Effect of Combined Inoculation on Biogas Production from Hardly Degradable Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculums and Substrate
2.2. Batch Tests
2.3. Analytical Methods
2.4. Kinetic Study
2.5. Statistical Analysis
3. Results
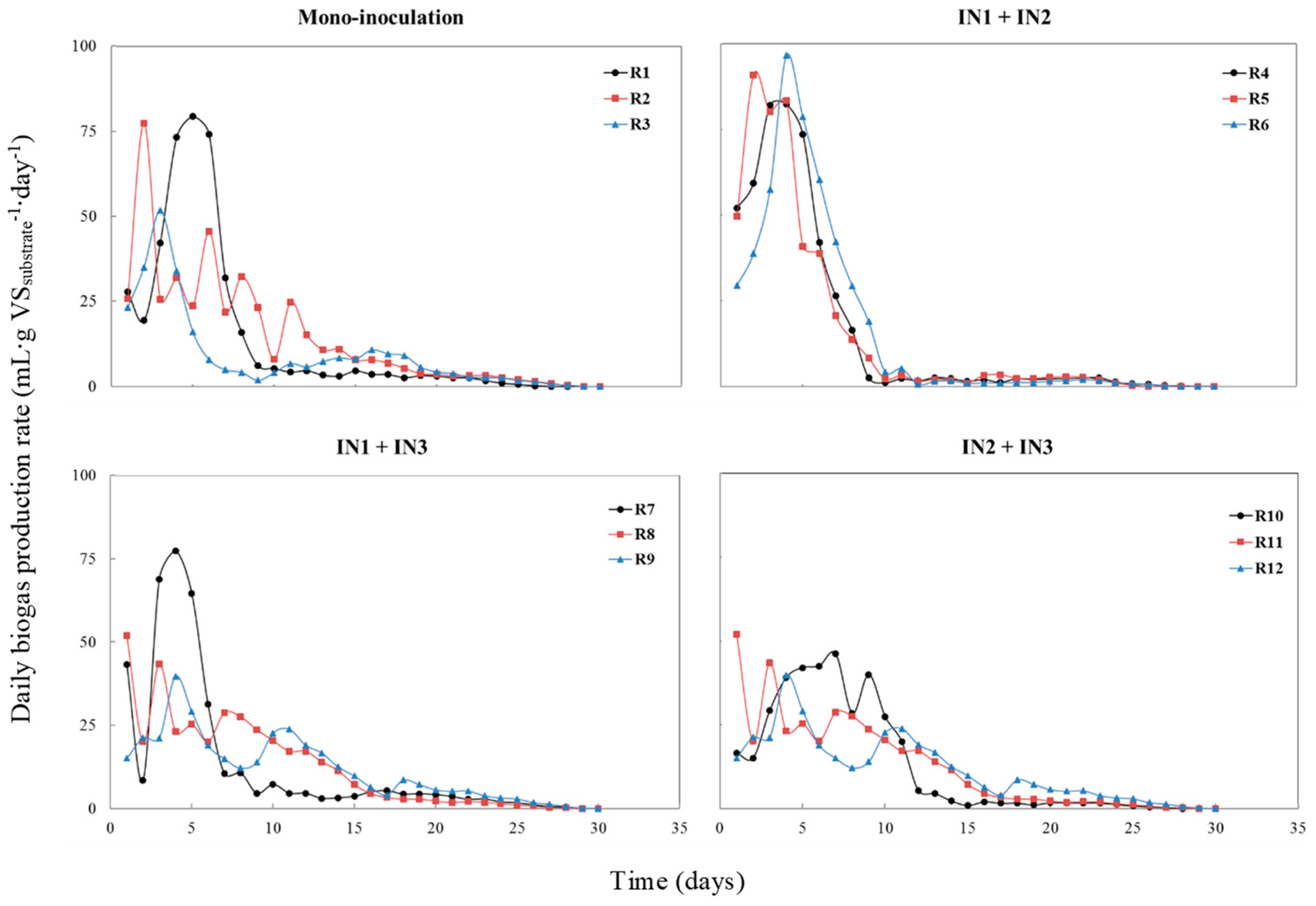
3.1. Biogas Production
3.2. pH and Alkalinity
3.3. VS% Removal
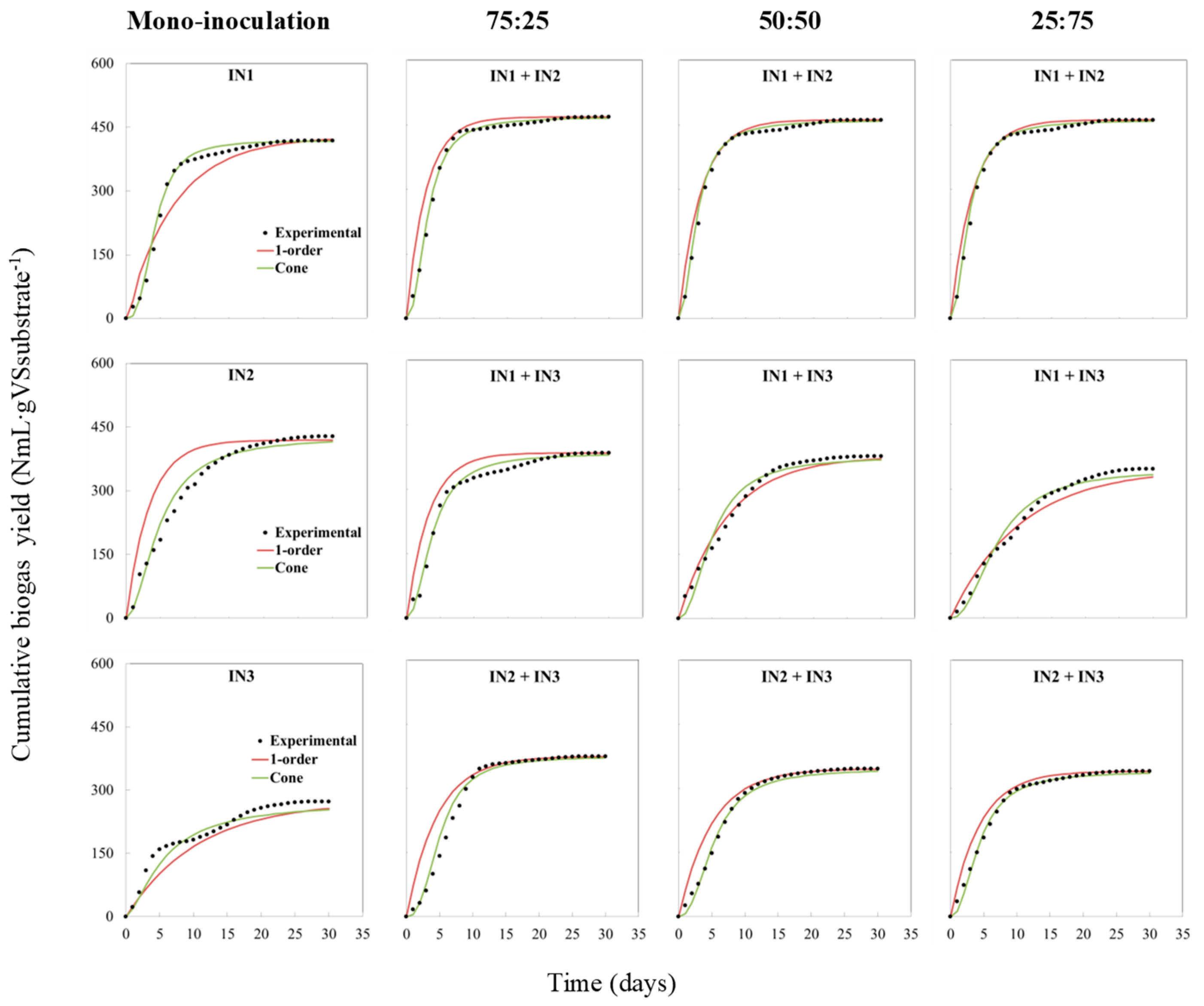
3.4. Kinetics Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sahajwalla, V. Green processes: Transforming waste into valuable resources. Engineering 2018, 4, 309–310. [Google Scholar] [CrossRef]
- Lauer, M.; Thrän, D. Flexible biogas in future energy systems—Sleeping beauty for a cheaper power generation. Energies 2018, 11, 761. [Google Scholar] [CrossRef]
- Davis, L.A. The shale oil and gas revolution. Engineering 2018, 4, 438–439. [Google Scholar] [CrossRef]
- Chen, P.; Anderson, E.; Addy, M.; Zhang, R.; Cheng, Y.; Peng, P.; Ma, Y.; Fan, L.; Zhang, Y.; Lu, Q.; et al. Breakthrough technologies for the biorefining of organic solid and liquid wastes. Engineering 2018, 4, 574–580. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopoulos, P. Green conversion of municipal solid wastes into fuels and chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar] [CrossRef]
- RedCorn, R.; Fatemi, S.; Engelberth, A.S. Comparing end-use potential for industrial food-waste sources. Engineering 2018, 4, 371–380. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A technological overview of biogas production from biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Chacón-Pérez, Y.; Cardona-Alzate, C.A. Evaluation of biogas and syngas as energy vectors for heat and power generation using lignocellulosic biomass as raw material. Electron. J. Biotechnol. 2018, 33, 52–62. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Fan, Y. Techno-economic challenges of fuel cell commercialization. Engineering 2018, 4, 352–360. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E. Membrane engineering for green process engineering. Engineering 2017, 3, 290–298. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V. Biogas combustion: An introductory briefing. In Biogas: Production, Applications and Global Developments; Vico, A., Artemio, N., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 179–193. [Google Scholar]
- Chen, J.F. Green chemical engineering. Engineering 2017, 3, 283–284. [Google Scholar] [CrossRef]
- Wen-Wei, L.; Han-Qing, Y. Advances in energy-producing anaerobic biotechnologies for municipal wastewater treatment. Engineering 2016, 2, 438–446. [Google Scholar]
- Chen, J.F. Green chemical engineering for a better life. Engineering 2017, 3, 279. [Google Scholar] [CrossRef]
- Nelson, M.J.; Nakhla, G.; Zhu, J. Fluidized-bed bioreactor applications for biological wastewater treatment: A review of research and developments. Engineering 2017, 3, 330–342. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Panico, A.; Pirozzi, F. Enhanced bio-methane production from co-digestion of different organic wastes. Environ. Technol. 2012, 33, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyo, S.; Sittijunda, S.; Reungsang, A. Co-digestion of napier grass with food waste and napier silage with food waste for methane production. Energies 2018, 11, 3200. [Google Scholar] [CrossRef]
- De Souza Guimarães, C.; da Silva Maia, D.R.; Gonçalves Serra, E. Construction of biodigesters to optimize the production of biogas from anaerobic co-digestion of food waste and sewage. Energies 2018, 11, 870. [Google Scholar] [CrossRef]
- EPA. Increasing Anaerobic Digester Performance with Codigestion. 2012. Available online: https://www.epa.gov/agstar (accessed on 7 September 2018).
- Cheng, L.; Charles, W.; Cord-Ruwisch, R. Automatic online buffer capacity (alkalinity) measurement of wastewater using an electrochemical cell. Environ. Technol. 2016, 37, 2467–2472. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from fresh spring and summer grass: Effect of the harvesting period. Energies 2018, 11, 1466. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Consolidated briefing of biochemical ethanol production from lignocellulosic biomass. Electron. J. Biotechnol. 2016, 23, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Pellera, F.M.; Gidarakos, E. Effect of the substrate to inoculum ratio and inoculum type on the biochemical methane potential of solid agroindustrial waste. J. Environ. Chem. Eng. 2016, 4, 3217–4229. [Google Scholar] [CrossRef]
- Gao, Y.; Kong, X.; Xing, T.; Sun, Y.; Zhang, Y.; Luo, X.; Sun, Y. Digestion performance and microbial metabolic mechanism in thermophilic and mesophilic anaerobic digesters exposed to elevated loadings of organic fraction of municipal solid waste. Energies 2018, 11, 952. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenerg. 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Nallathambi Gunaseelan, V. Effect of inoculum/substrate ratio and pretreatments on methane yield from Parthenium. Biomass Bioenerg. 1995, 8, 39–44. [Google Scholar] [CrossRef]
- Kasprzycka, A.; Kuna, J. Methodical aspects of biogas production in small-volume bioreactors in laboratory investigations. Energies 2018, 11, 1378. [Google Scholar] [CrossRef]
- Fabbri, A.; Serranti, S.; Bonifazi, G. Biochemical methane potential (BMP) of artichoke waste: The inoculum effect. Waste Manag. Res. 2014, 32, 207–214. [Google Scholar] [CrossRef]
- Eaton, A.D.; American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Lossie, U.; Pütz, P. Targeted Control of Biogas Plants with the Help of FOS/TAC. Pract. Rep. Hach Lange 2008. Available online: file:///D:/Downloads/14785938_DOC042.52.20011.Mar08.web.pdf (accessed on 24 October 2018).
- Palmowski, L.M.; Müller, J.A. Influence of the size reduction of organic waste on their anaerobic digestion. Water Sci. Technol. 2000, 41, 155–162. [Google Scholar] [CrossRef]
- VDI 4630, 2006. Fermentation of Organic Materials, Characterisation of Substrate, Sampling, Collection of Material Data, Fermentation Tests. Available online: https://standards.globalspec.com/std/10052171/vdi-4630 (accessed on 26 October 2018).
- Water Research Centre. Equipment for Measurement of Gas Production at Low Rates of Flow—Technical Memorandum TM104; Water Research Centre: Medmenham, UK, 1975. [Google Scholar]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Buck, A.L. New equations for computing vapor pressure and enhancement factor. J. Appl. Meteorol. 1981, 20, 1527–1532. [Google Scholar] [CrossRef]
- Luna-del Risco, M.; Normak, A.; Orupõld, K. Biochemical methane potential of different organic wastes and energy crops from Estonia. Agron. Res. 2011, 9, 331–342. [Google Scholar]
- Lay, J.; Li, Y.; Noike, T. Interaction between homoacetogens and methanogens in lake sediments. J. Ferment. Bioeng. 1998, 86, 467–471. [Google Scholar] [CrossRef]
- Bhattarai, S.; Oh, J.H.; Euh, S.H.; Kafle, G.K.; Kim, D.H. Simulation and model validation of sheet and tube type photovoltaic thermal solar system and conventional solar collecting system in transient states. Sol. Energy Mater. Sol. C 2012, 103, 184–193. [Google Scholar] [CrossRef]
- Ghasimi, D.S.M.; Zandvoort, M.H.; Adriaanse, M.; van Lier, J.B.; de Kreuk, M. Comparative analysis of the digestibility of sewage fine sieved fraction and hygiene paper produced from virgin fibers and recycled fibers. Waste Manag. 2016, 53, 156–164. [Google Scholar] [CrossRef]
- De Vrieze, J.; Gildemyn, S.; Vilchez-Vargas, R.; Jáuregui, R.; Pieper, D.H.; Verstraete, W.; Boon, N. Inoculum selection is crucial to ensure operational stability in anaerobic digestion. Appl. Microbiol. Biotechnol. 2015, 99, 189–199. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G.; Hafez, H. Biochemical methane potential (BMP) of food waste and primary sludge: Influence of inoculum pre-incubation and inoculum source. Bioresour. Technol. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- Ranieri, L.; Mossa, G.; Pellegrino, R.; Digiesi, S. Energy recovery from the organic fraction of Municipal Solid Waste: A real options-based facility assessment. Sustainability 2018, 10, 368. [Google Scholar] [CrossRef]
- Turovskiy, I.S.; Mathai, P.K. Wastewater Sludge Processing; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Carotenuto, C.; Guarino, G.; Mario Minale, M. Temperature and pH effect on methane production from buffalo manure anaerobic digestion. Int. J. Heat Technol. 2016, 34, 425–429. [Google Scholar] [CrossRef]
- Franchi, O.; Rosenkranz, F.; Chamy, R. Key microbial populations involved in anaerobic degradation of phenol and p-cresol using different inocula. Electron. J. Biotechnol. 2018, 35, 33–38. [Google Scholar] [CrossRef]
- Świątek, M.; Lewicki, A.; Szymanowska, D.; Kubiak, P. The effect of introduction of chicken manure on the biodiversity and performance of an anaerobic digester. Electron. J. Biotechnol. 2018, in press. [Google Scholar] [CrossRef]
- Anggarini, S.; Hidayat, N.; Sunyoto, N.M.S.; Wulandari, P.S. Optimization of hydraulic retention time (HRT) and inoculums addition in wastewater treatment using anaerobic digestion system. Agric. Agric. Sci. Prog. 2015, 3, 95–101. [Google Scholar] [CrossRef]
- Silva, A.J.; Pozzi, E.; Foresti, E.; Zaiat, M. The influence of the buffering capacity on the production of organic acids and alcohols from wastewater in anaerobic reactor. Appl. Biochem. Biotechnol. 2015, 175, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Triet, N.M.; Khang, D.N.; Preston, T.R. Improving the buffering capacity of biodigesters charged with cassava waste-water. Livest. Res. Rural Dev. 2017, 29. Available online: http://www.lrrd.org/lrrd29/2/trie29037.html (accessed on 26 October 2018).
- Gray, M.C.; Converse, A.O.; Wyman, C.E. Sugar Monomer and oligomer solubility: Data and predictions for application to biomass hydrolysis. Appl. Biochem. Biotechnol. 2003, 105–108, 179–193. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Müller, B.; Schnürer, A. Importance of inoculum source and initial community structure for biogas production from agricultural substrates. Bioresour. Technol. 2017, 245, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Pommier, S.; Llamas, A.M.; Lefebvre, X. Analysis of the outcome of shredding pretreatment on the anaerobic biodegradability of paper and cardboard materials. Bioresour. Technol. 2010, 101, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Influence of sheep manure addition on biogas potential and methanogenic communities during cow dung digestion under mesophilic conditions. Sustain. Environ. Res. 2018, 28, 240–246. [Google Scholar] [CrossRef]
- Ghasimi, D.S.M.; de Kreuk, M.; Maeng, S.K.; Zandvoort, M.H.; van Lier, J.B. High-rate thermophilic bio-methanation of the fine sieved fraction from Dutch municipal raw sewage: Cost-effective potentials for on-site energy recovery. Appl. Energy 2016, 165, 569–582. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Unit | IN1 | IN2 | IN3 | PFSF |
|---|---|---|---|---|---|
| pH | – | 7.56 | 7.21 | 6.72 | ND |
| TS | g∙kg−1 | 48.2 (1.1) | 117.7 (1.3) | 82.6 (1.9) | 193.2 (9.5) |
| VS | g∙kg−1 | 30.2 (0.8) | 64.8 (1.6) | 61.3 (1.3) | 174.8 (8.8) |
| VS/TS | – | 0.63 | 0.55 | 0.74 | 0.91 |
| COD | g∙kg−1 | 47.2 (1.7) | 83.9 (4.3) | 82.4 (5.2) | ND |
| VS/COD | – | 0.64 | 0.77 | 0.74 | ND |
| Reactors | IN1 | IN2 | IN3 | Organic load | ISR | Temperature (°C) | Replicates |
|---|---|---|---|---|---|---|---|
| R1 | 100 | 0 | 0 | 12 | 2 | 36 | 3 |
| R2 | 0 | 100 | 0 | 12 | 2 | 36 | 3 |
| R3 | 0 | 0 | 100 | 12 | 2 | 36 | 3 |
| R4 | 75 | 25 | 0 | 12 | 2 | 36 | 3 |
| R5 | 50 | 50 | 0 | 12 | 2 | 36 | 3 |
| R6 | 25 | 75 | 0 | 12 | 2 | 36 | 3 |
| R7 | 75 | 0 | 25 | 12 | 2 | 36 | 3 |
| R8 | 50 | 0 | 50 | 12 | 2 | 36 | 3 |
| R9 | 25 | 0 | 75 | 12 | 2 | 36 | 3 |
| R10 | 0 | 75 | 25 | 12 | 2 | 36 | 3 |
| R11 | 0 | 50 | 50 | 12 | 2 | 36 | 3 |
| R12 | 0 | 25 | 75 | 12 | 2 | 36 | 3 |
| Reactor | Biogas Yield (mL∙g VSadded−1) | VS Removal (%) | T80 (day) |
|---|---|---|---|
| R1 | 418.9 (26.4) | 41.7 (3.9) | 7 |
| R2 | 427.6 (19.7) | 43.5 (2.6) | 12 |
| R3 | 273.0 (12.9) | 30.8 (2.8) | 15 |
| R4 | 468.2 (24.6) | 45.4 (4.1) | 6 |
| R5 | 461.3 (26.8) | 44.0 (3.9) | 6 |
| R6 | 479.7 (27.0) | 48.2 (4.6) | 8 |
| R7 | 384.6 (17.2) | 36.1 (2.5) | 8 |
| R8 | 377.1 (16.8) | 35.6 (3.0) | 12 |
| R9 | 347.2 (16.3) | 31.9 (2.7) | 14 |
| R10 | 374.8 (18.4) | 37.5 (3.4) | 9 |
| R11 | 345.7 (20.3) | 38.6 (2.9) | 10 |
| R12 | 340.2 (19.5) | 35.3 (3.1) | 8 |
| Reactor | First-Order Model | Cone Model | |||||
|---|---|---|---|---|---|---|---|
| K (day−1) | R2 | RMSE | K (day−1) | n | R2 | RMSE | |
| R1 | 0.2967 | 0.9244 | 51.63 | 0.2419 | 2.91 | 0.9959 | 6.9 |
| R2 | 0.1421 | 0.9906 | 12.08 | 0.2094 | 1.911 | 0.981 | 13.91 |
| R3 | 0.0941 | 0.9292 | 27.67 | 0.1804 | 1.54 | 0.9148 | 13.09 |
| R4 | 0.346 | 0.9692 | 38.47 | 0.3213 | 2.312 | 0.9949 | 5.64 |
| R5 | 0.3066 | 0.9858 | 23.45 | 0.3755 | 2.098 | 0.9927 | 6.309 |
| R6 | 0.3619 | 0.9738 | 63.33 | 0.2461 | 2.833 | 0.9961 | 6.687 |
| R7 | 0.2239 | 0.9513 | 42.75 | 0.2651 | 2.114 | 0.9888 | 8.787 |
| R8 | 0.1377 | 0.9797 | 15.30 | 0.2016 | 2.118 | 0.9839 | 10.33 |
| R9 | 0.0969 | 0.9917 | 21.24 | 0.1447 | 2.196 | 0.9837 | 10.84 |
| R10 | 0.2161 | 0.9292 | 47.09 | 0.1992 | 2613 | 0.99 | 10.26 |
| R11 | 0.1992 | 0.9555 | 30.21 | 0.1891 | 2.348 | 0.9895 | 9.717 |
| R12 | 0.2294 | 0.9838 | 22.88 | 0.2315 | 2.194 | 0.9933 | 5.563 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achinas, S.; Euverink, G.J.W. Effect of Combined Inoculation on Biogas Production from Hardly Degradable Material. Energies 2019, 12, 217. https://doi.org/10.3390/en12020217
Achinas S, Euverink GJW. Effect of Combined Inoculation on Biogas Production from Hardly Degradable Material. Energies. 2019; 12(2):217. https://doi.org/10.3390/en12020217
Chicago/Turabian StyleAchinas, Spyridon, and Gerrit Jan Willem Euverink. 2019. "Effect of Combined Inoculation on Biogas Production from Hardly Degradable Material" Energies 12, no. 2: 217. https://doi.org/10.3390/en12020217




