Enhancing the Heat Transfer in an Active Barocaloric Cooling System Using Ethylene-Glycol Based Nanofluids as Secondary Medium
Abstract
:1. Introduction
2. Thermodynamic Cycle and the Tool of the Investigation
2.1. ABR Cycle: The Thermodynamic Cycle for Barocaloric Cooling
- Adiabatic compression;
- Heat vehiculation from Cold Heat EXchanger (CHEX) to Hot Heat EXchanger (HHEX);
- Adiabatic decompression;
- Heat vehiculation from HHEX to CHEX.
2.2. Numerical Model
3. Materials Employed in the Investigation
3.1. The Solid-State Barocaloric Refrigerant
3.2. Nanofluids as Heat Transfer Termvectorial Fluid
4. Working Conditions of the Investigation
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Roman symbols | |
| C | specific heat, J kg−1 K−1 |
| h | convective heat transfer coefficient, W m−2 K−1 |
| k | thermal conductivity, W m−1 K−1 |
| L | length of the regenerator in fluid flow direction, m |
| n | empirical shape factor |
| p | pressure, Pa |
| Q | power density associated to barocaloric effect, W m−3 |
| q | number of ABR cycles |
| convective heat flux, W m−2 | |
| T | temperature, K |
| t | time, s |
| u | longitudinal fluid velocity, m s−1 |
| v | orthogonal fluid velocity, m s−1 |
| x | longitudinal spatial coordinate, m |
| y | orthogonal spatial coordinate, m |
| Greek symbols | |
| Δ | finite difference |
| partial derivative | |
| δ | infinitesimal difference |
| infinitesimal quantity | |
| θ | period of the ABR cycle, s |
| μ | dynamic viscosity, Pa s |
| ν | cinematic viscosity, m2 s−1 |
| ρ | density, kg m−3 |
| φ | volume fraction |
| τ | period of each step of the ABR cycle, s |
| Subscripts | |
| ad | adiabatic |
| ABR | active barocaloric refrigerator |
| bf | base fluid |
| C | cold heat exchanger |
| c | convective |
| D | decompression |
| H | hot heat exchanger |
| nf | nanofluid |
| np | nanoparticles |
| s | solid |
References
- World Bank Open Data—Electric Power Consumption (kWh per Capita). Available online: https://data.worldbank.org/indicator/EG.USE.ELEC.KH.PC (accessed on 29 May 2019).
- Protocol, Montreal. Montreal Protocol on Substances that Deplete the Ozone Layer; United Nation Environment Program (UN): New York, NY, USA, 1987. [Google Scholar]
- Protocol, Kyoto. Kyoto Protocol to the United Nation Framework Convention on Climate Change; United Nation Environment Program (UN): Kyoto, Japan, 1997. [Google Scholar]
- Heath, E.A. Amendment to the Montreal Protocol on Substances that Deplete the Ozone Layer (Kigali Amendment). Int. Legal Mater. 2017, 56, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Greco, A.; Mastrullo, R.; Palombo, A. R407C as an alternative to R22 in vapour compression plant: An experimental study. Int. J. Energy Res. 1997, 21, 1087–1098. [Google Scholar] [CrossRef]
- Greco, A.; Vanoli, G.P. Flow boiling heat transfer with HFC mixtures in a smooth horizontal tube. Part II: Assessment of predictive methods. Exp. Therm. Fluid Sci. 2005, 29, 199–208. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A. The substitution of R134a with R744: An exergetic analysis based on experimental data. Int. J. Refrig. 2013, 36, 2148–2159. [Google Scholar] [CrossRef]
- Brown, D.; Stout, T.; Dirks, J.; Fernandez, N. The Prospects of Alternatives to Vapor Compression Technology for Space Cooling and Food Refrigeration Applications. Energy Eng. 2012, 109, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Goetzler, W.; Zogg, R.; Young, J.; Johnson, C. Alternatives to vapor-compression HVAC technology. ASHRAE J. 2014, 56, 12. [Google Scholar]
- Bellos, E.; Tzivanidis, C. CO2 Transcritical Refrigeration Cycle with Dedicated Subcooling: Mechanical Compression vs. Absorption Chiller. Appl. Sci. 2019, 9, 1605. [Google Scholar] [CrossRef]
- Li, S.; Jeong, J.W. Energy Performance of Liquid Desiccant and Evaporative Cooling-Assisted 100% Outdoor Air Systems under Various Climatic Conditions. Energies 2018, 11, 1377. [Google Scholar] [CrossRef]
- Brown, J.S.; Domanski, P.A. Review of alternative cooling technologies. Appl. Therm. Eng. 2014, 64, 252–262. [Google Scholar] [CrossRef]
- Czernuszewicz, A.; Kaleta, J.; Lewandowski, D. Multicaloric effect: Toward a breakthrough in cooling technology. Energy Convers. Manag. 2018, 178, 335–342. [Google Scholar] [CrossRef]
- Fähler, S.; Rößler, U.K.; Kastner, O.; Eckert, J.; Eggeler, G.; Emmerich, H.; Entel, P.; Müller, S.; Quandt, E.; Albe, K. Caloric Effects in Ferroic Materials: New Concepts for Cooling. Adv. Eng. Mater. 2012, 14, 10–19. [Google Scholar] [CrossRef]
- Kitanovski, A.; Plaznik, U.; Tomc, U.; Poredoš, A. Present and future caloric refrigeration and heat-pump technologies. Int. J. Refrig. 2015, 57, 288–298. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Solid-state refrigeration: A comparison of the energy performances of caloric materials operating in an active caloric regenerator. Energy 2018, 165, 439–455. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A., Jr. Magnetocaloric effect and magnetic refrigeration. J. Magn. Magn. Mater. 1999, 200, 44–56. [Google Scholar] [CrossRef]
- Ohnishi, T.; Soejima, K.; Yamashita, K.; Wada, H. Magnetocaloric Properties of (MnFeRu) 2 (PSi) as Magnetic Refrigerants near Room Temperature. Magnetochemistry 2017, 3, 6. [Google Scholar] [CrossRef]
- Thang, N.; Dijk, N.; Brück, E. Tuneable giant magnetocaloric effect in (Mn, Fe) 2 (P, Si) materials by Co-B and Ni-B co-doping. Materials 2016, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.F. Electrocaloric materials. Annu. Rev. Mater. Res. 2011, 41, 229–240. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Jafri, H.M.; Wang, J.; Wen, Y.; Dang, Z.M. Wide Electrocaloric Temperature Range Induced by Ferroelectric to Antiferroelectric Phase Transition. Appl. Sci. 2019, 9, 1672. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Zheng, G.P.; Zheng, X.C.; Wang, H. Exceptionally high negative electro-caloric effects of poly (VDF–co–TrFE) based nanocomposites tuned by the geometries of barium titanate nanofillers. Polymers 2017, 9, 315. [Google Scholar] [CrossRef]
- Wu, Y.; Ertekin, E.; Sehitoglu, H. Elastocaloric cooling capacity of shape memory alloys—Role of deformation temperatures, mechanical cycling, stress hysteresis and inhomogeneity of transformation. Acta Mater. 2017, 135, 158–176. [Google Scholar] [CrossRef]
- Strässle, T.; Furrer, A. Cooling by adiabatic (DE) pressurization—The barocaloric effect. High Press. Res. 2000, 17, 325–333. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Magnetic refrigeration: An eco-friendly technology for the refrigeration at room temperature. J. Phys. Conf. Ser. 2015, 655, 012026. [Google Scholar] [CrossRef]
- Saito, A.T.; Kobayashi, T.; Kaji, S.; Li, J.; Nakagome, H. Environmentally Friendly Magnetic Refrigeration Technology Using Ferromagnetic Gd Alloys. Int. J. Environ. Sci. Dev. 2016, 7, 316–320. [Google Scholar] [CrossRef] [Green Version]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Electrocaloric refrigeration: An innovative, emerging, eco-friendly refrigeration technique. J. Phys. Conf. Ser. 2017, 796, 012019. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. The environmental impact of solid-state materials working in an active caloric refrigerator compared to a vapor compression cooler. Int. J. Heat Technol. 2018, 36, 1155–1162. [Google Scholar] [CrossRef]
- Kawanami, T.; Hirano, S.; Fumoto, K.; Hirasawa, S. Evaluation of fundamental performance on magnetocaloric cooling with active magnetic regenerator. Appl. Therm. Eng. 2011, 31, 1176–1183. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. A comparison between rare earth and transition metals working as magnetic materials in an AMR refrigerator in the room temperature range. Appl. Therm. Eng. 2015, 91, 767–777. [Google Scholar] [CrossRef]
- Valant, M. Electrocaloric materials for future solid-state refrigeration technologies. Prog. Mater. Sci. 2012, 57, 980–1009. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. A comparison between different materials in an active electrocaloric regenerative cycle with a 2D numerical model. Int. J. Refrig. 2016, 69, 369–382. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Xie, J. Effects of Strain Rate and Measuring Temperature on the Elastocaloric Cooling in a Columnar-Grained Cu71Al17. 5Mn11. 5 Shape Memory Alloy. Metals 2017, 7, 527. [Google Scholar] [CrossRef]
- Tušek, J.; Engelbrecht, K.; Millán-Solsona, R.; Mañosa, L.; Vives, E.; Mikkelsen, L.P.; Pryds, N. The Elastocaloric Effect: A Way to Cool Efficiently. Adv. Energy Mater. 2015, 5, 1500361. [Google Scholar] [CrossRef]
- Strässle, T.; Furrer, A.; Dönni, A.; Komatsubara, T. Barocaloric effect: The use of pressure for magnetic cooling in Ce 3 Pd 20 Ge 6. J. Appl. Phys. 2002, 91, 8543–8545. [Google Scholar] [CrossRef]
- Plaznik, U.; Tušek, J.; Kitanovski, A.; Poredoš, A. Numerical and experimental analyses of different magnetic thermodynamic cycles with an active magnetic regenerator. Appl. Therm. Eng. 2013, 59, 52–59. [Google Scholar] [CrossRef]
- Tušek, J.; Kitanovski, A.; Poredoš, A. Geometrical optimization of packed-bed and parallel-plate active magnetic regenerators. Int. J. Refrig. 2013, 36, 1456–1464. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A. An application of the artificial neural network to optimise the energy performances of a magnetic refrigerator. Int. J. Refrig. 2017, 82, 238–251. [Google Scholar] [CrossRef]
- Teyber, R.; Trevizoli, P.V.; Christiaanse, T.V.; Govindappa, P.; Niknia, I.; Rowe, A. Semi-analytic AMR element model. Appl. Therm. Eng. 2018, 128, 1022–1029. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A., Jr. Advanced magnetocaloric materials: What does the future hold? Int. J. Refrig. 2006, 29, 1239–1249. [Google Scholar] [CrossRef]
- Crossley, S.; Mathur, N.D.; Moya, X. New developments in caloric materials for cooling applications. AIP Adv. 2015, 5, 067153. [Google Scholar] [CrossRef]
- Moya, X.; Kar-Narayan, S.; Mathur, N.D. Caloric materials near ferroic phase transitions. Nat. Mater. 2014, 13, 439–450. [Google Scholar] [CrossRef]
- Liu, Y.; Scott, J.F.; Dkhil, B. Some strategies for improving caloric responses with ferroelectrics. APL Mater. 2016, 4, 064109. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Liu, M.; Egolf, P.W.; Kitanovski, A. A review of magnetic refrigerator and heat pump prototypes built before the year 2010. Int. J. Refrig. 2010, 33, 1029–1060. [Google Scholar] [CrossRef]
- Sari, O.; Balli, M. From conventional to magnetic refrigerator technology. Int. J. Refrig. 2014, 37, 8–15. [Google Scholar] [CrossRef]
- Franco, V.; Blazquez, J.S.; Amiano, A.C. Field dependence of the magnetocaloric effect in materials with a second order phase transition: A master curve for the magnetic entropy change. Appl. Phys. Lett. 2006, 89, 222512. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A. The use of the first and of the second order phase magnetic transition alloys for an AMR refrigerator at room temperature: A numerical analysis of the energy performances. Energy Convers. Manag. 2013, 70, 40–55. [Google Scholar] [CrossRef]
- Correia, T.; Zhang, Q. Electrocaloric Materials; Springer: Berlin, Germany, 2014. [Google Scholar]
- Qian, S.; Geng, Y.; Wang, Y.; Ling, J.; Hwang, Y.; Radermacher, R.; Takeuchi, I.; Cui, J. A review of elastocaloric cooling: Materials, cycles and system integrations. Int. J. Refrig. 2016, 64, 1–19. [Google Scholar] [CrossRef]
- Greco, A.; Aprea, C.; Maiorino, A.; Masselli, C. A review of the state of the art of solid-state caloric cooling processes at room-temperature before 2019. Int. J. Refrig. 2019. [Google Scholar] [CrossRef]
- Lu, B.; Liu, J. Mechanocaloric materials for solid-state cooling. Sci. Bull. 2015, 60, 1638–1643. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. A comparison between different materials with mechanocaloric effect. Int. J. Heat Technol. 2018, 36, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Bom, N.M.; Imamura, W.; Usuda, E.O.; Paixão, L.S.; Carvalho, A.M.G. Giant Barocaloric Effects in Natural Rubber: A Relevant Step toward Solid-State Cooling. ACS Macro Lett. 2017, 7, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Bom, N.M.; Usuda, E.O.; Guimarães, G.M.; Coelho, A.A.; Carvalho, A.M.G.; Carvalho, A. Note: Experimental setup for measuring the barocaloric effect in polymers: Application to natural rubber. Rev. Sci. Instrum. 2017, 88, 046103. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U.S. Review and Comparison of Nanofluid Thermal Conductivity and Heat Transfer Enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Choi, S.U.S. Enhancing thermal conductivity of fluids with nanoparticles. Dev. Appl. Non-Newton. Flows 1995, 66, 99–105. [Google Scholar]
- Mohamad, N.A.; Azis, N.; Jasni, J.; Kadir, A.; Abidin, M.Z.; Yunus, R.; Yaakub, Z. Impact of Fe3O4, CuO and Al2O3 on the AC Breakdown Voltage of Palm Oil and Coconut Oil in the Presence of CTAB. Energies 2019, 12, 1605. [Google Scholar] [CrossRef]
- Irfan, S.A.; Shafie, A.; Yahya, N.; Zainuddin, N. Mathematical Modeling and Simulation of Nanoparticle-Assisted Enhanced Oil Recovery—A Review. Energies 2019, 12, 1575. [Google Scholar] [CrossRef]
- Hussain, M.I.; Kim, J.H.; Kim, J.T. Nanofluid-Powered Dual-Fluid Photovoltaic/Thermal (PV/T) System: Comparative Numerical Study. Energies 2019, 12, 775. [Google Scholar] [CrossRef]
- Fal, J.; Mahian, O.; Żyła, G. Nanofluids in the Service of High Voltage Transformers: Breakdown Properties of Transformer Oils with Nanoparticles, a Review. Energies 2018, 11, 2942. [Google Scholar] [CrossRef]
- Kristiawan, B.; Santoso, B.; Wijayanta, A.; Aziz, M.; Miyazaki, T. Heat transfer enhancement of TiO2/water nanofluid at laminar and turbulent flows: A numerical approach for evaluating the effect of nanoparticle loadings. Energies 2018, 11, 1584. [Google Scholar] [CrossRef]
- Sun, X.H.; Yan, H.; Massoudi, M.; Chen, Z.H.; Wu, W.T. Numerical simulation of nanofluid suspensions in a geothermal heat exchanger. Energies 2018, 11, 919. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C. Optimization of a solar-driven trigeneration system with nanofluid-based parabolic trough collectors. Energies 2017, 10, 848. [Google Scholar] [CrossRef]
- Kumar, S.A.; Meenakshi, K.S.; Narashimhan, B.R.V.; Srikanth, S.; Arthanareeswaran, G. Synthesis and characterization of copper nanofluid by a novel one-step method. Mater. Chem. Phys. 2009, 113, 57–62. [Google Scholar] [CrossRef]
- Chiba, Y. Enhancements of thermal performances of an active magnetic refrigeration device based on nanofluids. Mechanics 2017, 23, 31–38. [Google Scholar] [CrossRef]
- Mugica, I.; Roy, S.; Poncet, S.; Bouchard, J.; Nesreddine, H. Exergy analysis of a parallel-plate active magnetic regenerator with nanofluids. Entropy 2017, 19, 464. [Google Scholar] [CrossRef]
- Aprea, C.; Cardillo, G.; Greco, A.; Maiorino, A.; Masselli, C. A comparison between experimental and 2D numerical results of a packed-bed active magnetic regenerator. Appl. Therm. Eng. 2015, 90, 376–383. [Google Scholar] [CrossRef]
- Aprea, C.; Cardillo, G.; Greco, A.; Maiorino, A.; Masselli, C. A rotary permanent magnet magnetic refrigerator based on AMR cycle. Appl. Therm. Eng. 2016, 101, 699–703. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Energy performances and numerical investigation of solid-state magnetocaloric materials used as refrigerant in an active magnetic regenerator. Therm. Sci. Eng. Prog. 2018, 6, 370–379. [Google Scholar] [CrossRef]
- Imamura, W.; Usuda, E.O.; Paixão, L.S.; Bom, N.M.; Gomes, A.M.; Carvalho, A.M.G. Supergiant barocaloric effects in acetoxy silicone rubber around room temperature. arXiv 2017, arXiv:1710.01761. [Google Scholar]
- Ashrae. 2009 ASHRAE Handbook—Fundamentals (SI Edition); Ashrae: Atlanta, GA, USA, 2009. [Google Scholar]
- Bianco, V.; Vafai, K.; Manca, O.; Nardini, S. Heat Transfer Enhancement with Nanofluids; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Brinkman, H.C. The viscosity of concentrated suspensions and solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fund. 1962, 1, 187–191. [Google Scholar] [CrossRef]



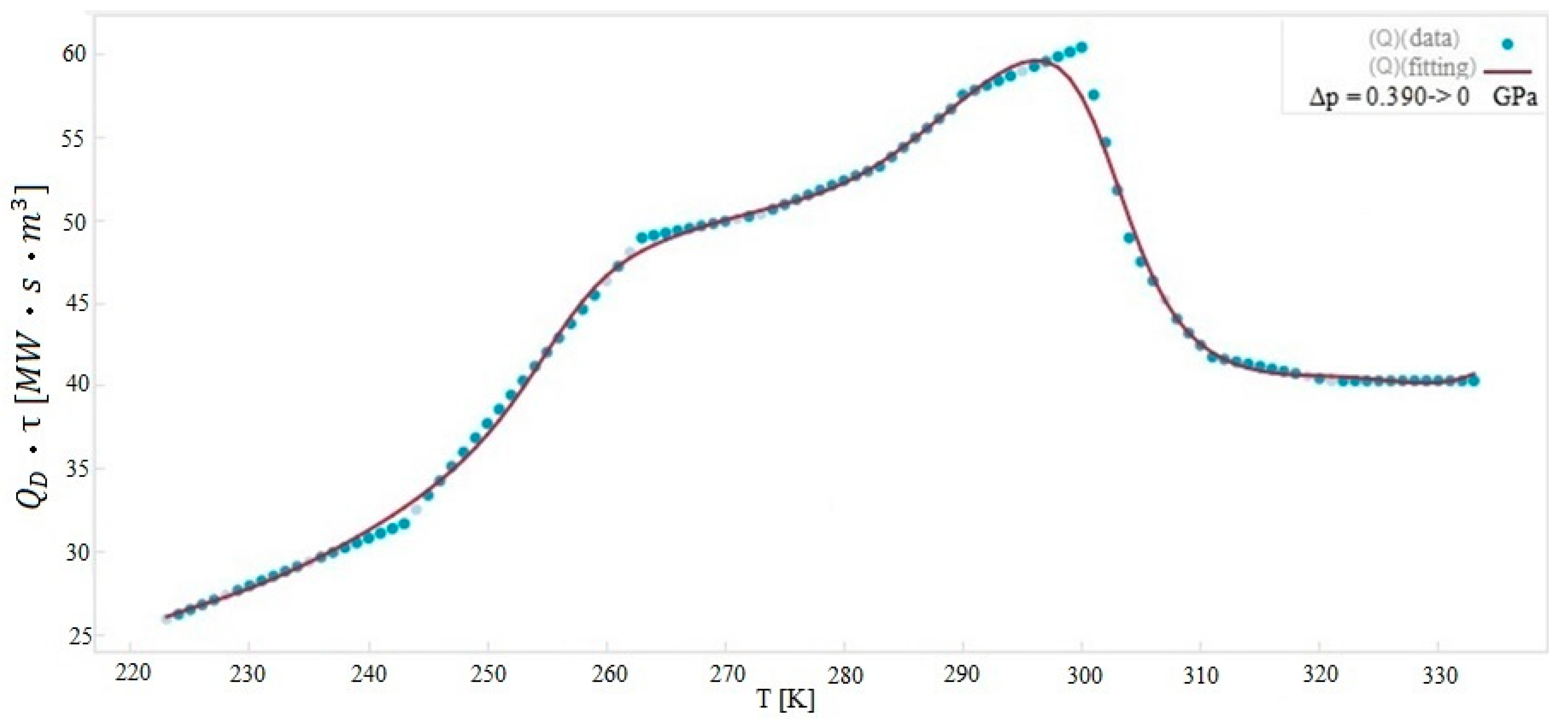
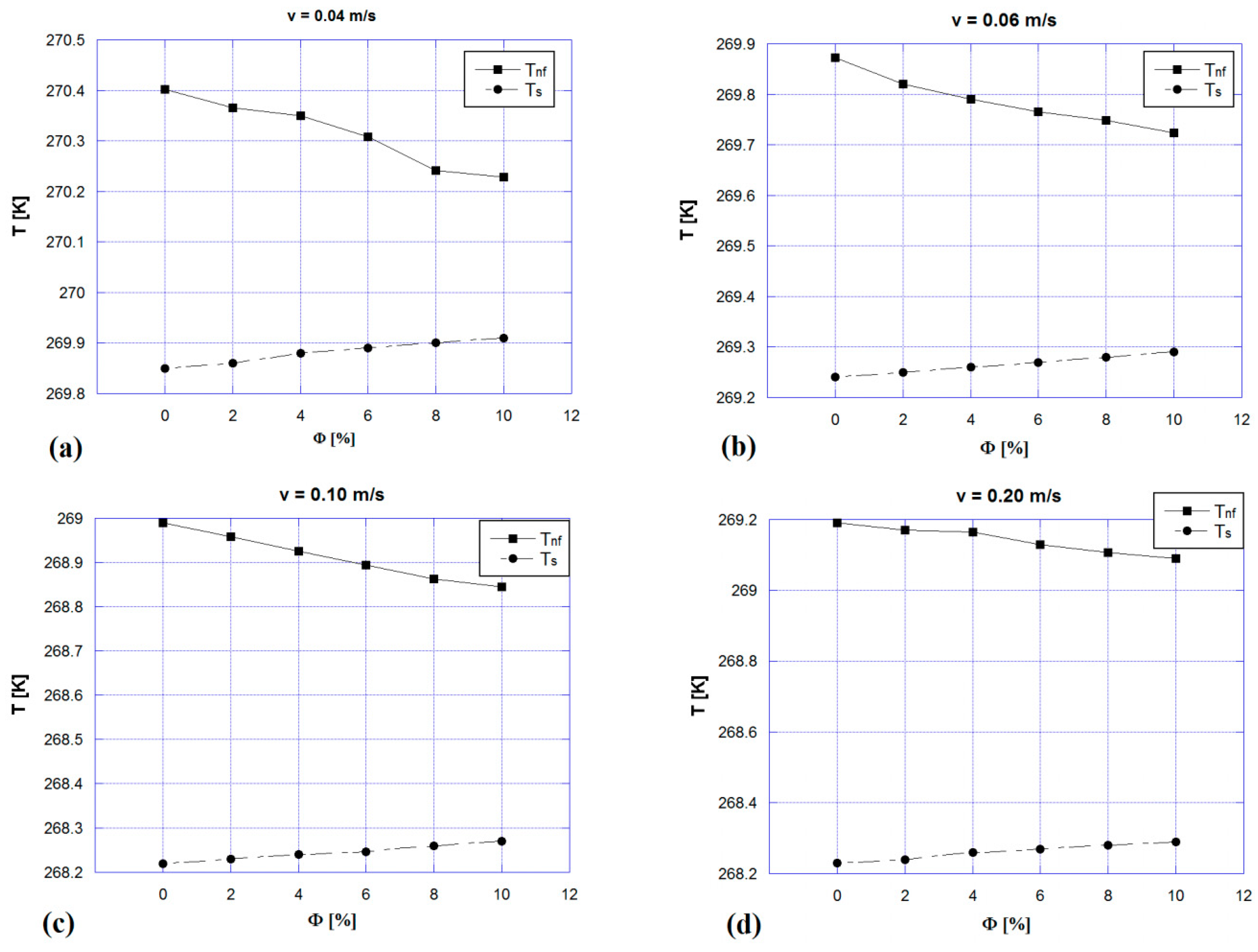
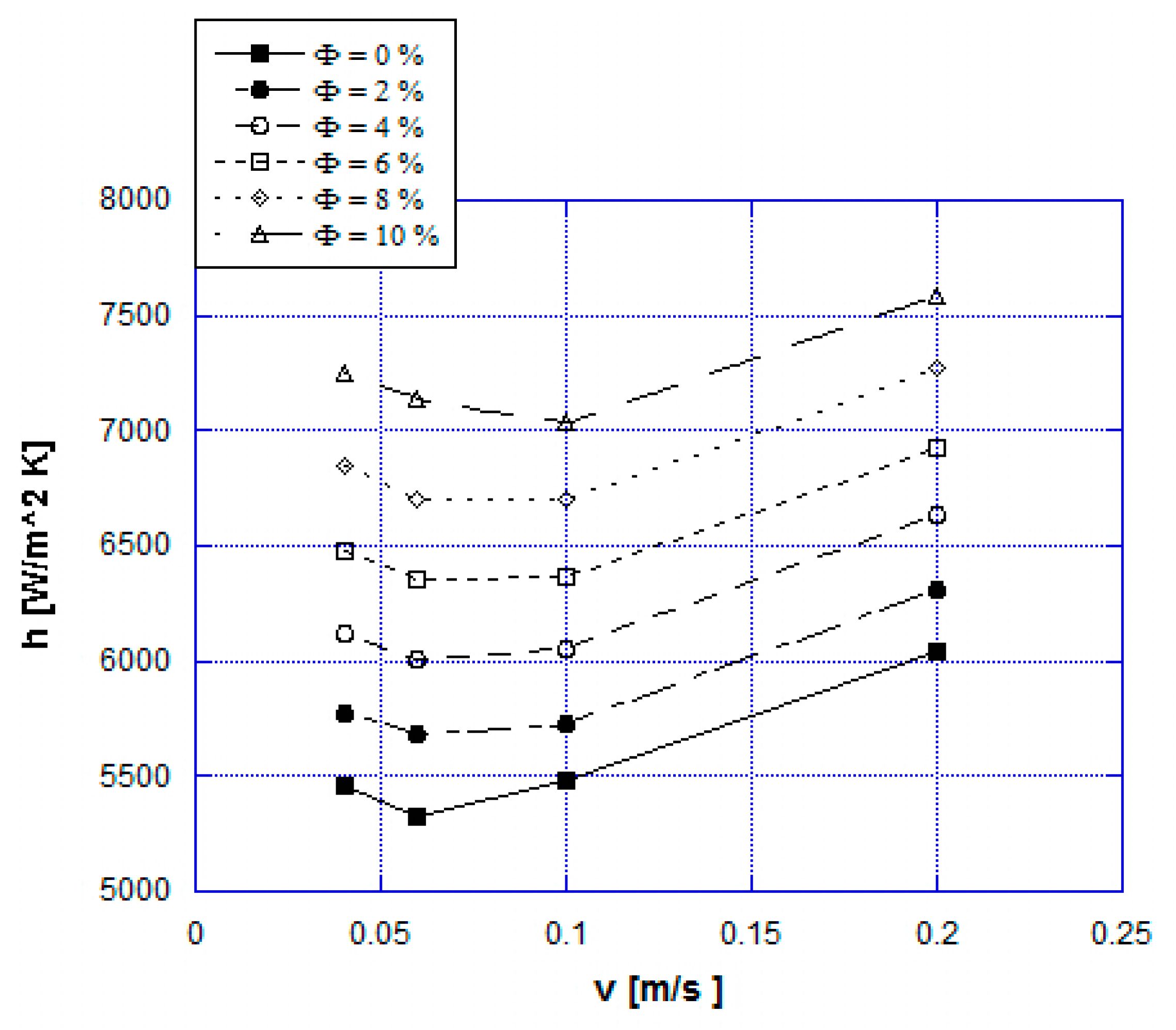
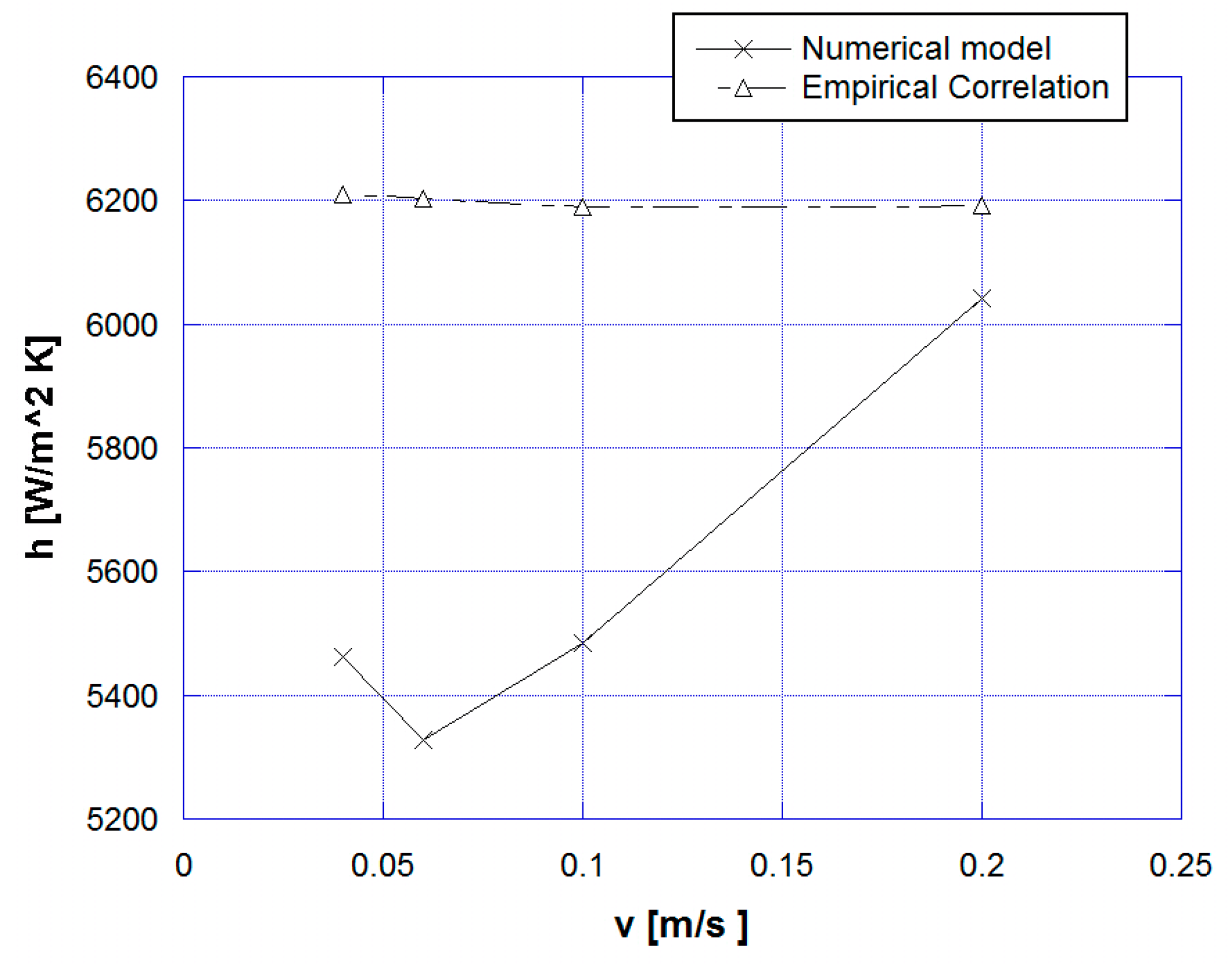

| Material | Tpeak [K] | Δp [GPa] | ∆Tad,max [K] | Density [kg/m3] | Thermal Conductivity [W/mK] |
|---|---|---|---|---|---|
| ASR | 298 | 0.390 | 41.1 | 960 | 1.48 |
| Material | Specific Heat [J/kgK] | Density [kg/m3] | Thermal Conductivity [W/mK] |
|---|---|---|---|
| Cu | 383 | 8933 | 401 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Enhancing the Heat Transfer in an Active Barocaloric Cooling System Using Ethylene-Glycol Based Nanofluids as Secondary Medium. Energies 2019, 12, 2902. https://doi.org/10.3390/en12152902
Aprea C, Greco A, Maiorino A, Masselli C. Enhancing the Heat Transfer in an Active Barocaloric Cooling System Using Ethylene-Glycol Based Nanofluids as Secondary Medium. Energies. 2019; 12(15):2902. https://doi.org/10.3390/en12152902
Chicago/Turabian StyleAprea, Ciro, Adriana Greco, Angelo Maiorino, and Claudia Masselli. 2019. "Enhancing the Heat Transfer in an Active Barocaloric Cooling System Using Ethylene-Glycol Based Nanofluids as Secondary Medium" Energies 12, no. 15: 2902. https://doi.org/10.3390/en12152902







