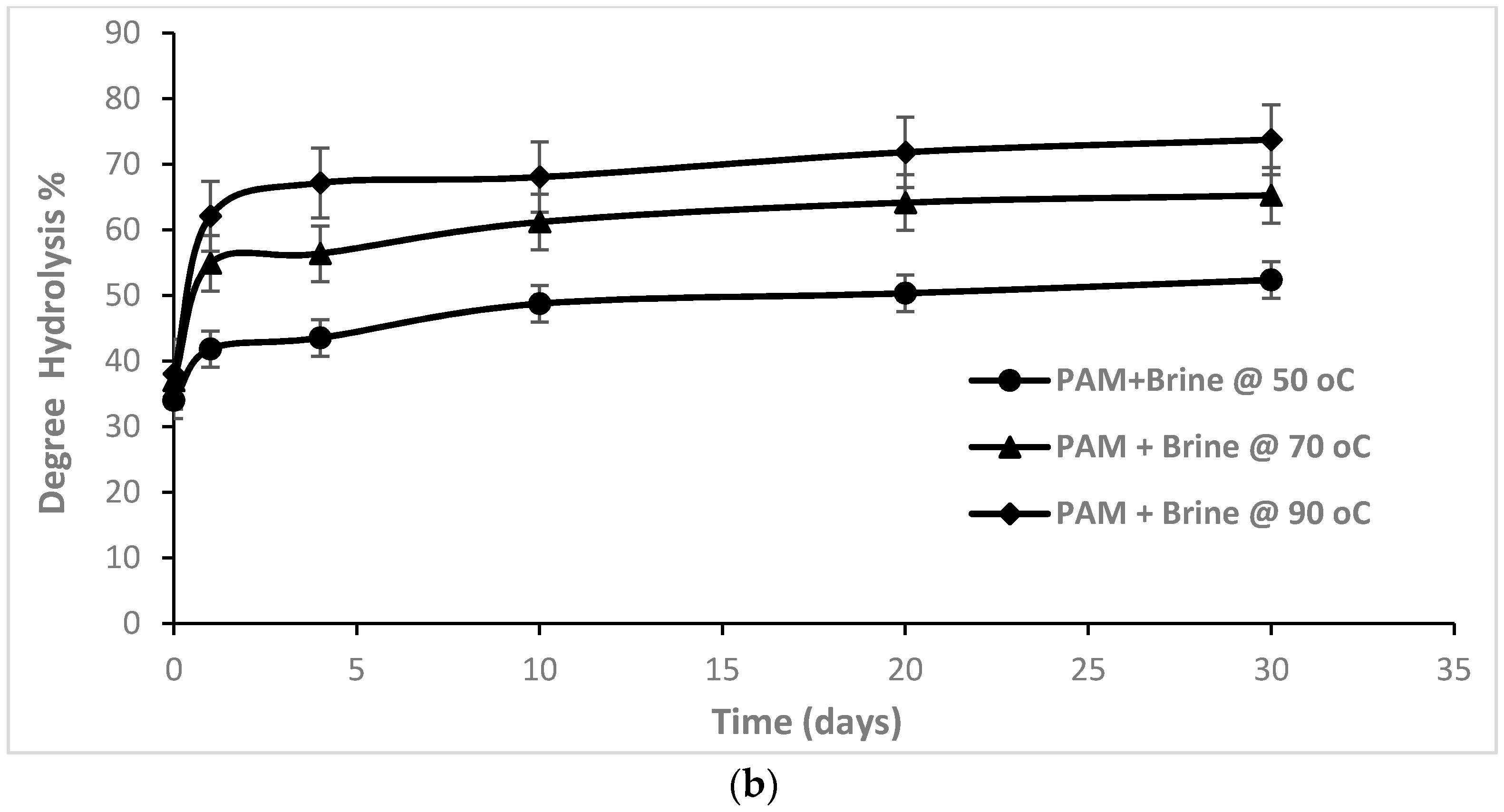
3.1. Degree of hydrolysis of PAM in Thermally Aged Samples
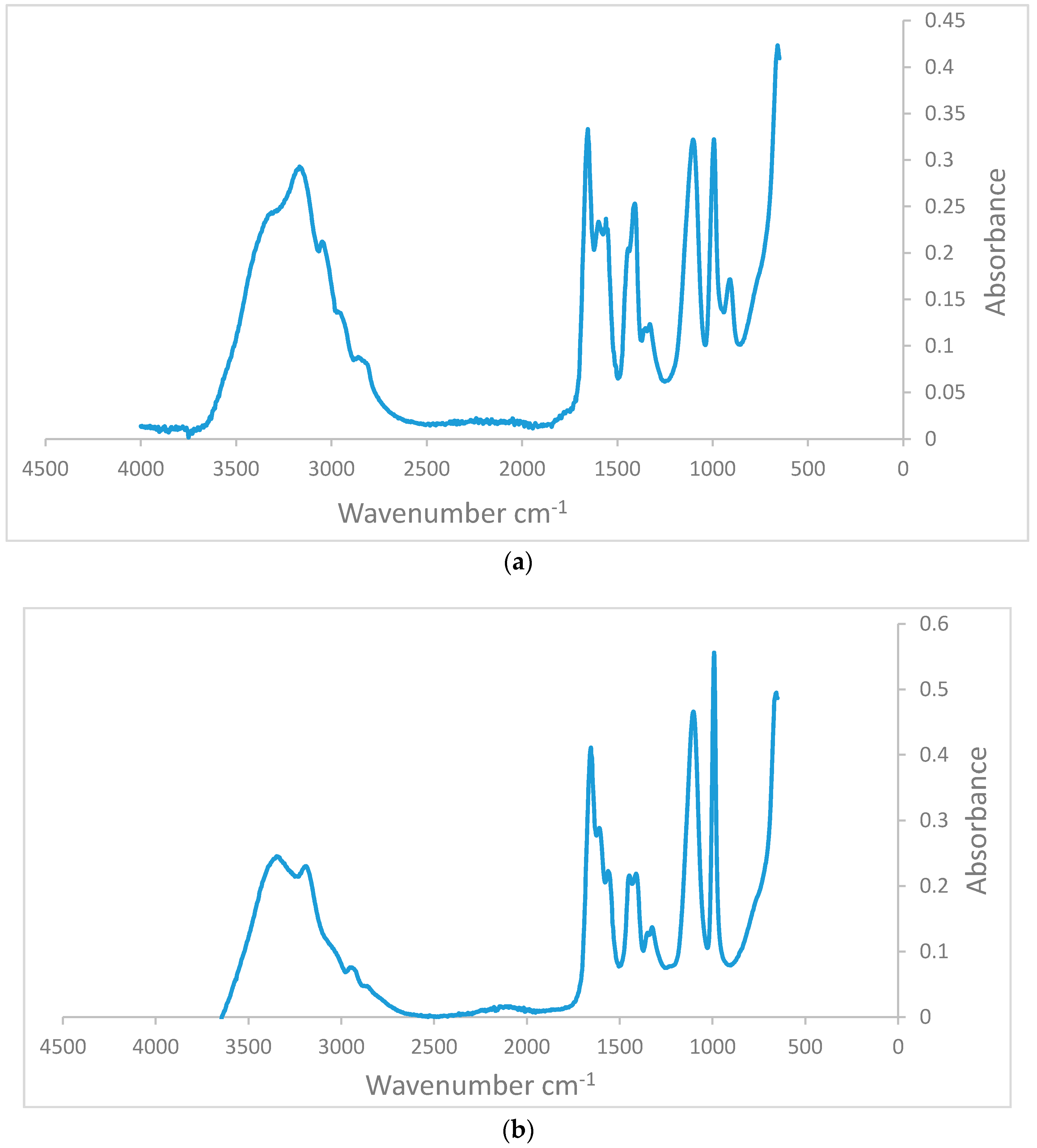
3.1.1. FTIR Measurements to Determine the Change in Degree of Hydrolysis in the Aged Samples
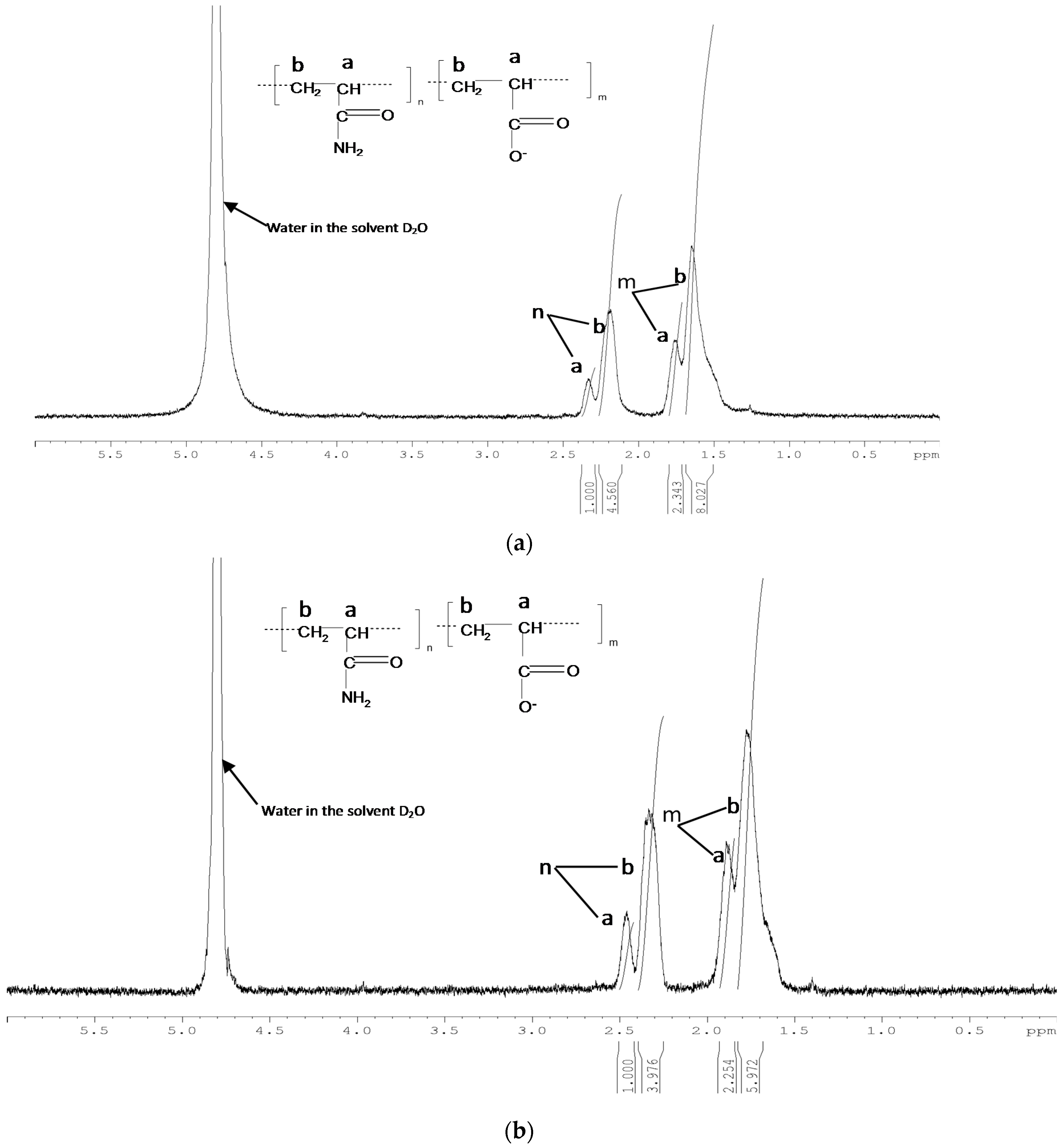
3.1.2. NMR Measurements on Time Zero Samples
3.2. Rheological Characterization of PAM
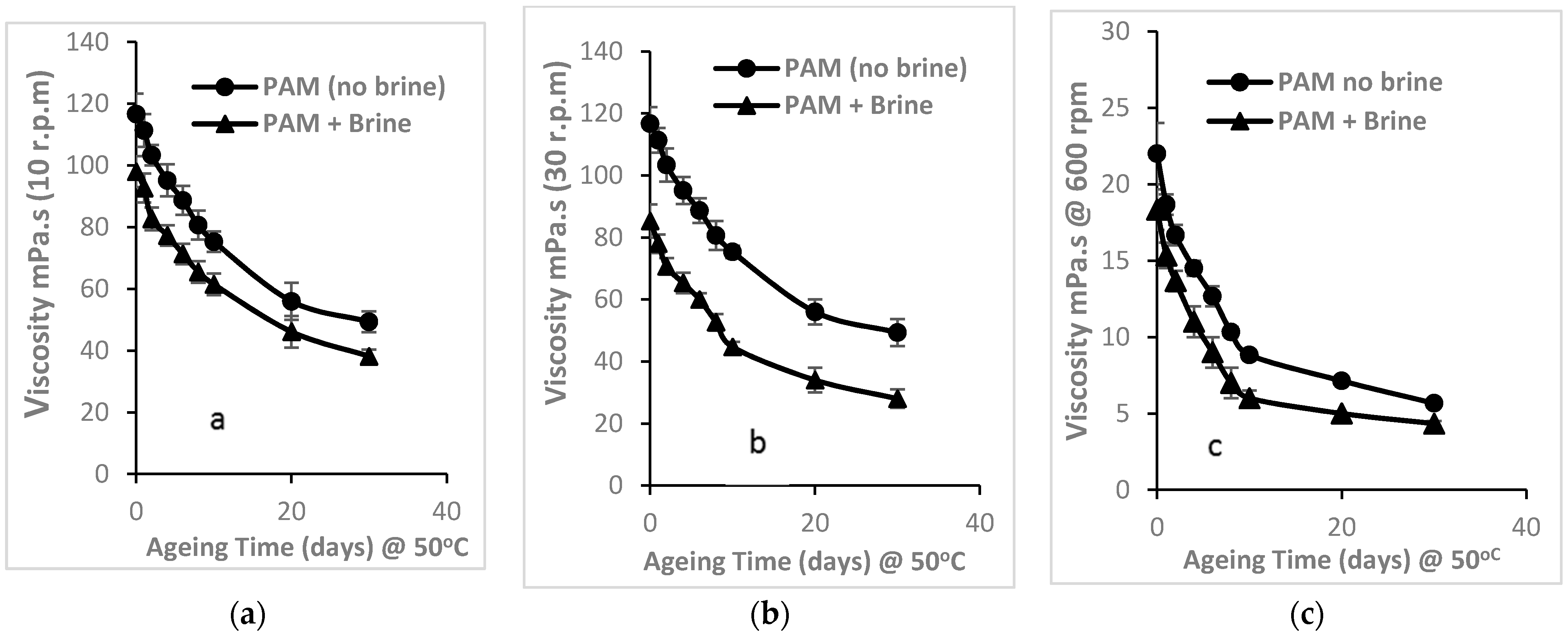
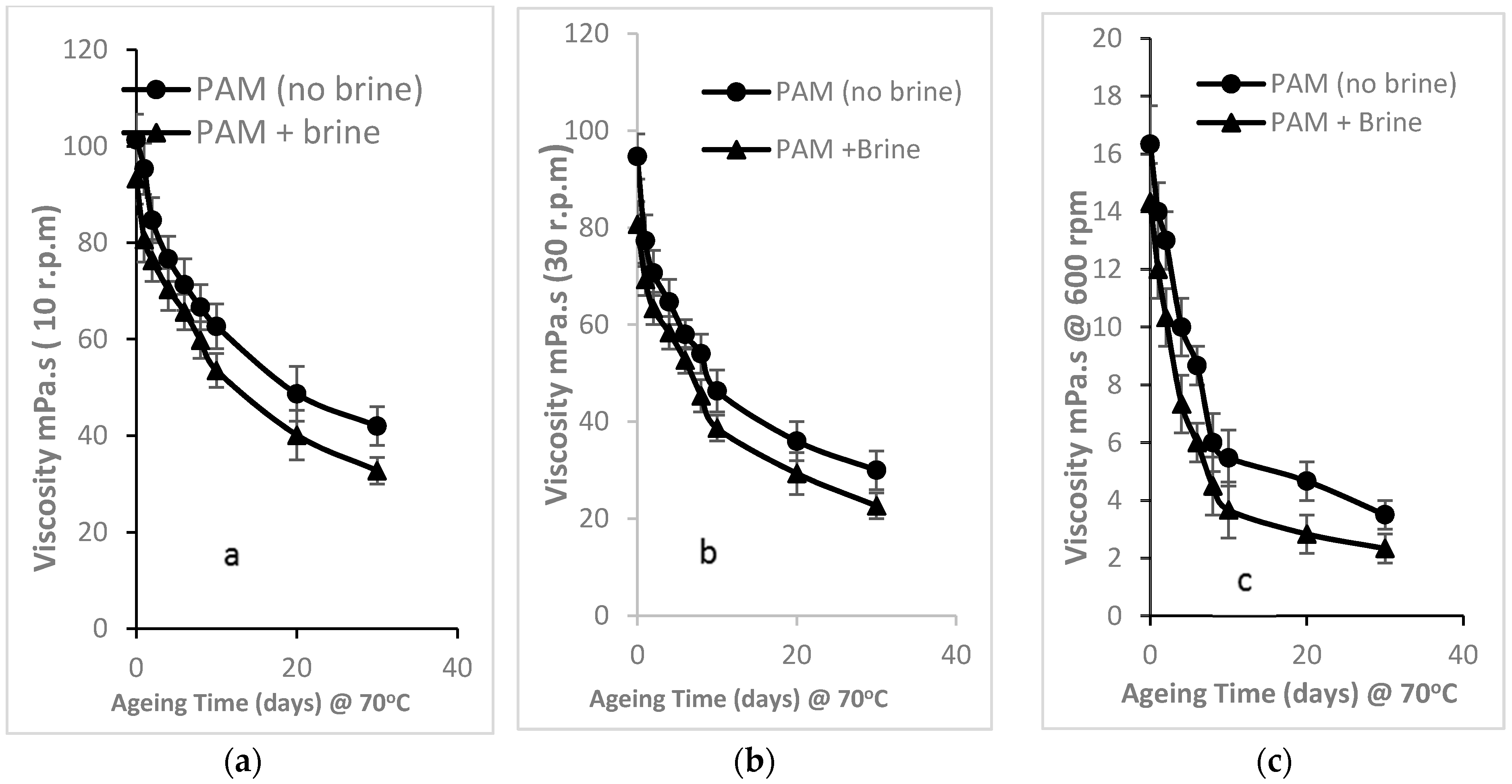
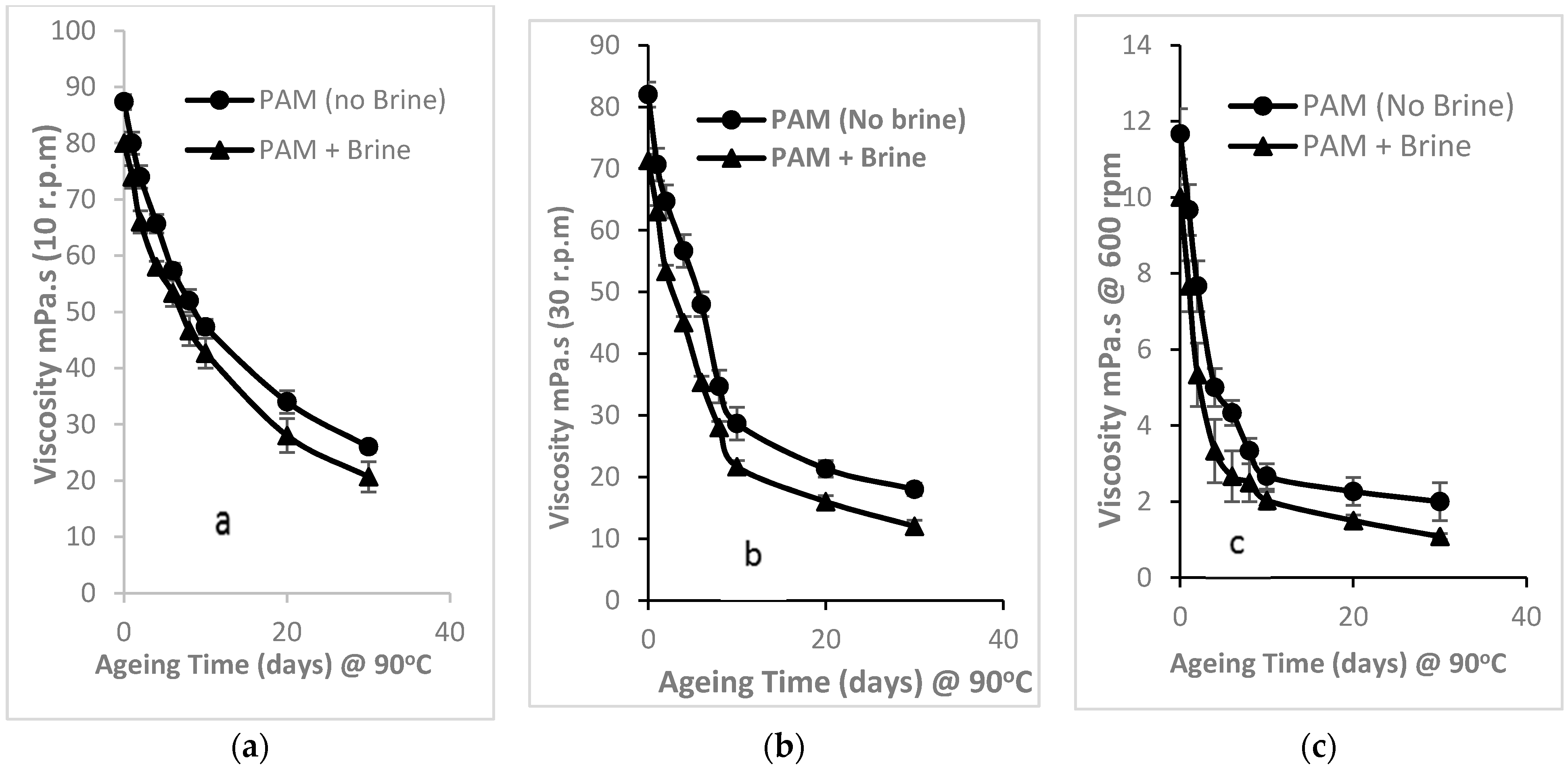
3.2.1. Time-Dependent Effects on Thermal Stability of PAM Viscosity
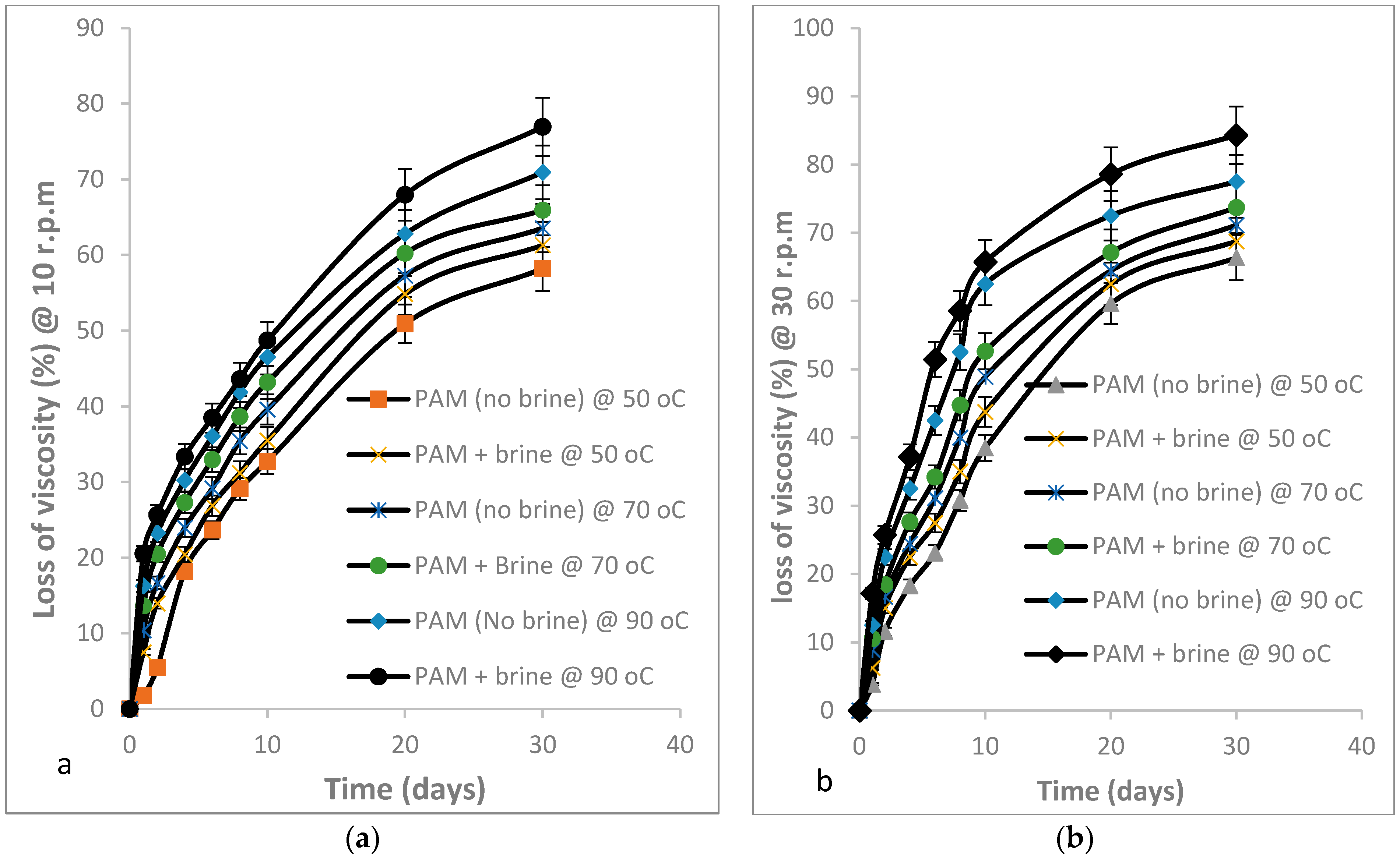
3.2.2. Percentage Change in Viscosity of PAM Solution
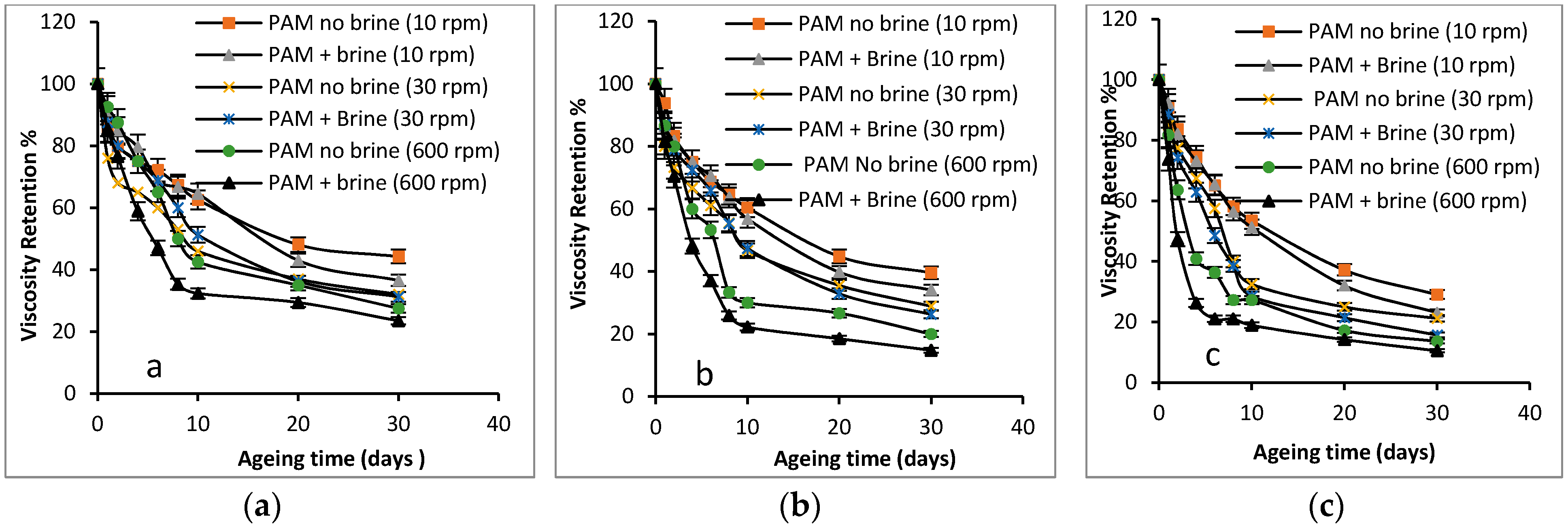
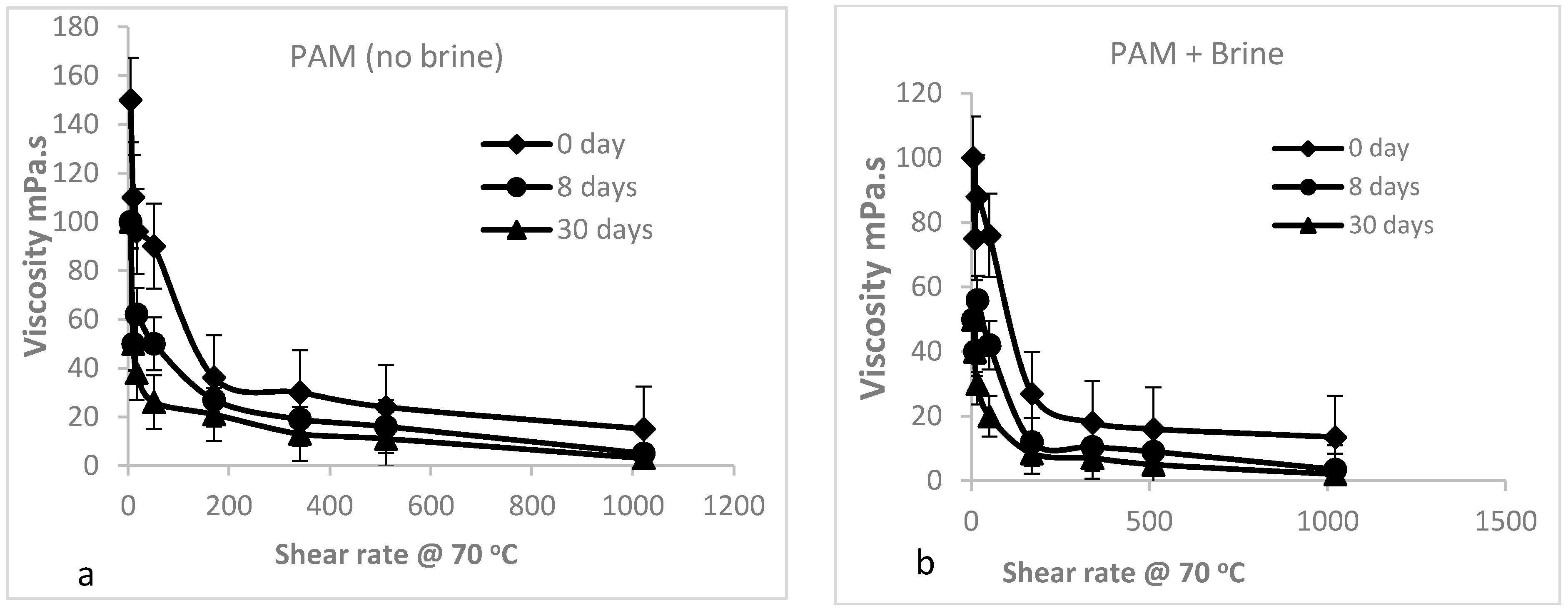
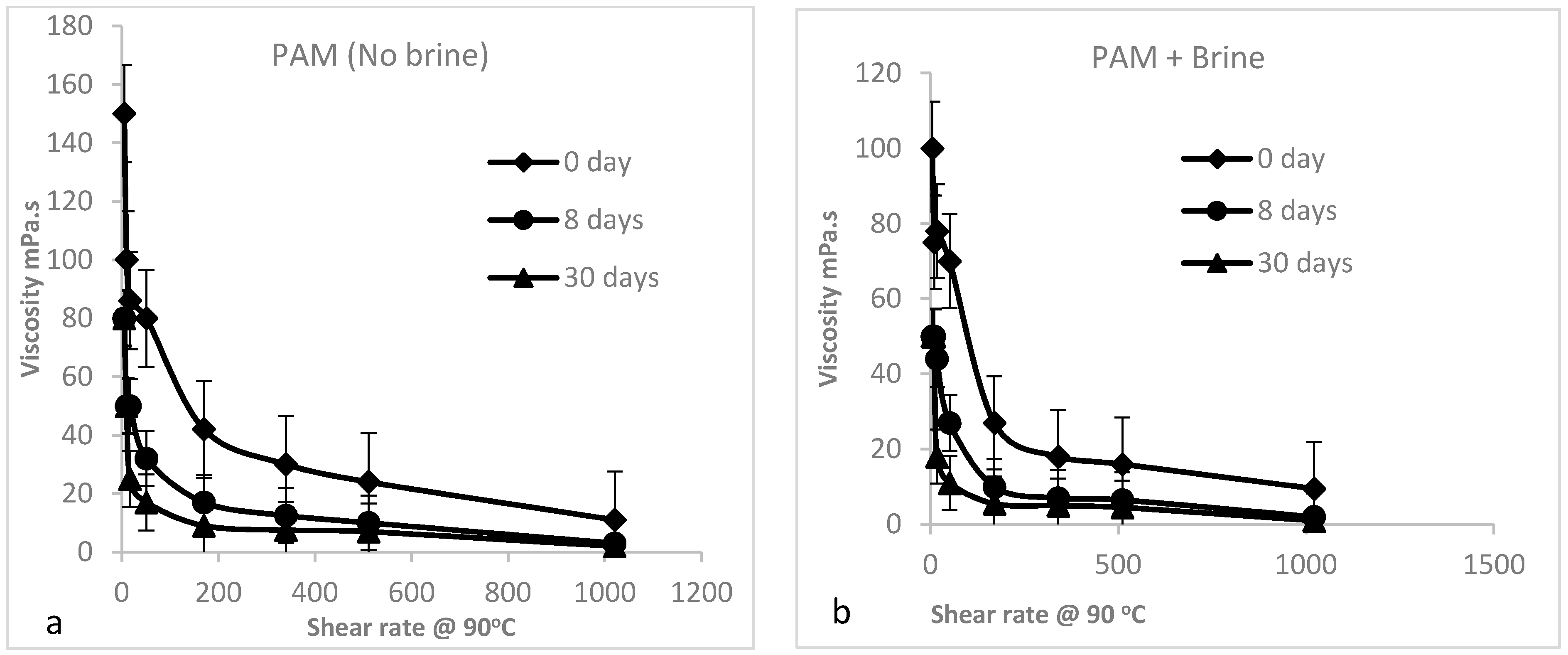
3.2.3. Shear Dependence of the Viscosity of PAM Solution (Mechanical Degradation)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Xu, Z.; Yin, H.; Feng, Y.; Quan, H. Comparative studies on enhanced oil recovery: Thermoviscosifying polymer versus polyacrylamide. Energy Fuels 2017, 31, 2479–2487. [Google Scholar] [CrossRef]
- Nwidee, L.N.; Barifcani, A.; Maxim, L.; Sarmadivaleh, M.; Iglauer, S. A realistic look at nanostructured material as an innovative approach for enhanced oil recovery process upgrading. Recent insights in petroleum science and engineering. InTechOpen 2018, 155–188. [Google Scholar] [CrossRef]
- Sharma, T.; Suresh Kumar, G.; Sangwai, J.S. Enhanced oil recovery using oil-in-water (o/w) emulsion stabilized by nanoparticle, surfactant and polymer in the presence of NaCl. Geosyst. Eng. 2014, 17, 195–205. [Google Scholar] [CrossRef]
- Al Anssari, S.; Arif, M.; Wang, S.; Barifcani, A.; Lebedev, M.; Iglauer, S. Wettability of nanofluid–modified oil–wet calcite at reservoir conditions. Fuel 2018, 211, 405–414. [Google Scholar] [CrossRef]
- Sandiford, B. Laboratory and field studies of water floods using polymer solutions to increase oil recoveries. J. Petrol. Technol. 1964, 16, 917–922. [Google Scholar] [CrossRef]
- Jennings, R.R.; Rogers, J.H.; West, T.J. Factors influencing mobility control by polymer solutions. J. Petrol. Technol. 1971, 23, 391–401. [Google Scholar] [CrossRef]
- Mungan, N. Shear viscosities of ionic polyacrylamide solutions. Soc. Petrol. Eng. J. 1972, 12, 469–473. [Google Scholar] [CrossRef]
- Martin, F.D.; Sherwood, N.S. The effect of hydrolysis of polyacrylamide on solution viscosity, polymer retention and flow resistance properties. SPE 5339. In Proceedings of the Rocky Mountain Regional Meeting of the Society of Petroleum Engineers of AIME, Denver, CO, USA, 7–9 April 1975. [Google Scholar]
- Sorbie, K.S. Polymer-Improved Oil Recovery, 1st ed.; Blackie and Son Glasgow: Glasgow, Scotland, 1991. [Google Scholar]
- Zhu, Y.; Lei, M.; Zhu, Z. Development and performance of salt–resistant polymers for chemical flooding. In Proceedings of the Middle East Oil and Gas Show and Conference, Manama, Bahrain, 8–11 March 2015; pp. 1–14. [Google Scholar]
- Zhu, D.; Wei, L.; Wang, B.; Feng, Y. Aqueous hybrids of silica nanoparticles and hydrophobically associating hydrolysed Polyacrylamide used for EOR in high–temperature and high–salinity reservoirs. Energies 2013, 7, 3858–3871. [Google Scholar] [CrossRef]
- Lockhart, T.P.; Burrafato, G. Water production control with relative permeability modifiers. In Proceedings of the 16th World Petroleum Congress (WPC-30134), Calgary, AB, Canada, 11–15 June 2000; pp. 196–197. [Google Scholar]
- Sun, Y.; Saleh, L.; Bai, B. Measurement and impact factors of polymer rheology in porous media, Rheology, Dr. Juan De Vicente (Ed.). InTechOpen 2012, 187–202. [Google Scholar] [CrossRef]
- Levitt, D.B.; Pope, G.A.; Jouenne, S. Chemical degradation of polyacrylamide polymers under alkaline conditions. SPE 129879. In Proceedings of the Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2011. [Google Scholar]
- Moradi–Araghi, A.; Doe, P.H. Hydrolysis and precipitation of polyacrylamides in hard brines at elevated temperatures. SPE Reserv. Eng. 1987, 2, 189–198. [Google Scholar] [CrossRef]
- Glover, P. Reservoir Fluids. 2012. Available online: http://homepages.see.leeds.ac.uk/~earpwjg/PG_EN/CD%20Contents (accessed on 10 February 2017).
- Muller, G.; Fenyo, J.C.; Seleny, E. High molecular weight hydrolysed polyacrylamides. III. Effect of temperature on chemical stability. J. Appl. Polym. Sci. 1980, 25, 627–633. [Google Scholar] [CrossRef]
- Zaitoun, A.; Potie, B. Limiting Conditions for the use of Hydrolysed Polyacrylamides in Brines Containing Divalent Ions. SPE 11785. In Proceedings of the International Symposium on Oilfield and Geothermal Chemistry, Denver, CO, USA, 1–3 June 1983; pp. 143–150. [Google Scholar]
- Ryles, R.G. Chemical stability limits of water-soluble polymers used in oil recovery processes. SPE Reserv. Eng. 1988, 3, 23–34. [Google Scholar] [CrossRef]
- Albonico, P.; Lockhart, T.P. Divalent ion–resistant polymer gels for high–temperature applications: Syneresis-inhibiting additives. SPE-25220. In Proceedings of the SPE International Symposium on Oilfield Chemistry, New Orleans, LA, USA, 2–5 March 1993. [Google Scholar]
- Seright, R.S.; Campbell, A.R.; Mozley, P.S.; Han, P. Stability of partially hydrolysed polyacrylamides at elevated temperatures in the absence of divalent cations. SPE 121460. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 April 2010; Volume 15, pp. 341–348. [Google Scholar]
- Uranta, K.G.; Gomari, S.R.; Russel, P.A.; Hamad, F. Determining safe maximum temperature point (SMTP) for Polyacrylamide polymer (PAM) in saline solutions. J. Oil Gas Petrochem. Sci. 2018, 1, 26–33. [Google Scholar] [CrossRef]
- Knight, B.L. Reservoir stability of polymer solutions. J. Petrol. Technol. 1973, 25, 618–626. [Google Scholar] [CrossRef]
- Lewandowska, K. Comparative studies of rheological properties of polyacrylamide and partially hydrolyzed polyacrylamide solutions. J. Appl. Polym. Sci. 2007, 103, 2235–2241. [Google Scholar] [CrossRef]
- Borling, D.; Chan, K.; Hughes, T.; Sydansk, R. Pushing out the Oil with conformance control. Oilfield Rev. 1994, 6, 44–58. [Google Scholar]
- Akbari, S.; Mahmood, S.M.; Tan, I.M.; Hosein, G.; Ling, O.L. Assessment of polyacrylamide based Co–Polymers enhanced by functional group modifications with regards to salinity and hardness. Polymers 2017, 9, 647. [Google Scholar] [CrossRef]
- Stuart, B.H. Modern Infrared Spectroscopy, 2nd ed.; John Wiley & Sons: Chichester, UK, 1996; pp. 81–83. [Google Scholar]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological characterisation of polymer gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Meyer, B., Jr. Polymer Science Textbook, 1st ed.; John Wiley & Sons: New York, NY, USA, 1971; pp. 185–191. [Google Scholar]
- Thomas, A.; Gaillard, N.; Favero, C. Some key features to consider when studying acrylamide–based polymers for chemical enhanced oil recovery. Oil Gas Sci. Technol.–Rev. IFP Energies Nouv. 2012, 67, 887–902. [Google Scholar] [CrossRef]





| Components | Concentration (g) | Concentration (ppm) |
|---|---|---|
| NaCl | 34,700 | 34,700 |
| CaCl2∙6H2O | 4900 | 4900 |
| MgCl2∙6H2O | 2700 | 2700 |
| KCl | 0.4 | 400 |
| NaHCO3 | 0.4 | 400 |
| SrCl2∙6H2O | 0.12 | 120 |
| BaCl2∙6H2O | 0.06 | 60 |
| Total dissolved salts (TDS) | 43,280 | 43,280 |
| Frequency | Assignment |
|---|---|
| 3340–3332 | Primary amide NH2 asymmetric stretching |
| 3300–3250 | Secondary amide N–H stretching |
| 3190–3170 | Primary amide NH2 symmetric stretching |
| 3100–3060 | Secondary amide II overtone |
| 1680–1630 | Primary amide C=O stretching |
| 1630–1603 | Secondary amide C=O stretching |
| Carbonyl Containing Compound | |
| The major bands that appear in the infrared spectra of carboxylic acids (which contain the COOH group) summarized below: | |
| 1603–1330 | COO− stretching |
| 1330–1300 | C–O stretching |
| 1300–1000 | C–O–H in plane bending |
| 900–992 | C–O–H out of plane bending |
| Temperature | ||
|---|---|---|
| 50 °C | 30% | 34% |
| 70 °C | 31% | 37% |
| 90 °C | 33% | 38% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godwin Uranta, K.; Rezaei-Gomari, S.; Russell, P.; Hamad, F. Studying the Effectiveness of Polyacrylamide (PAM) Application in Hydrocarbon Reservoirs at Different Operational Conditions. Energies 2018, 11, 2201. https://doi.org/10.3390/en11092201
Godwin Uranta K, Rezaei-Gomari S, Russell P, Hamad F. Studying the Effectiveness of Polyacrylamide (PAM) Application in Hydrocarbon Reservoirs at Different Operational Conditions. Energies. 2018; 11(9):2201. https://doi.org/10.3390/en11092201
Chicago/Turabian StyleGodwin Uranta, Kingsley, Sina Rezaei-Gomari, Paul Russell, and Faik Hamad. 2018. "Studying the Effectiveness of Polyacrylamide (PAM) Application in Hydrocarbon Reservoirs at Different Operational Conditions" Energies 11, no. 9: 2201. https://doi.org/10.3390/en11092201





