Investigation on Blending Effects of Gasoline Fuel with N-Butanol, DMF, and Ethanol on the Fuel Consumption and Harmful Emissions in a GDI Vehicle
Abstract
:1. Introduction
2. Experimental Apparatus and Methods
2.1. Experimental Equipment
2.2. Experimental Methods
2.3. Test Fuels
3. Results and Discussions
3.1. Ignition Timing Comparison
3.2. Energy Consumption Comparison
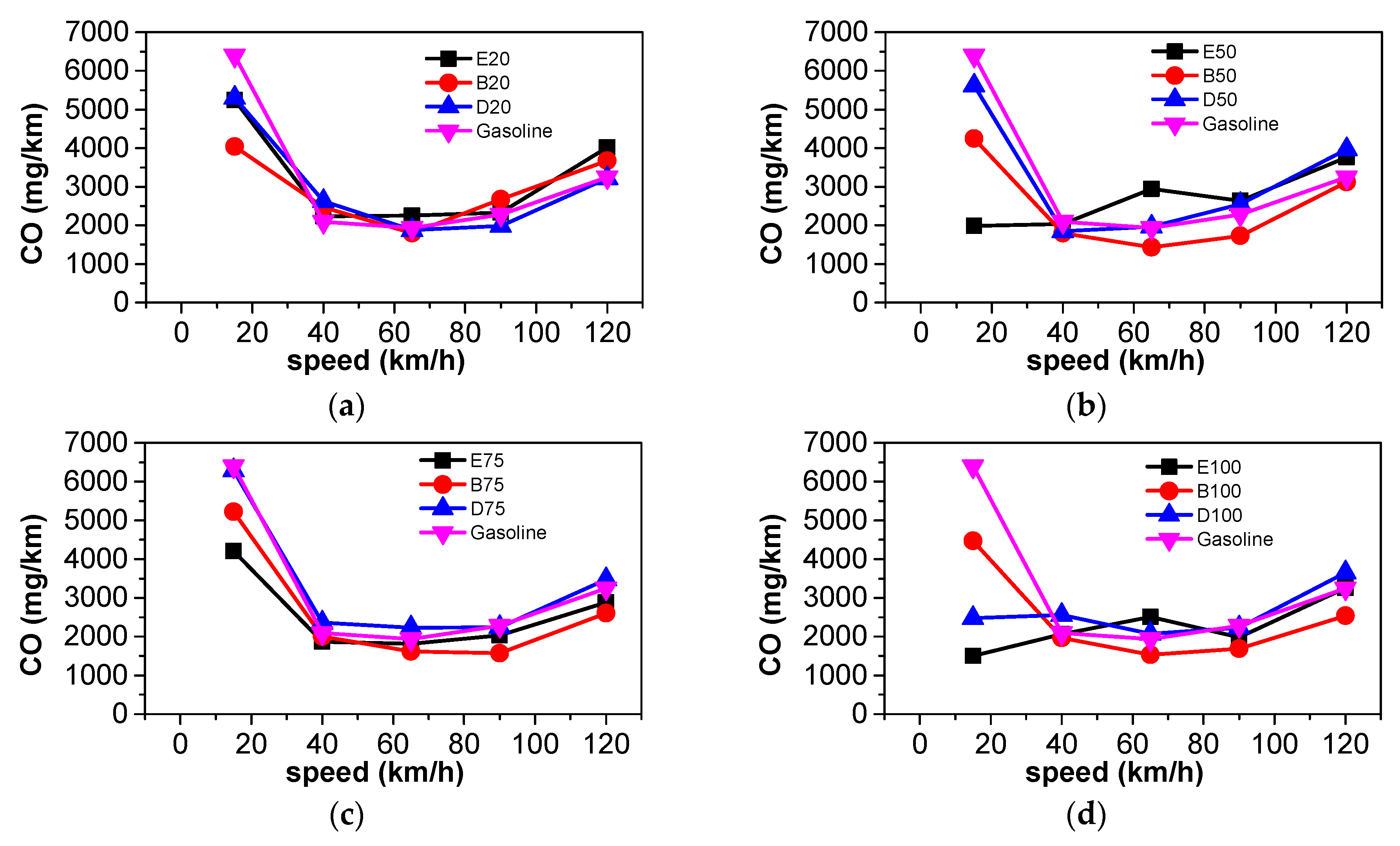
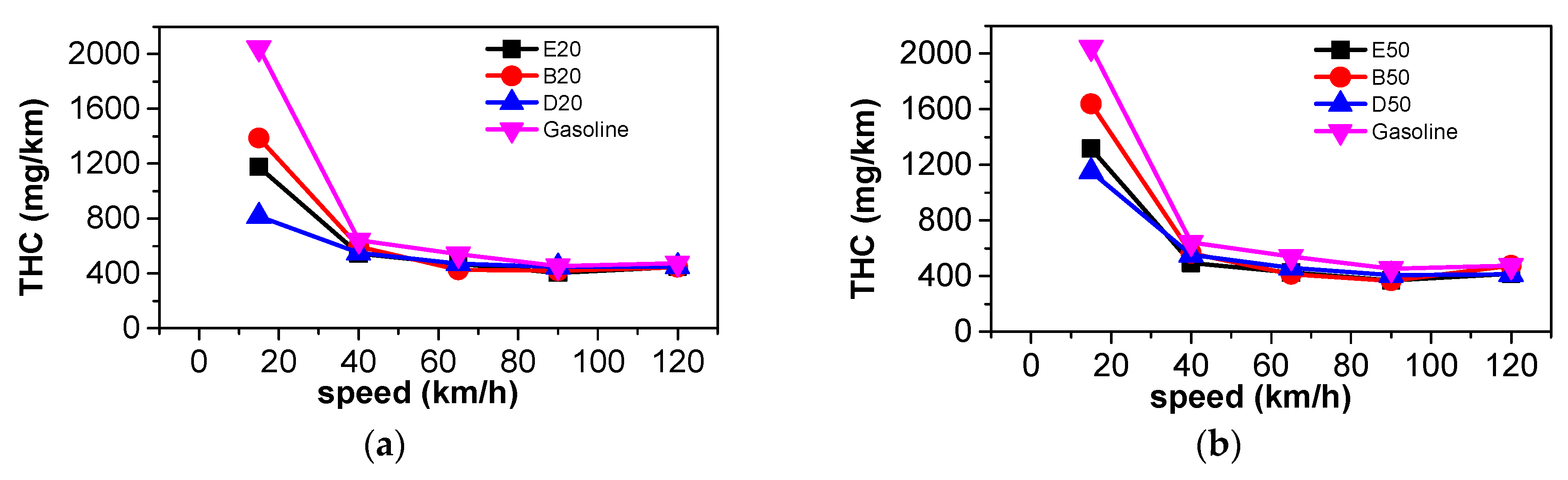
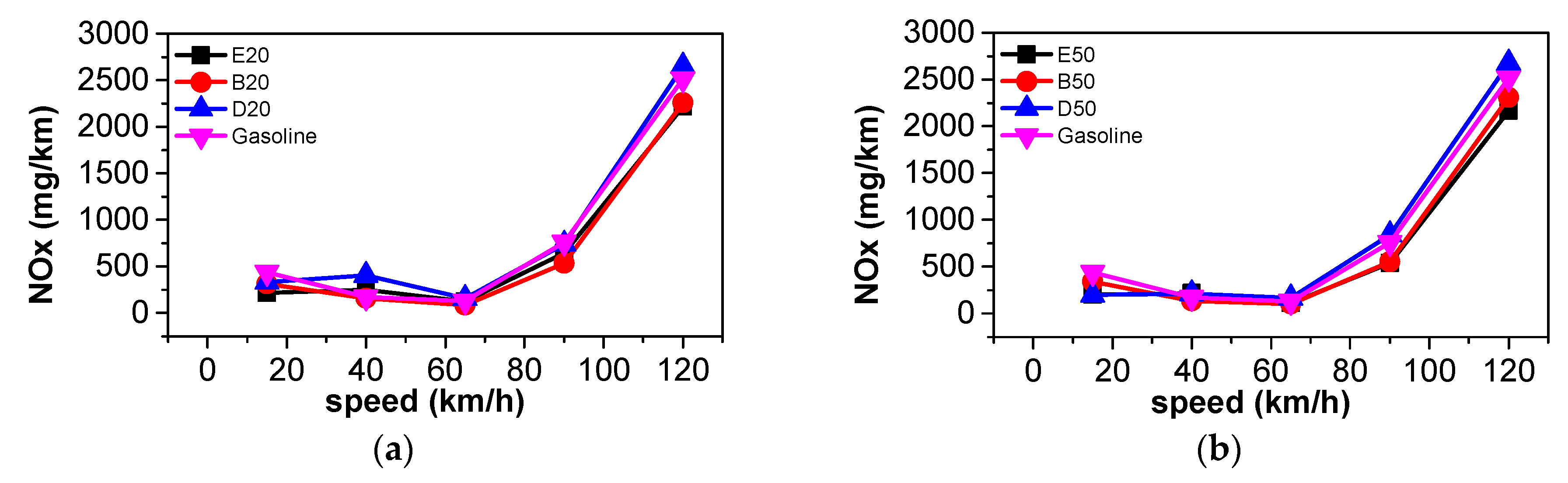
3.3. Comparison of Harmful Gaseous Emissions
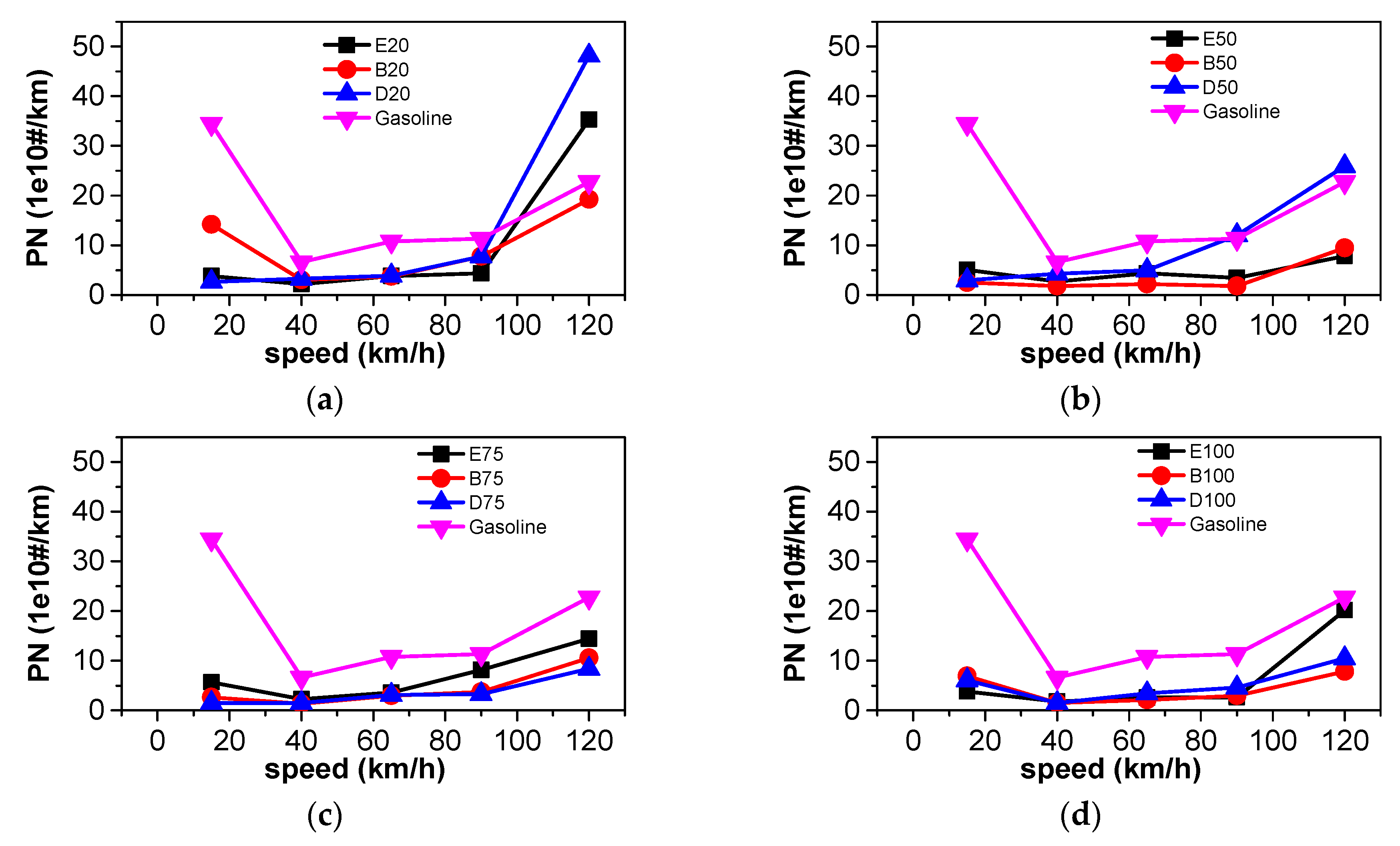
3.4. Particle Number Emissions
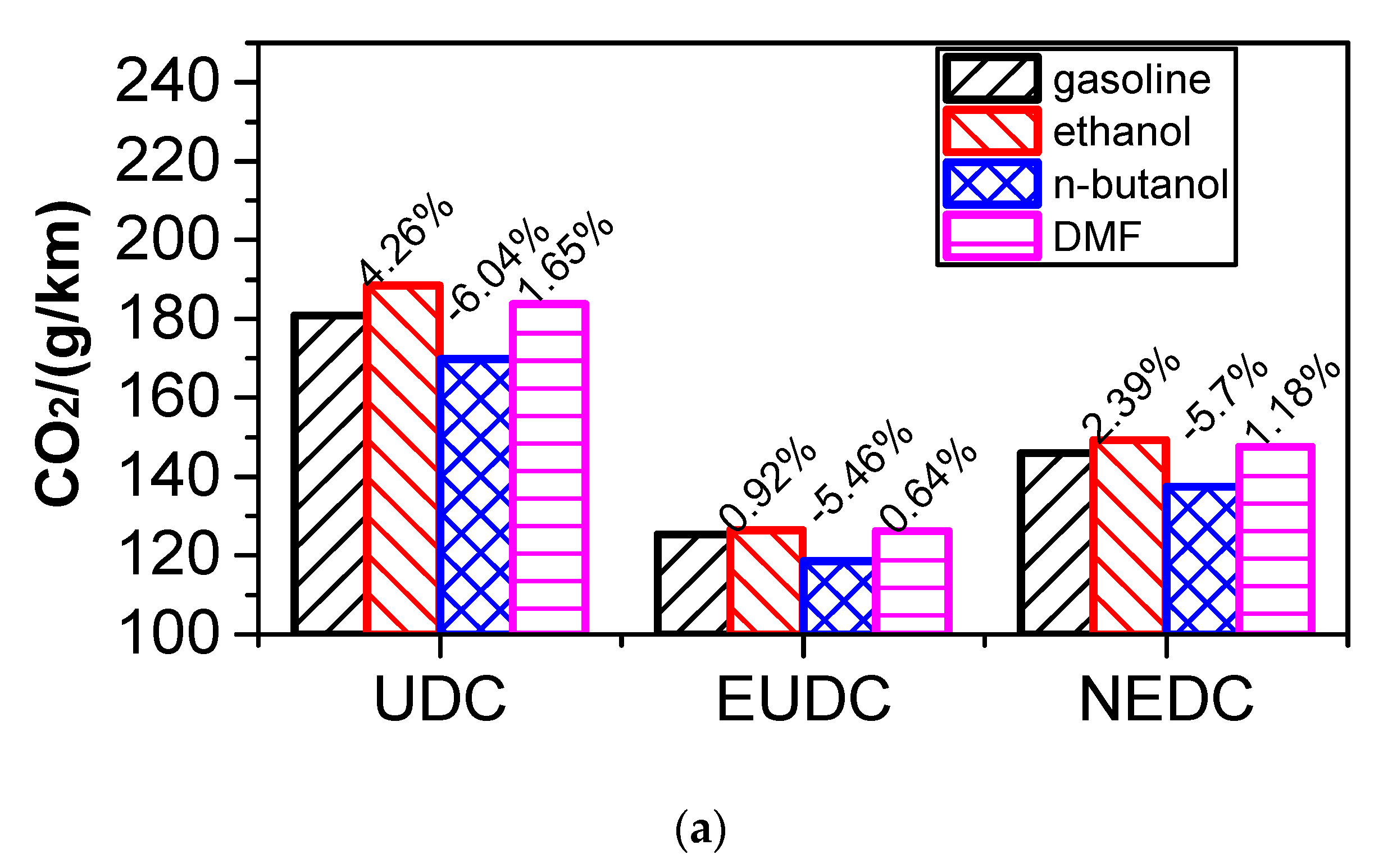
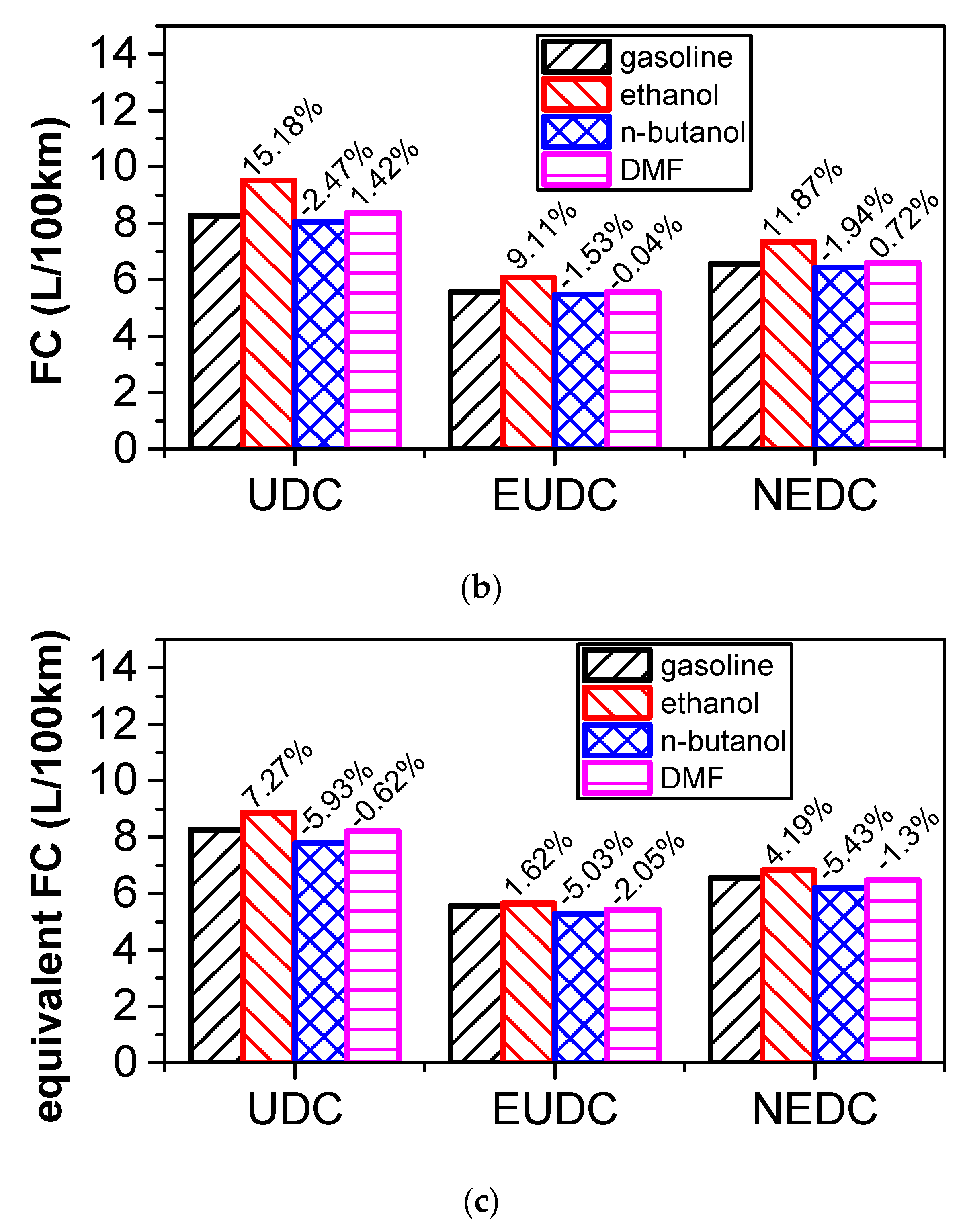
3.5. Effect of Blending Small Fraction Oxygenated Fuel in Gasoline on Fuel Consumption and Emissions of NEDC Cycle
3.5.1. Comparison of Energy Consumption
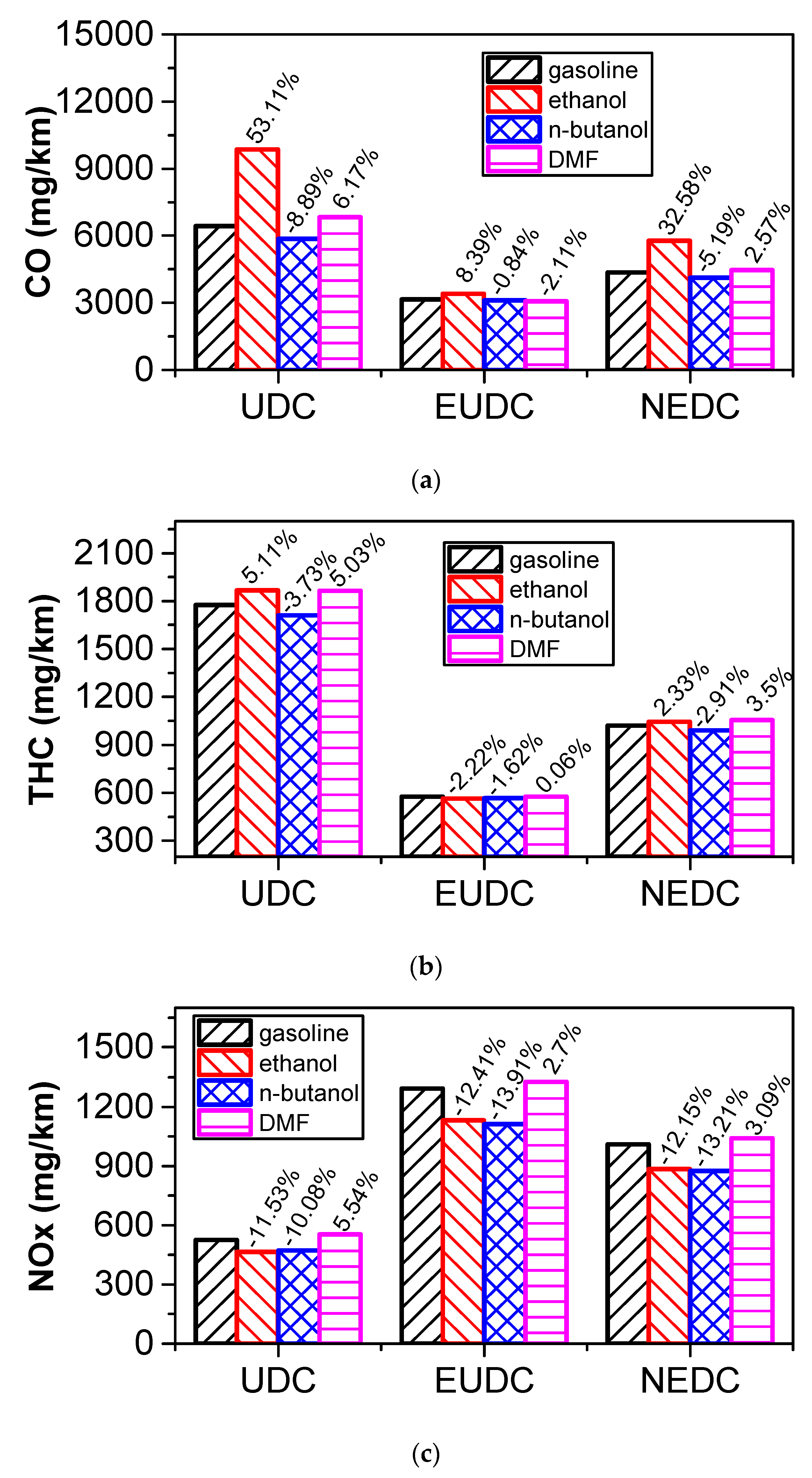
3.5.2. Comparison of Harmful Emissions
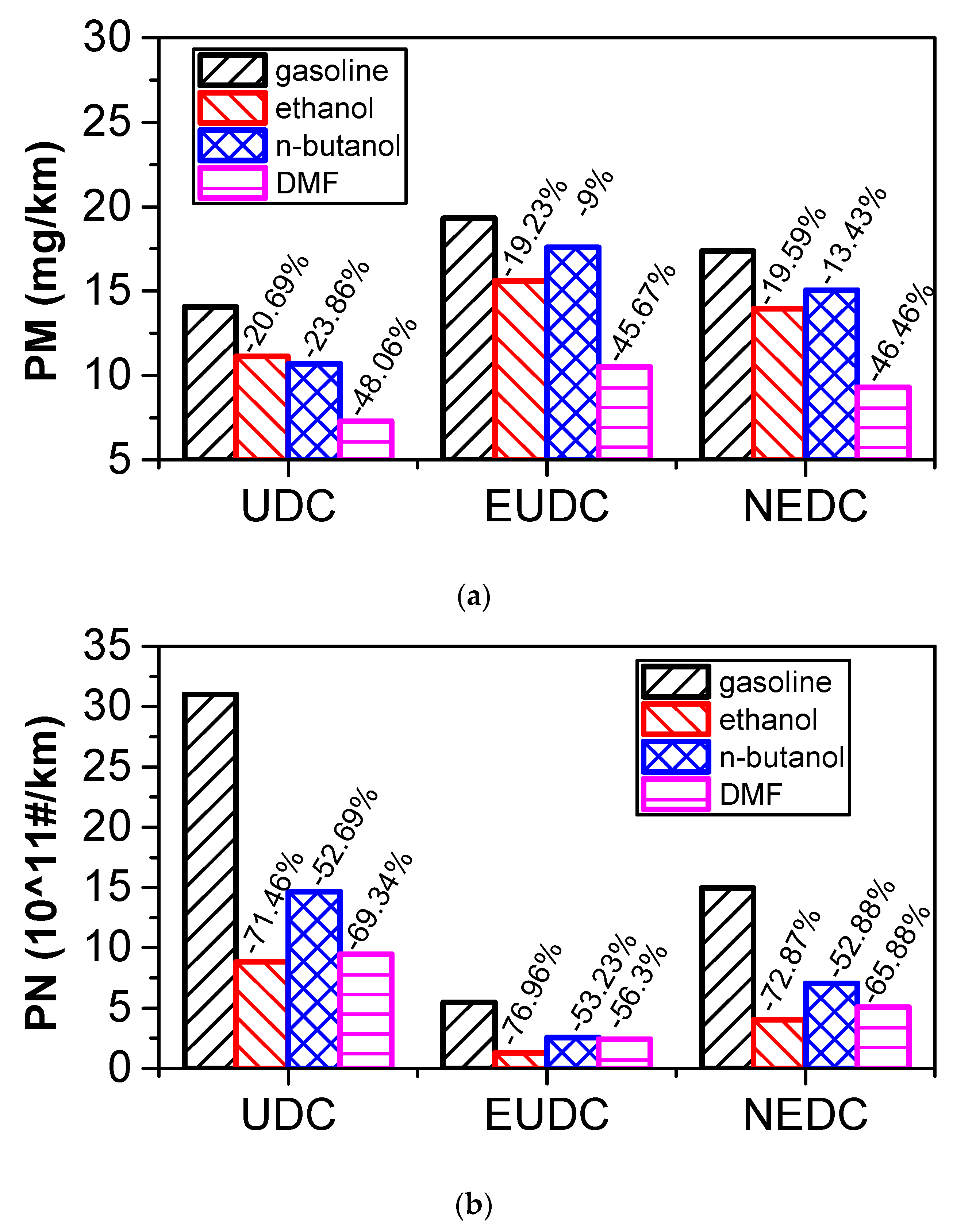
3.5.3. Comparison of Particle Emissions
3.6. Comparison of Acceleration Performance
4. Conclusions
- (1)
- At steady state, CO2 emissions and equivalent fuel consumption were reduced when n-butanol was blended into gasoline. The emissions of CO, THC, NOX, and PN also decreased as a large proportion of n-butanol was used. Blending DMF into gasoline has little effect on CO2 emissions. A large proportion of DMF resulted in the decreased THC and PN emissions and increased NOX emissions. When a large proportion of ethanol was blended into gasoline, THC, NOX, and PN emissions simultaneously decreased. Among the three oxygenated blended fuels, n-butanol–gasoline is the best for improving harmful emissions and CO2 emissions.
- (2)
- According to the results of the NEDC cycle, when blending 20% n-butanol into gasoline, the CO2 emissions, volumetric fuel consumption, and equivalent fuel consumption are reduced by 5.7%, 1.94%, and 5.434%, respectively, compared with gasoline. Meanwhile, CO, THC, and NOX emissions decreased. However, when blending 20% DMF into gasoline, the CO2 emissions and volumetric fuel consumption were higher than those of gasoline in the NEDC cycle, and CO, THC, and NOX emissions increased, while the equivalent fuel consumption was lower than that of gasoline. When blending 20% ethanol into gasoline, the CO2 emissions, volumetric fuel consumption, and equivalent fuel consumption were higher than that of gasoline. Ethanol–gasoline blends increased CO and THC emissions in all stages in the cycle, but NOX emissions decreased. Blending 20% of the three oxygenated fuels significantly reduced PN and PM emissions in the NEDC cycle.
- (3)
- The acceleration performance of three, four, and five gears is improved by blending oxygenated fuel into gasoline. As the DMF blending ratio increases, the acceleration time decreases gradually. Blending a large proportion of n-butanol also improved the acceleration performance. Overall, the acceleration performance of ethanol–gasoline was the best among the three kinds of blended fuels at five gears, although its acceleration time at three and four gears under 100% blending was slightly longer than that of the other two oxygenated fuels.
- (4)
- The impacts of the addition of ethanol, n-butanol, and DMF on the steady-state performance of the vehicle were positive. However, when the blending ratio was over 30%, the cold start performance of the vehicle will be challenging without changing the engine. So, the coupling of fuel and the engine needs to be further studied.
Author Contributions
Funding
Conflicts of Interest
References
- Giakoumis, E.G.; Rakopoulos, C.D.; Dimaratos, A.M.; Rakopoulos, D.C. Exhaust emissions with ethanol or n-butanol diesel fuel blends during transient operation: A review. Renew. Sustain. Energy Rev. 2012, 17, 170–190. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Komninos, N.P.; Kosmadakis, G.M.; Papagiannakis, R.G. Comparative Evaluation of Ethanol, n-Butanol, and Diethyl Ether Effects as Biofuel Supplements on Combustion Characteristics, Cyclic Variations, and Emissions Balance in Light-Duty Diesel Engine. J. Energy Eng. 2017, 143, 1–8. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel: A Realistic Fuel Alternative for Diesel Engines; Springer: London, UK, 2008. [Google Scholar]
- Jin, C.; Pang, X.T.; Zhang, X.Y.; Wu, S.; Ma, M.X.; Xiang, Y.; Ma, J.S.; Ji, J.; Wang, G.; Liu, H.F. Effects of C3-C5 alcohols on solubility of alcohols/diesel blends. Fuel 2019, 236, 65–74. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, J.; Zhu, Z.; Wei, H.; Lv, D.; Zhang, P.; Sun, H. Development and validation of a new reduced diesel-n-butanol blends mechanism for engine applications. Energy Convers. Manag. 2017, 149, 553–563. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Liu, J.; Lee, C. Combustion and emissions characteristics of high n-butanol/diesel ratio blend in a heavy-duty diesel engine and EGR impact. Energy Convers. Manag. 2014, 78, 787–795. [Google Scholar] [CrossRef]
- Jin, C.; Yao, M.; Liu, H.; Lee, C.F.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, C.; Liu, Q.; Wang, Q.; Wang, X. An experimental study on the combustion and emission characteristics of a diesel engine under low temperature combustion of diesel/gasoline/n-butanol blends. Appl. Energy 2016, 170, 219–231. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Wu, Z.; Lee, C. Effects of port fuel injection (PFI) of n-butanol and EGR on combustion and emissions of a direct injection diesel engine. Energy Convers. Manag. 2013, 76, 725–731. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Gurbuz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Lovell, W.G. Knocking Characteristics of Pure Hydrocarbons. Ind. Eng. Chem. 1948, 40, 2388–2438. [Google Scholar] [CrossRef]
- Rothamer, D.A.; Jennings, J.H. Study of the knocking propensity of 2,5-dimethylfuran–gasoline and ethanol–gasoline blends. Fuel 2012, 98, 203–212. [Google Scholar] [CrossRef]
- Zhao, F.; Lai, M.C.; Harrington, D.L. Automotive spark-ignited direct-injection gasoline engines. Prog. Energy Combust. 1999, 25, 437–562. [Google Scholar] [CrossRef]
- Hasan, A.O.; Abu-Jrai, A.; Turner, D.; Tsolakis, A.; Xu, H.; Golunski, S.E.; Herreros, J.M. Control of harmful hydrocarbon species in the exhaust of modern advanced GDI engines. Atmos. Environ. 2016, 129, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Marseglia, G.; Costa, M.; Catapano, F.; Sementa, P.; Vaglieco, B.M. Study about the link between injection strategy and knock onset in an optically accessible multi-cylinder GDI engine. Energy Convers. Manag. 2017, 134, 1–19. [Google Scholar] [CrossRef]
- Costa, R.C.; Sodré, J.R. Hydrous ethanol vs. gasoline-ethanol blend: Engine performance and emissions. Fuel 2010, 89, 287–293. [Google Scholar] [CrossRef]
- Palmer, F.H. Vehicle performance of gasoline containing oxygenates. Inst. Mech. Eng. 1986, 319, 33–46. [Google Scholar]
- Hsieh, W.D.; Chen, R.H.; Wu, T.L.; Lin, T.H. Engine performance and pollutant emission of an SI engine using ethanol–gasoline blended fuels. Atmos. Environ. 2002, 36, 403–410. [Google Scholar] [CrossRef]
- Chen, R.H.; Chiang, L.B.; Chen, C.N.; Lin, T.H. Cold-start emissions of an SI engine using ethanol–gasoline blended fuel. Appl. Eng. 2011, 31, 1463–1467. [Google Scholar] [CrossRef]
- Costagliola, M.A.; De, S.L.; Iannaccone, S.; Prati, M.V. Combustion efficiency and engine out emissions of a S.I. engine fueled with alcohol/gasoline blends. Appl. Energy 2013, 111, 1162–1171. [Google Scholar] [CrossRef]
- Stępień, Z.; Czerwinski, J.; Comte, P.; Oleksiak, S. Nanoparticle and Non-legislated Gaseous Emissions from a Gasoline Direct-Injection Car with Ethanol Blend Fuels and Detergent Additives. Energy Fuels 2016, 30, 7268–7276. [Google Scholar] [CrossRef]
- Ericsson, P.; Holmström, M.; Amberntsson-Carlsson, A.; Ohlson, C.; Skoglundh, M.; Andersson, B.; Carlsson, P. Characterization of Particulate Emissions and Methodology for Oxidation of Particulates from Non-Diesel Combustion Systems. SAE Pap. 2008. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Vu, D.; Villela, M.; Asa-Awuku, A.; Durbin, T.D. Evaluating the regulated emissions, air toxics, ultrafine particles, and black carbon from SI-PFI and SI-DI vehicles operating on different ethanol and iso-butanol blends. Fuel 2014, 128, 410–421. [Google Scholar] [CrossRef]
- Gu, X.; Huang, Z.; Cai, J.; Gong, J.; Wu, X.; Lee, C.F. Emission characteristics of a spark-ignition engine fuelled with gasoline-n-butanol blends in combination with EGR. Fuel 2012, 93, 611–617. [Google Scholar] [CrossRef]
- Dernotte, J.; Mounaim-Rousselle, C.; Halter, F.; Seers, P. Evaluation of butanol-gasoline blends in a port fuel-injection, spark-ignition engine. Oil Gas Sci. Technol. 2010, 65, 345–351. [Google Scholar] [CrossRef]
- Papagiannakis, R.G.; Rakopoulos, D.C.; Rakopoulos, C.D. Theoretical Study of the Effects of Spark Timing on the Performance and Emissions of a Light-Duty Spark Ignited Engine Running under Either Gasoline or Ethanol or Butanol Fuel Operating Modes. Energies 2017, 10, 1198. [Google Scholar] [CrossRef]
- Wigg, B.; Coverdill, R.; Lee, C.F.; Kyritsis, D. Emissions Characteristics of Neat Butanol Fuel Using a Port Fuel-Injected, Spark-Ignition Engine. SAE Tech. Pap. 2011. [Google Scholar] [CrossRef]
- Wallner, T.; Frazee, R. Study of Regulated and Non-Regulated Emissions from Combustion of Gasoline, Alcohol Fuels and their Blends in a DI-SI Engine. SAE Tech. Pap. 2010. [Google Scholar] [CrossRef]
- He, B.Q.; Liu, M.B.; Yuan, J.; Zhao, H. Combustion and emission characteristics of a HCCI engine fuelled with n-butanol–gasoline blends. Fuel 2013, 108, 668–674. [Google Scholar] [CrossRef]
- He, B.Q.; Yuan, J.; Liu, M.B.; Zhao, H. Combustion and emission characteristics of a n-butanol HCCI engine. Fuel 2014, 115, 758–764. [Google Scholar] [CrossRef]
- Venugopal, T.; Ramesh, A. Effective utilisation of butanol along with gasoline in a spark ignition engine through a dual injection system. Appl. Eng. 2013, 59, 550–558. [Google Scholar] [CrossRef]
- Singh, S.B.; Dhar, A.; Agarwal, A.K. Technical feasibility study of butanol-gasoline blends for powering medium-duty transportation spark ignition engine. Renew. Energy 2015, 76, 706–716. [Google Scholar] [CrossRef]
- Bata, R.M.; Elrod, A.C.; Lewandowskia, T.P. Butanol as a blending agent with gasoline for IC engines. SAE Tech. Pap. 1989. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, F.; Xue, S.; Wu, Z.; Liu, J. Impact of higher n-butanol addition on combustion and performance of GDI engine in stoichiometric combustion. Energy Convers. Manag. 2015, 106, 385–392. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Wei, X.; Zhang, Q.; Wu, Z.; Liu, J. Thermodynamic process and performance of high n-butanol/gasoline blends fired in a GDI production engine running wide-open throttle (WOT). Energy Convers. Manag. 2017, 152, 57–64. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhang, P.; Liu, X.L.; Chen, B.L.; Geng, C.; Li, B.; Wang, H.; Li, Z.S.; Yao, M.F. Laser diagnostics and chemical kinetic analysis of PAHs and soot in co-flow partially premixed flames using diesel surrogate and oxygenated additives of n-butanol and DMF. Combust. Flame 2018, 188, 129–141. [Google Scholar] [CrossRef]
- Chen, B.L.; Liu, X.L.; Liu, H.F.; Wang, H.; Kyritsis, D.C.; Yao, M.F. Soot reduction effects of the addition of four butanol isomers on partially premixed flames of diesel surrogates. Combust. Flame 2017, 177, 123–136. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Wang, X.F.; Zhong, X.F.; Hu, B.; Liu, H.F.; Yao, M.F. Experimental study on the combustion and emissions fueling biodiesel/n-butanol, biodiesel/ethanol and biodiesel/2,5-dimethylfuran on a diesel engine. Energy 2016, 115, 539–549. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Li, C.L.; Liu, H.F.; Zhang, Y.; Zhong, X.F.; Yao, M.F. Experimental study on diesel conventional and low temperature combustion by fueling four isomers of butanol. Fuel 2015, 141, 109–119. [Google Scholar] [CrossRef]
- Christensen, E.; Yanowitz, J.; Ratcliff, M.; McCormick, R.L. Renewable oxygenate blending effects on gasoline properties. Energy Fuel 2011, 25, 4723–4733. [Google Scholar] [CrossRef]
- Daniel, R.; Xu, H.; Wang, C.; Richardson, D.; Shuai, S. Combustion performance of 2,5-dimethylfuran blends using dual-injection compared to direct-injection in a SI engine. Appl. Energy 2012, 98, 59–68. [Google Scholar] [CrossRef]
- Daniel, R.; Wang, C.; Xu, H.; Tian, G. Effects of combustion phasing, injection timing, relative air-fuel ratio and variable valve timing on SI engine performance and emissions using 2,5-Dimethylfuran. SAE Int. 2012, 5, 855–866. [Google Scholar] [CrossRef]
- Shukla, M.K.; Singh, E.; Singh, N.; Singal, S.K. Prospects of 2,5-dimethylfuran as a fuel: Physico-chemical and engine performance characteristics evaluation. J. Mater. Cycles Waste Manag. 2015, 17, 459–464. [Google Scholar] [CrossRef]
- Daniel, R.; Tian, G.; Xu, H.; Wyszynski, M.L.; Wu, X.; Huang, Z. Effect of spark timing and load on a DISI engine fueled with 2,5-dimethylfuran. Fuel 2011, 90, 449–458. [Google Scholar] [CrossRef]
- Tian, G.; Daniel, R.; Li, H.; Xu, H.; Shuai, S.; Richards, P. Laminar burning velocities of 2,5-dimethylfuran compared with ethanol and gasoline. Energy Fuels 2010, 24, 3898–3905. [Google Scholar] [CrossRef]
- Tornatore, C.; Marchitto, L.; Valentino, G.; Corcione, F.E.; Merola, S.S. Optical diagnostics of the combustion process in a PFI SI boosted engine fueled with butanol-gasoline blend. Energy 2012, 45, 277–287. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Thomson, M.J.; Togbé, C.; Dagaut, P.; Halter, F.; Mounaim-Rousselle, C. An experimental and kinetic modeling study of n-butanol combustion. Combust. Flame 2009, 156, 852–864. [Google Scholar] [CrossRef]
- Yang, B.; Osswald, P.; Li, Y.; Wang, J.; Wei, L.; Tian, Z.; Qi, F.; Kohse-Höinghaus, K. Identification of combustion intermediates in isomeric fuel-rich premixed butanol-oxygen flames at low pressure. Combust. Flame 2007, 148, 198–209. [Google Scholar] [CrossRef]
- Szwaja, S.; Naber, J.D. Combustion of n-butanol in a spark-ignition IC engine. Fuel 2010, 89, 1573–1582. [Google Scholar] [CrossRef]

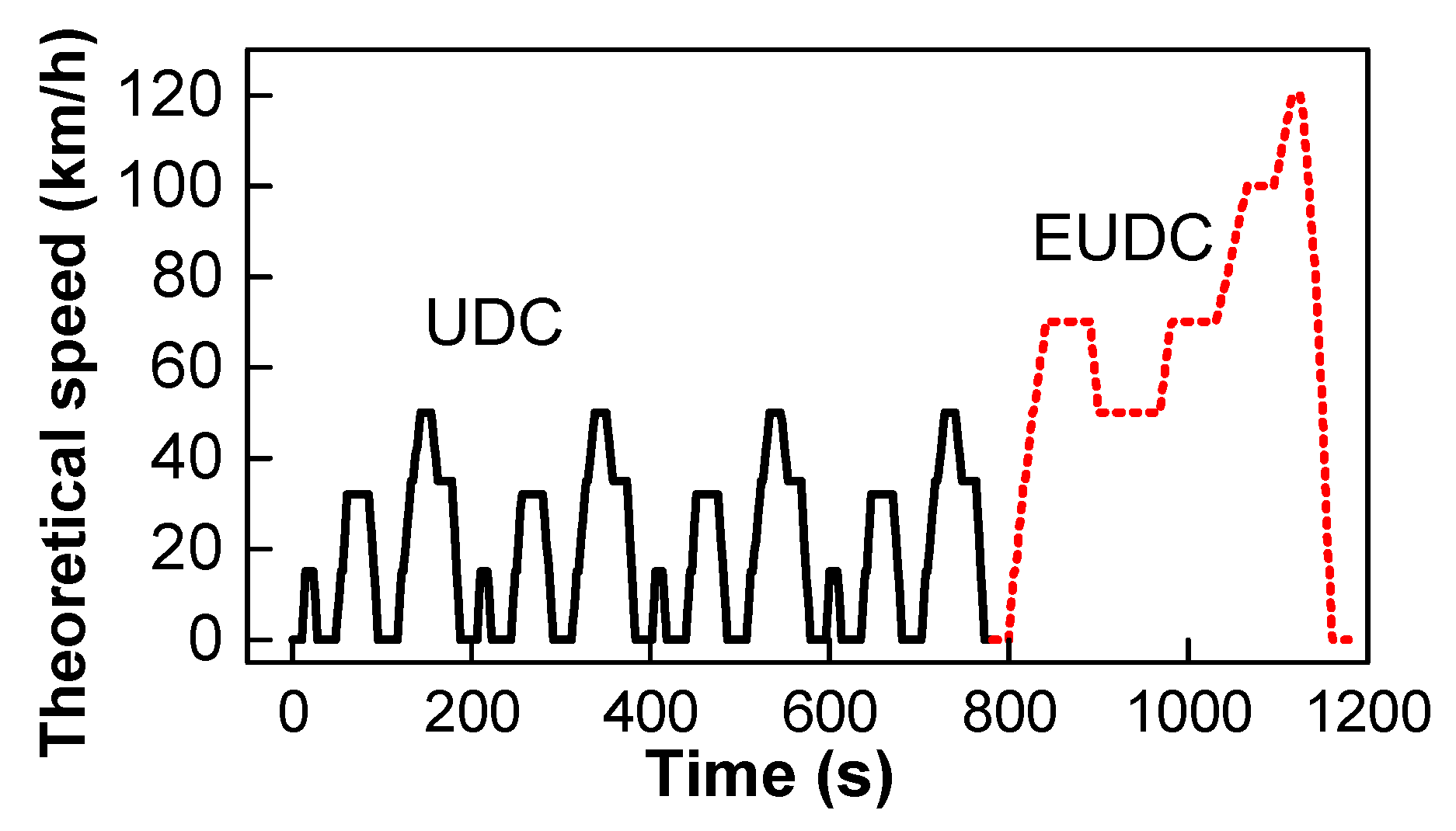
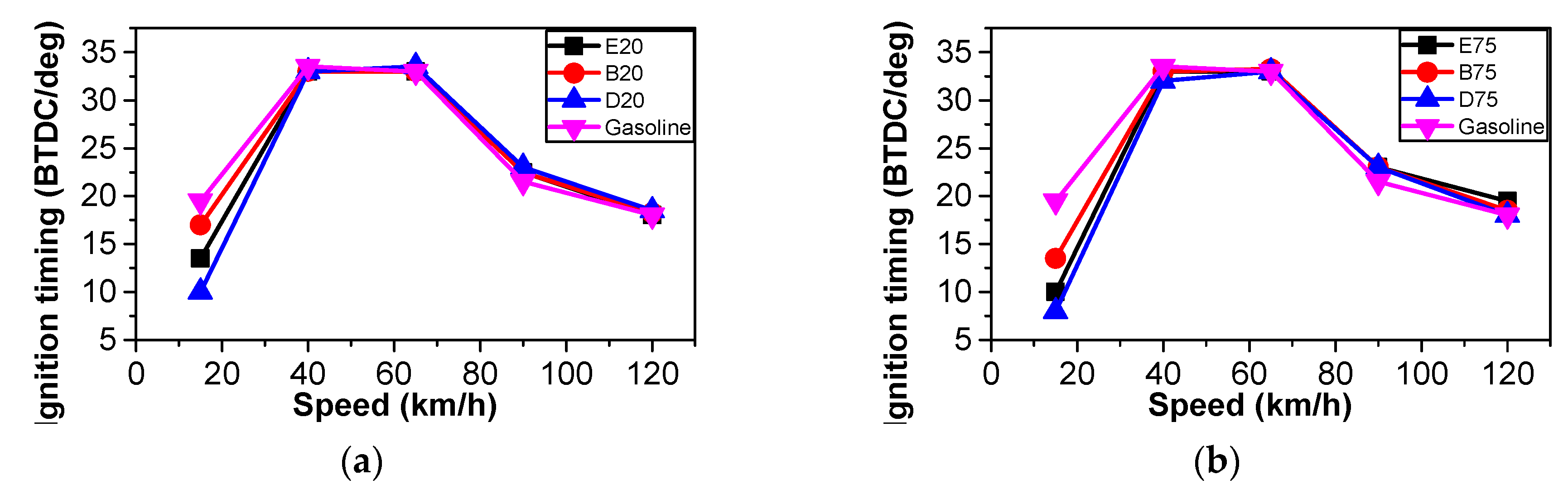
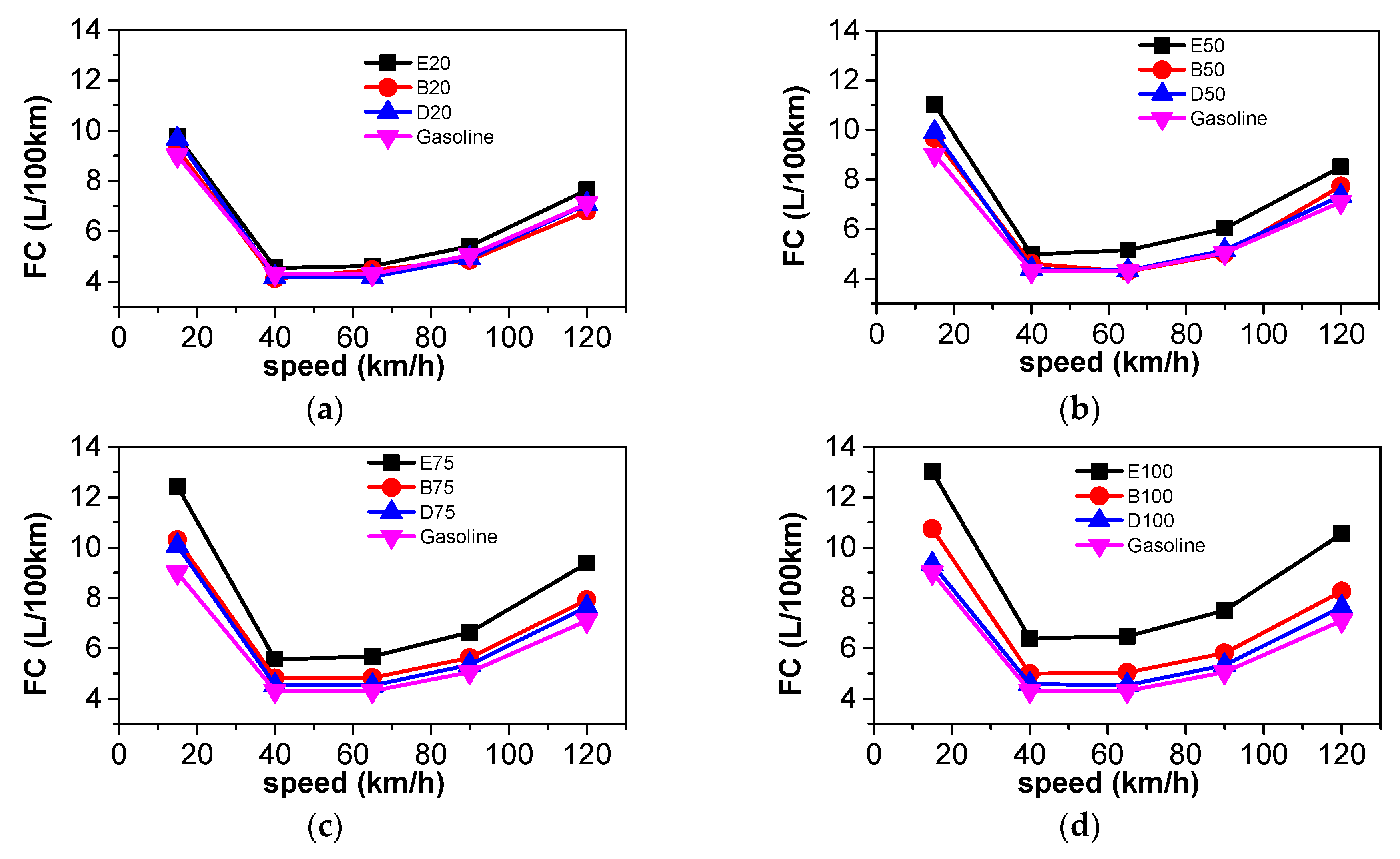
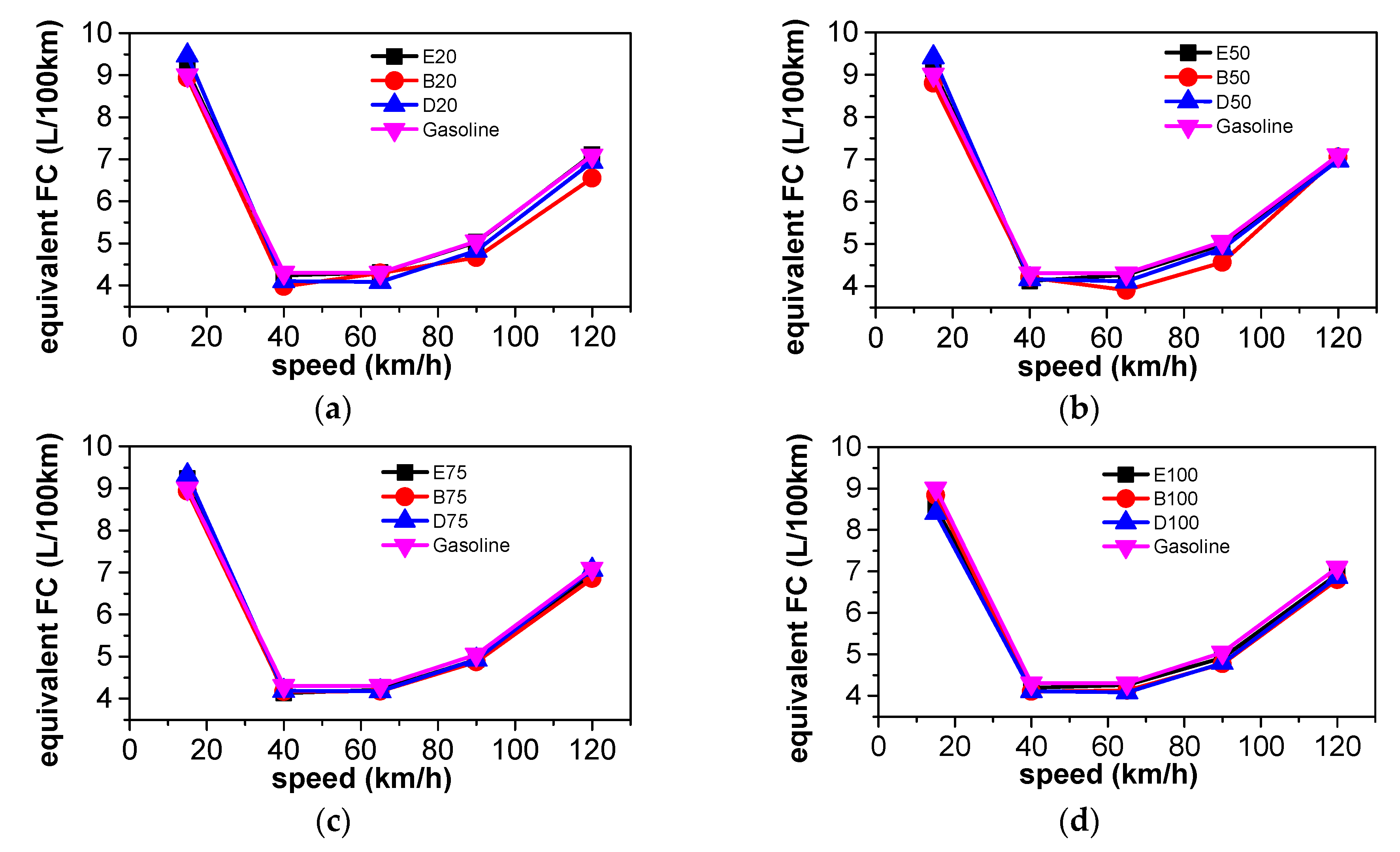




| Parameter | GDI Car |
|---|---|
| Engine type | Four cylinders, four valves per cylinder |
| Displacement | 1395 mL |
| Maximum power | 96 kW |
| Air intake system | Turbocharged |
| Compression ratio | 10.5 |
| Fuel supply method | Direct injection in cylinder |
| Exhaust treatment | No |
| Knock sensor | Exists |
| Emission standards | China V |
| Gasoline recommended | 95# |
| Maximum design total mass | 1765 kg |
| Name | Type | Manufacturer |
|---|---|---|
| Chassis dynamometer | Roadsim48"compact | AVL |
| Emission analyzer | MEXA-7200H | Horiba |
| Dilution sampling system | CVS-7200T | Horiba |
| Dilution channel | DLS-7100E | Horiba |
| Particle counter | MEXA-2000SPCS | Horiba |
| High precision electronic balance | MSE6.6S-000-DF | Sartorius |
| Equipment | Test Items | Measurement Error |
|---|---|---|
| Emission analyzer | CO2 | ≤1% Full scale or 2% measurements, take the minimum |
| CO | ||
| THC | ||
| NOX | ||
| Particle counter | PN | <10% |
| Chassis dynamometer | Speed constant adjustment difference | <0.05% Full scale |
| Time measurement tolerance | 0.00005% | |
| Traction constant tolerance | <0.2% Full scale | |
| Precision electronic balance | Filter paper quality (PM) | ±1 µg (5% load) |
| Fuel | Gasoline | Ethanol | n-Butanol | 2,5-Dimethylfuran |
|---|---|---|---|---|
| Molecular formula | C2–C14 | C2H5OH | C4H9OH | C6H8O |
| Molecular weight (g/mol) | 110.8 | 46.07 | 74.12 | 96.1 |
| Research octane number | 93.1 | 107 | 96 | 119 |
| Density at 20 °C (g/mL) | 0.745 | 0.789 | 0.81 | 0.89 |
| Lower heating value (MJ/L) | 32.9 | 21.3 | 26.9 | 29.3 |
| Laminar flame burning speed at 1 bar, 390K (cm/s) | 52 | 63 | 57 | 50 |
| Viscosity at 20 °C (cSt) | 0.4–0.8 | 1.52 | 3.35 | 0.57 |
| Surface Tension at 20 °C (mN/m) | 20–25 | 22.39 | 24. 6 | 25.9 |
| Vapor Pressure (kPa) | 55–103 | 18 | 4.08 | 1.253 |
| Boiling point (°C) | 35–210 | 78 | 117 | 92 |
| Latent heat of vaporization (kJ/kg) | 180–373 | 840 | 546 | 332 |
| H/C ratio | 1.8 | 3 | 2.5 | 1.3 |
| O/C ratio | 0 | 0.5 | 0.25 | 0.167 |
| Stoichiometric A/F ratio | 14.56 | 8.95 | 11.13 | 10.72 |
| Stoichiometric CO2 (kg/L, fuel) | 2.38 | 1.51 | 1.93 | 2.45 |
| Fuel | Density at 20 °C (g/mL) | Lower Heating Value (MJ/L) | H/C Ratio | O/C Ratio | Stoichiometric A/F Ratio |
|---|---|---|---|---|---|
| E20 | 0.754 | 30.6 | 2.0 | 0.07 | 13.27 |
| E50 | 0.767 | 27.1 | 2.3 | 0.19 | 11.61 |
| E75 | 0.778 | 24.2 | 2.6 | 0.33 | 10.26 |
| B20 | 0.758 | 31.7 | 1.9 | 0.04 | 13.72 |
| B50 | 0.778 | 29.9 | 2.1 | 0.11 | 12.71 |
| B75 | 0.794 | 28.4 | 2.3 | 0.18 | 11.91 |
| D20 | 0.774 | 32.2 | 1.7 | 0.03 | 13.57 |
| D50 | 0.818 | 31.1 | 1.6 | 0.08 | 12.41 |
| D75 | 0.854 | 30.2 | 1.5 | 0.13 | 11.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, X.; Zhang, D.; Dong, F.; Liu, X.; Yang, Y.; Huang, H.; Wang, Y.; Wang, Q.; Zheng, Z. Investigation on Blending Effects of Gasoline Fuel with N-Butanol, DMF, and Ethanol on the Fuel Consumption and Harmful Emissions in a GDI Vehicle. Energies 2019, 12, 1845. https://doi.org/10.3390/en12101845
Liu H, Wang X, Zhang D, Dong F, Liu X, Yang Y, Huang H, Wang Y, Wang Q, Zheng Z. Investigation on Blending Effects of Gasoline Fuel with N-Butanol, DMF, and Ethanol on the Fuel Consumption and Harmful Emissions in a GDI Vehicle. Energies. 2019; 12(10):1845. https://doi.org/10.3390/en12101845
Chicago/Turabian StyleLiu, Haifeng, Xichang Wang, Diping Zhang, Fang Dong, Xinlu Liu, Yong Yang, Haozhong Huang, Yang Wang, Qianlong Wang, and Zunqing Zheng. 2019. "Investigation on Blending Effects of Gasoline Fuel with N-Butanol, DMF, and Ethanol on the Fuel Consumption and Harmful Emissions in a GDI Vehicle" Energies 12, no. 10: 1845. https://doi.org/10.3390/en12101845





