Study of Solid Calcium Diglyceroxide for Biodiesel Production from Waste Cooking Oil Using a High Speed Homogenizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Equipment
2.3. Experimental Procedure
2.3.1. Synthesis Procedure for the Calcium Diglyceroxide Catalyst
2.3.2. Transesterification Reaction Procedure
2.4. Analytical Methods
3. Results and Discussion
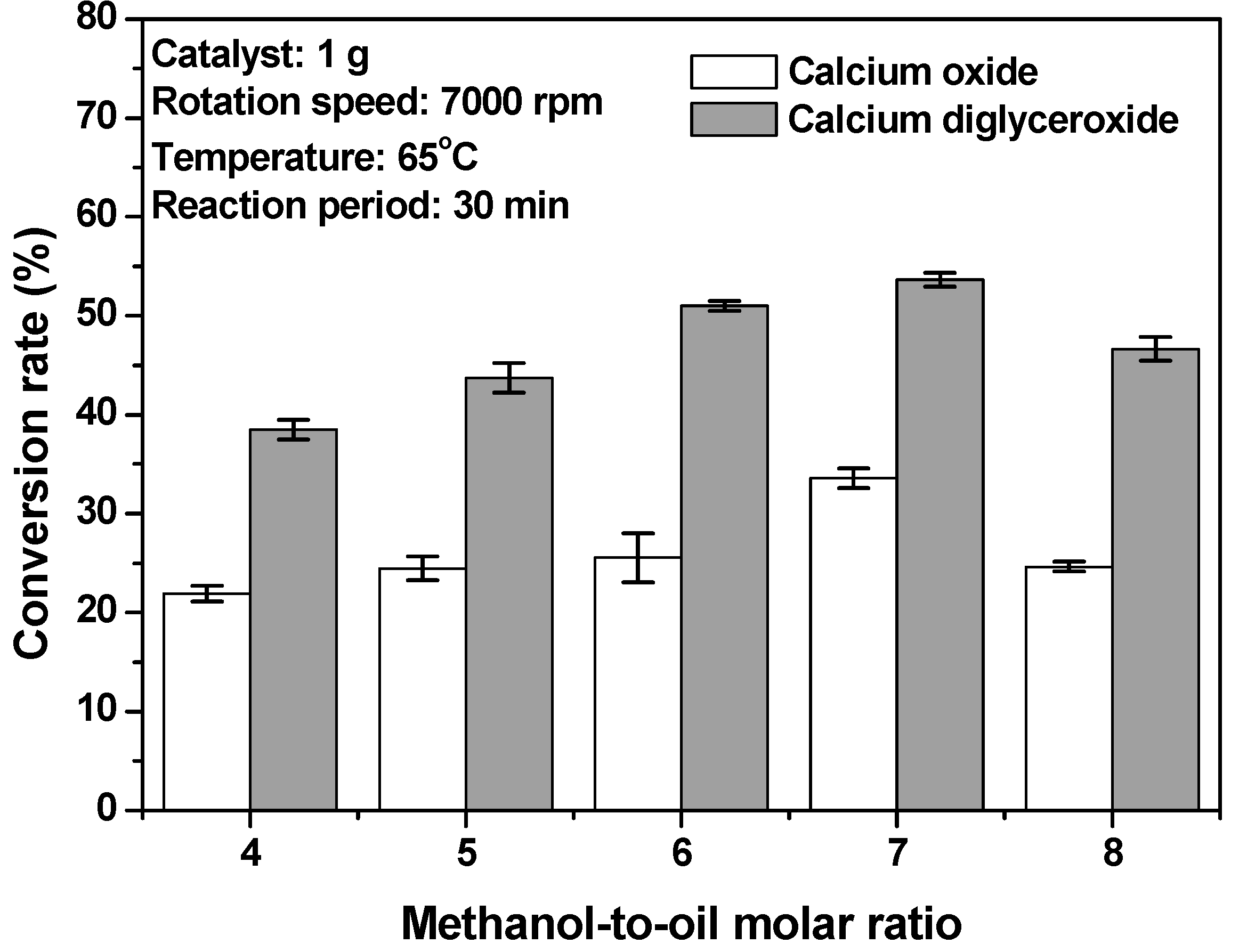
3.1. Effects of Methanol-to-Oil Molar Ratio and Catalyst Type on the Conversion Rate of Biodiesel
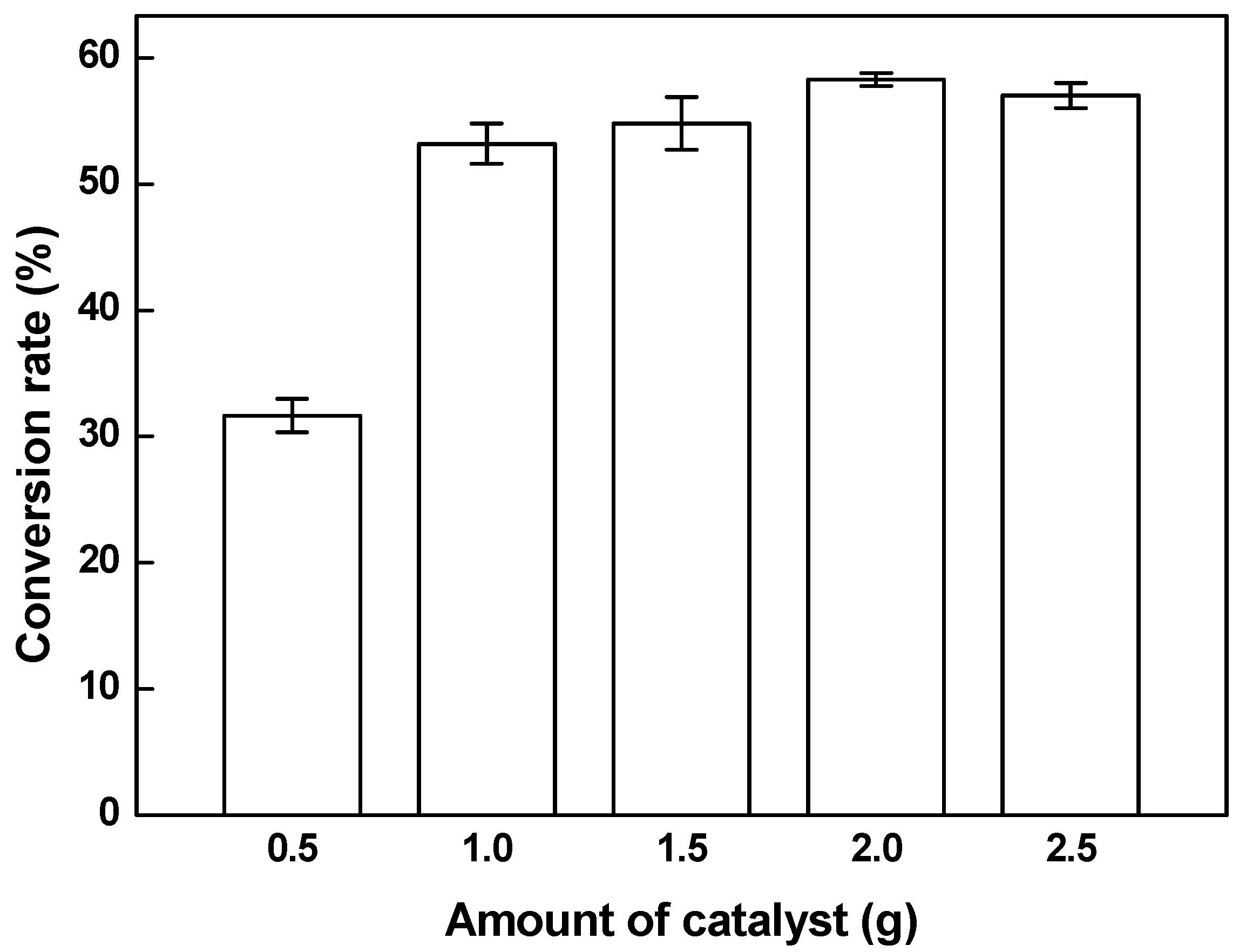
3.2. Effects of the Amount of Catalyst on the Conversion Rate of Biodiesel
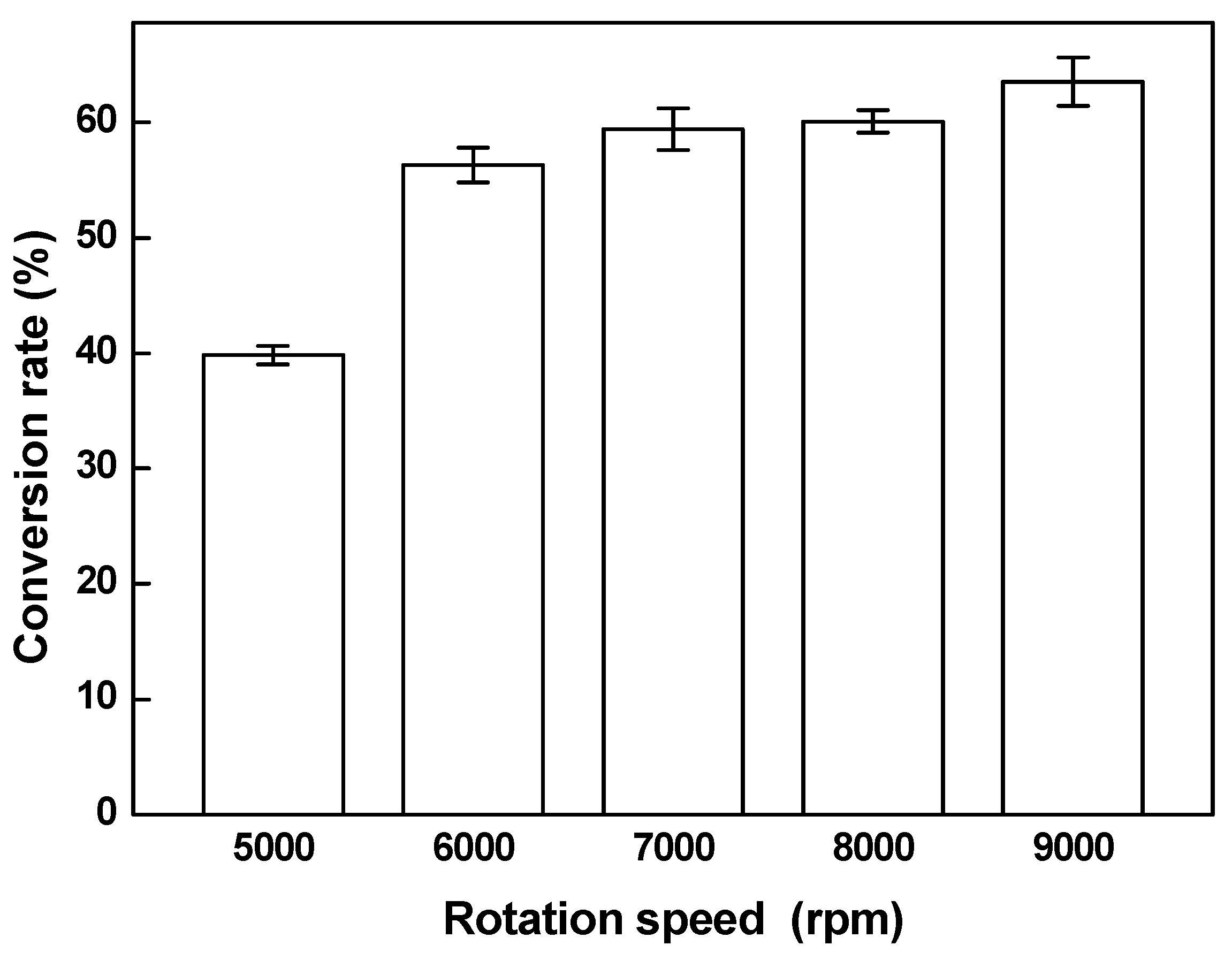
3.3. Effect of Rotation on the Conversion Rate of Biodiesel
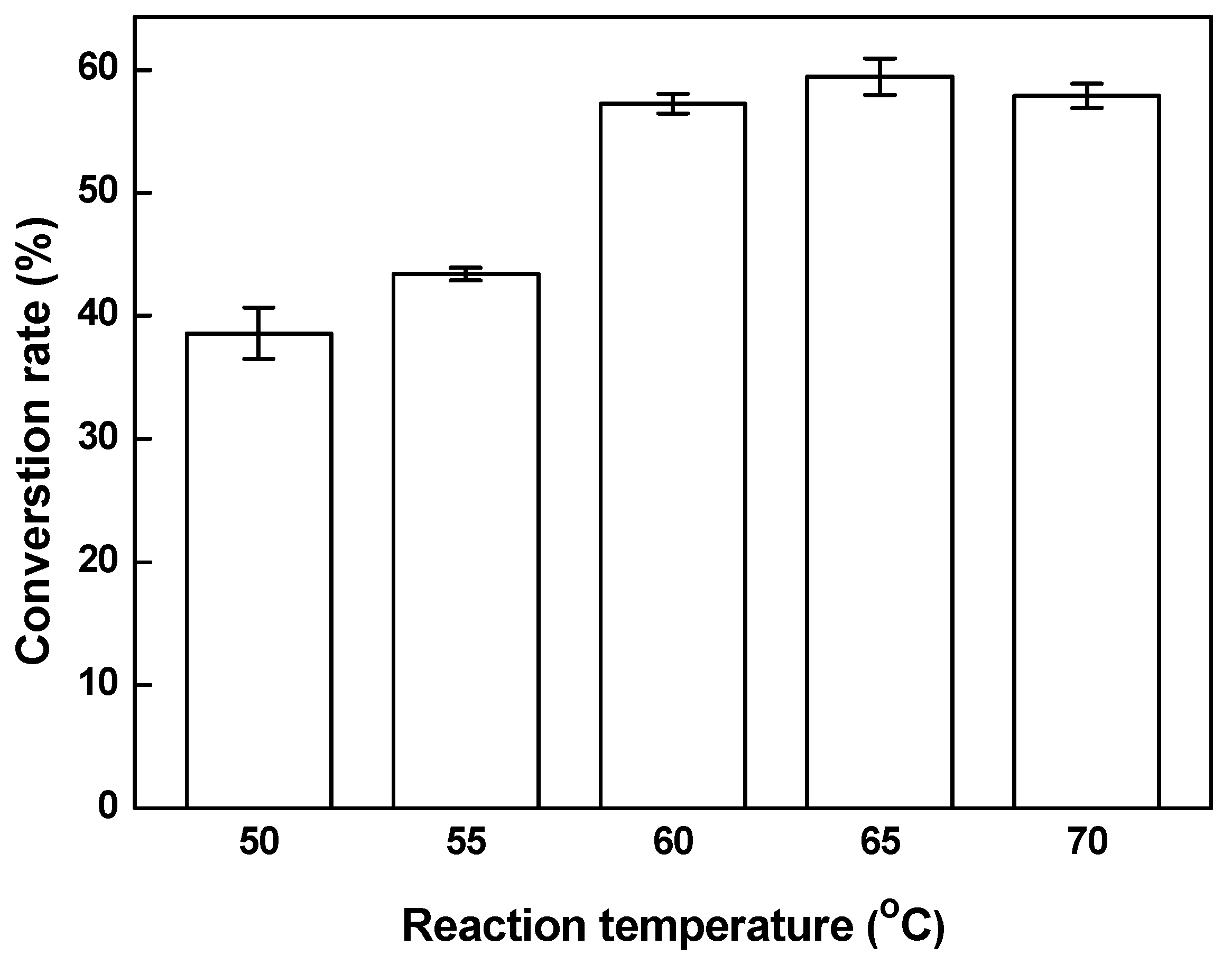
3.4. Effect of Reaction Temperature on the Conversion Rate of Biodiesel
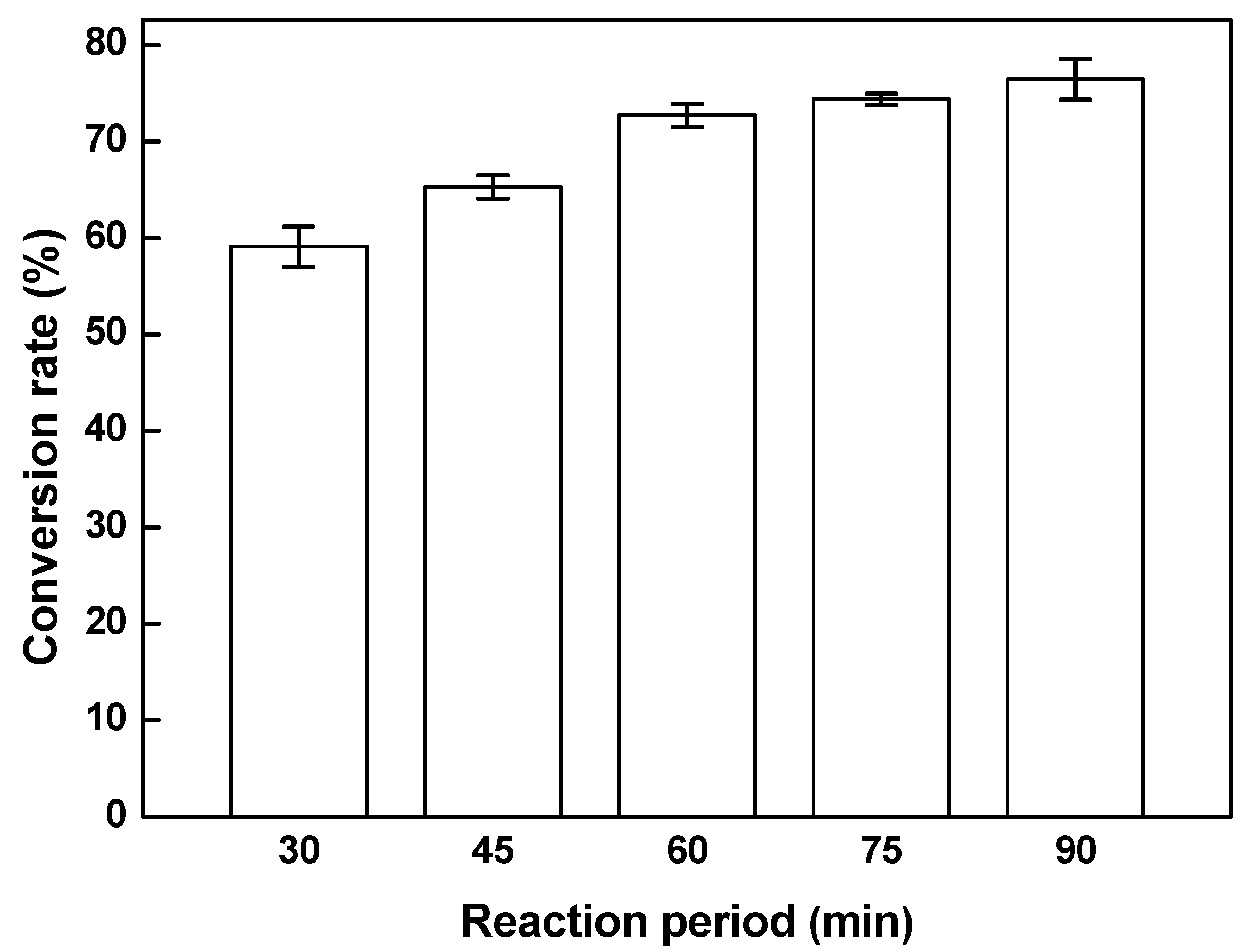
3.5. Effect of Reaction Period on the Conversion Rate of Biodiesel
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbaszadeh-Mayvan, A.; Ghobadian, B.; Najafi, G.; Yusaf, T. Intensification of continuous biodiesel production from waste cooking oils using shockwave power reactor: Process evaluation and optimization through response surface methodology (RSM). Energies 2018, 11, 2845. [Google Scholar] [CrossRef]
- Kesic, Z.; Lukic, I.; Zdujic, M.; Mojovic, L.; Skala, D. Calcium oxide based catalysts for biodiesel production: A review. Chem. Ind. Chem. Eng. Q. 2016, 22, 391–408. [Google Scholar] [CrossRef]
- Sirisomboonchai, S.; Abuduwayiti, M.; Guan, G.; Samart, C.; Abliz, S.; Hao, X.; Kusakabe, K.; Abudula, A. Biodiesel production from waste cooking oil using calcined scallop shell as catalyst. Energy Convers. Manag. 2015, 95, 242–247. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Egg shell waste as heterogeneous nanocatalyst for biodiesel production: Optimized by response surface methodology. J. Environ. Manag. 2017, 198, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Asikin-Mijan, N.; Lee, H.V.; Taufiq-Yap, Y.H. Synthesis and catalytic activity of hydration–dehydration treated clamshell derived CaO for biodiesel production. Chem. Eng. Res. Des. 2015, 102, 368–377. [Google Scholar] [CrossRef]
- Kostić, M.D.; Bazargan, A.; Stamenković, O.S.; Veljković, V.B.; McKay, G. Optimization and kinetics of sunflower oil methanolysis catalyzed by calcium oxide-based catalyst derived from palm kernel shell biochar. Fuel 2016, 163, 304–313. [Google Scholar] [CrossRef]
- Viriya-Empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef]
- Yoo, S.J.; Lee, H.S.; Veriansyah, B.; Kim, J.; Kim, J.D.; Lee, Y.W. Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts. Bioresour. Technol. 2010, 101, 8686–8689. [Google Scholar] [CrossRef] [PubMed]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Kouzu, M.; Tsunomori, M.; Yamanaka, S.; Hidaka, J. Solid base catalysis of calcium oxide for a reaction to convert vegetable oil into biodiesel. Adv. Powder Technol. 2010, 21, 488–494. [Google Scholar] [CrossRef]
- Lukić, I.; Kesić, Ž.; Zdujić, M.; Skala, D. Calcium diglyceroxide synthesized by mechanochemical treatment, its characterization and application as catalyst for fatty acid methyl esters production. Fuel 2016, 165, 159–165. [Google Scholar] [CrossRef]
- Sánchez-Cantú, M.; Reyes-Cruz, F.M.; Rubio-Rosas, E.; Pérez-Díaz, L.M.; Ramírez, E.; Valente, J.S. Direct synthesis of calcium diglyceroxide from hydrated lime and glycerol and its evaluation in the transesterification reaction. Fuel 2014, 138, 126–133. [Google Scholar] [CrossRef]
- León-Reina, L.; Cabeza, A.; Rius, J.; Maireles-Torres, P.; Alba-Rubio, A.C.; Granados, M.L. Structural and surface study of calcium glyceroxide, an active phase for biodiesel production under heterogeneous catalysis. J. Catal. 2013, 300, 30–36. [Google Scholar] [CrossRef]
- Gupta, A.R.; Yadav, S.V.; Rathod, V.K. Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Calcium diglyceroxide catalyzed biodiesel production from waste cooking oil in the presence of microwave: Optimization and kinetic studies. Renew. Energy 2018, 121, 757–767. [Google Scholar] [CrossRef]
- Esipovich, A.; Rogozhin, A.; Danov, S.; Belousov, A.; Kanakov, E. The structure, properties and transesterification catalytic activities of the calcium glyceroxide. Chem. Eng. J. 2018, 339, 303–316. [Google Scholar] [CrossRef]
- Yunus Khan, T.M.; Badruddin, I.A.; Ankalgi, R.F.; Badarudin, A.; Hungund, B.S.; Ankalgi, F.R. Biodiesel Production by Direct Transesterification Process via Sequential Use of Acid–Base Catalysis. Arab. J. Sci. Eng. 2018, 43, 5929–5936. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Gogate, P.R.; Pandit, A.B. Intensified synthesis of biodiesel using hydrodynamic cavitation reactors based on the interesterification of waste cooking oil. Fuel 2014, 137, 285–292. [Google Scholar] [CrossRef]
- Joshi, S.; Gogate, P.R.; Moreira, P.F., Jr.; Giudici, R. Intensification of biodiesel production from soybean oil and waste cooking oil in the presence of heterogeneous catalyst using high speed homogenizer. Ultrason. Sonochem. 2017, 39, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.C.; Kuo, J.Y.; Hsieh, P.H.; Hou, S.S. Improving biodiesel conversions from blends of high-and low-acid-value waste cooking oils using sodium methoxide as a catalyst based on a high speed homogenizer. Energies 2018, 11, 2298. [Google Scholar] [CrossRef]
- Ding, J.; Xia, Z.; Lu, J. Esterification and deacidification of a waste cooking oil (TAN 68.81 mg KOH/g) for biodiesel production. Energies 2012, 5, 2683–2691. [Google Scholar] [CrossRef]
- Nkafamiya, I.I.; Maina, H.M.; Osemeahon, S.A.; Modibbo, U.U. Percentage oil yield and physiochemical properties of different groundnut species (Arachis hypogaea). Afr. J. Food Sci. 2010, 4, 418–421. [Google Scholar]
- Zhu, H.; Wu, Z.; Chen, Y.; Zhang, P.; Duan, S.; Liu, X.; Mao, Z. Preparation of biodiesel catalyzed by solid super base of calcium oxide and its refining process. Chin. J. Catal. 2006, 27, 391–396. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Kuan, I.C.; Kao, W.C.; Chen, C.L.; Yu, C.Y. Microbial biodiesel production by direct transesterification of Rhodotorula glutinis biomass. Energies 2018, 11, 1036. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, S.; Liu, P.; Xue, F.; Tang, S. Comparison of two different processes to synthesize biodiesel by waste cooking oil. J. Mol. Catal. Chem. 2006, 252, 107–112. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H. Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel 2011, 90, 1963–1967. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H.; Chen, L.C. Ultrasonic mixing and closed microwave irradiation-assisted transesterification of soybean oil. Fuel 2010, 89, 3618–3622. [Google Scholar] [CrossRef]
- Mohamad, M.; Ngadi, N.; Wong, S.L.; Jusoh, M.; Yahya, N.Y. Prediction of biodiesel yield during transesterification process using response surface methodology. Fuel 2017, 190, 104–112. [Google Scholar] [CrossRef]
- Pal, A.; Verma, A.; Kachhwaha, S.S.; Maji, S. Biodiesel production through hydrodynamic cavitation and performance testing. Renew. Energy 2010, 35, 619–624. [Google Scholar] [CrossRef]
- Mohod, A.V.; Gogate, P.R.; Viel, G.; Firmino, P.; Giudici, R. Intensification of biodiesel production using hydrodynamic cavitation based on high speed homogenizer. Chem. Eng. J. 2017, 316, 751–757. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, M.-C.; Chang, L.-W.; Hou, S.-S. Study of Solid Calcium Diglyceroxide for Biodiesel Production from Waste Cooking Oil Using a High Speed Homogenizer. Energies 2019, 12, 3205. https://doi.org/10.3390/en12173205
Hsiao M-C, Chang L-W, Hou S-S. Study of Solid Calcium Diglyceroxide for Biodiesel Production from Waste Cooking Oil Using a High Speed Homogenizer. Energies. 2019; 12(17):3205. https://doi.org/10.3390/en12173205
Chicago/Turabian StyleHsiao, Ming-Chien, Li-Wen Chang, and Shuhn-Shyurng Hou. 2019. "Study of Solid Calcium Diglyceroxide for Biodiesel Production from Waste Cooking Oil Using a High Speed Homogenizer" Energies 12, no. 17: 3205. https://doi.org/10.3390/en12173205





