Creep Behaviors of Methane Hydrate-Bearing Frozen Sediments
Abstract
:1. Introduction
2. Experimental Methods
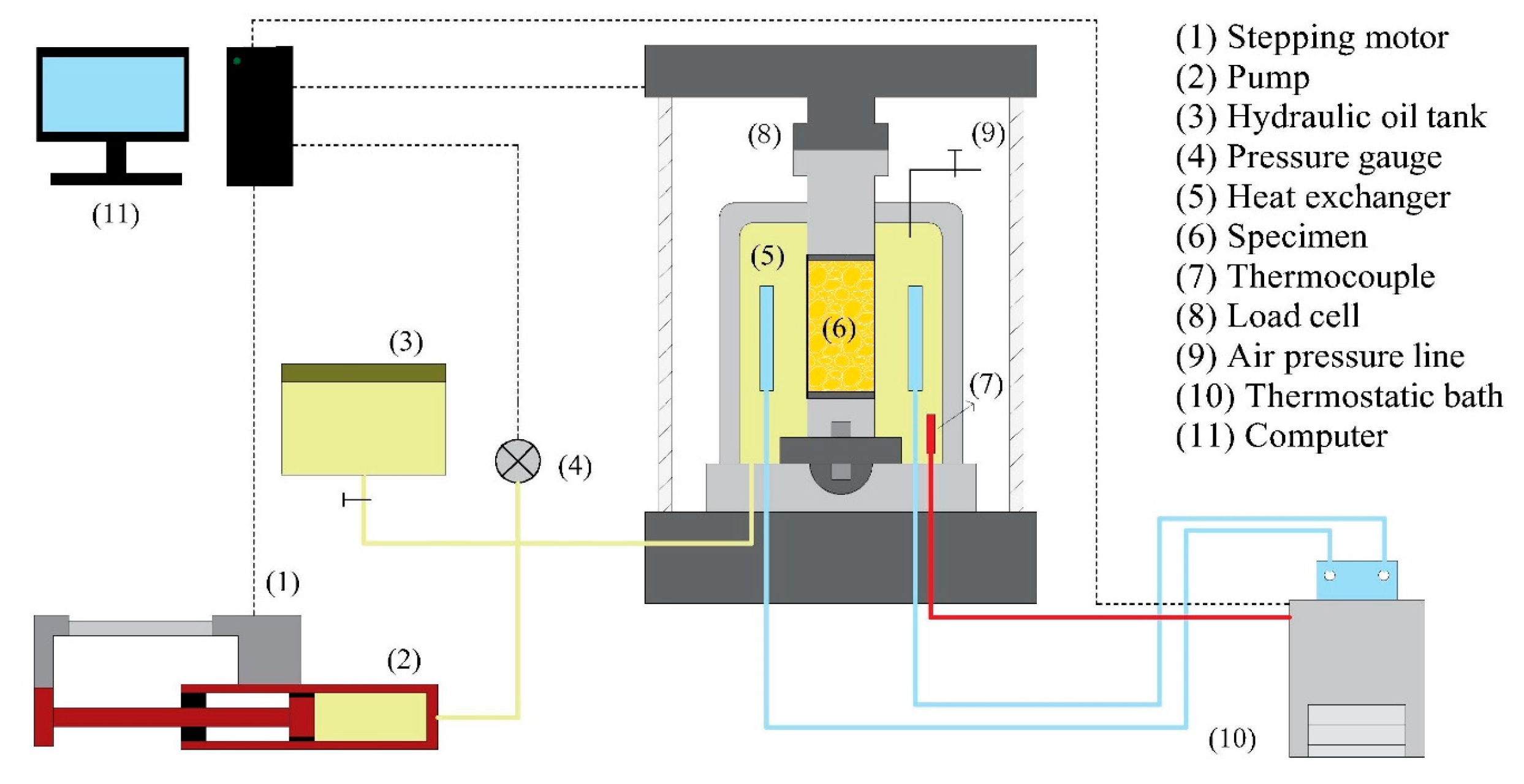
2.1. Experimental Apparatus
2.2. Experimental Procedure and Test Conditions
3. Results and Discussion
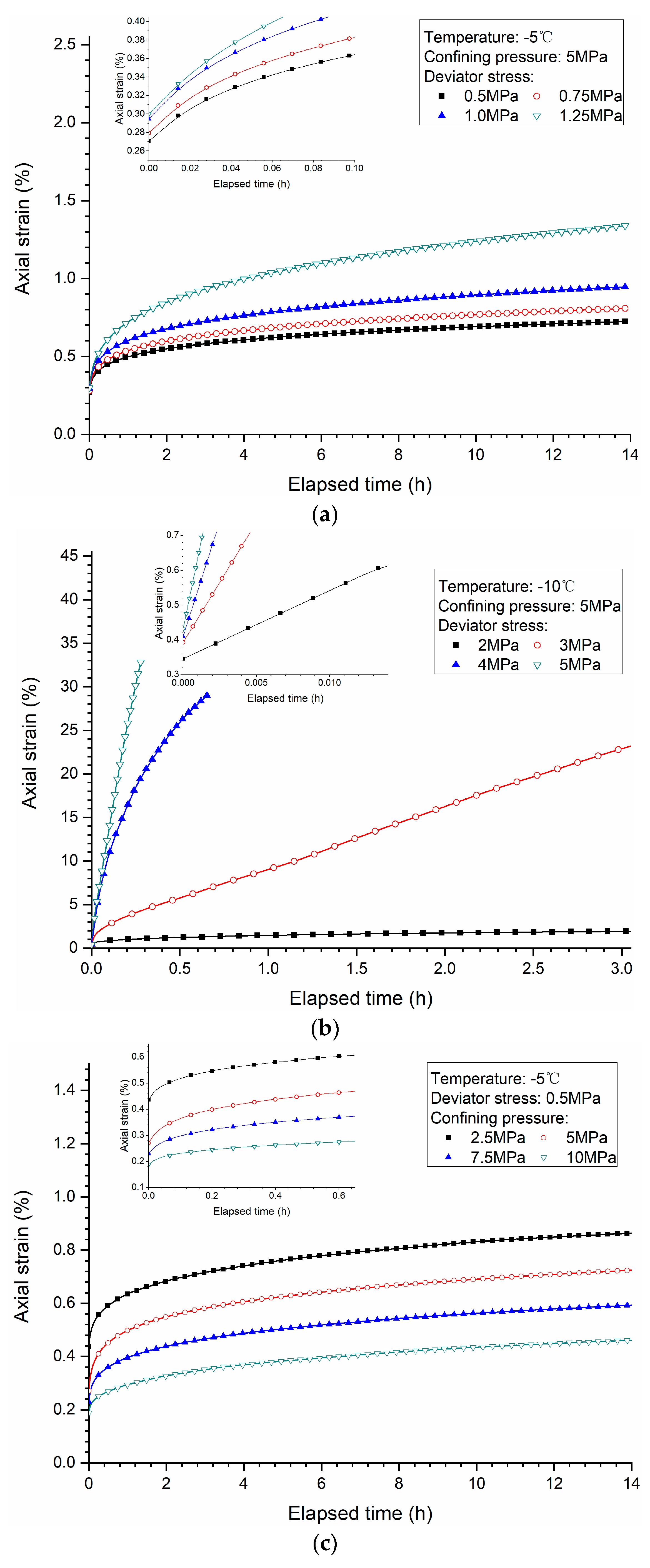
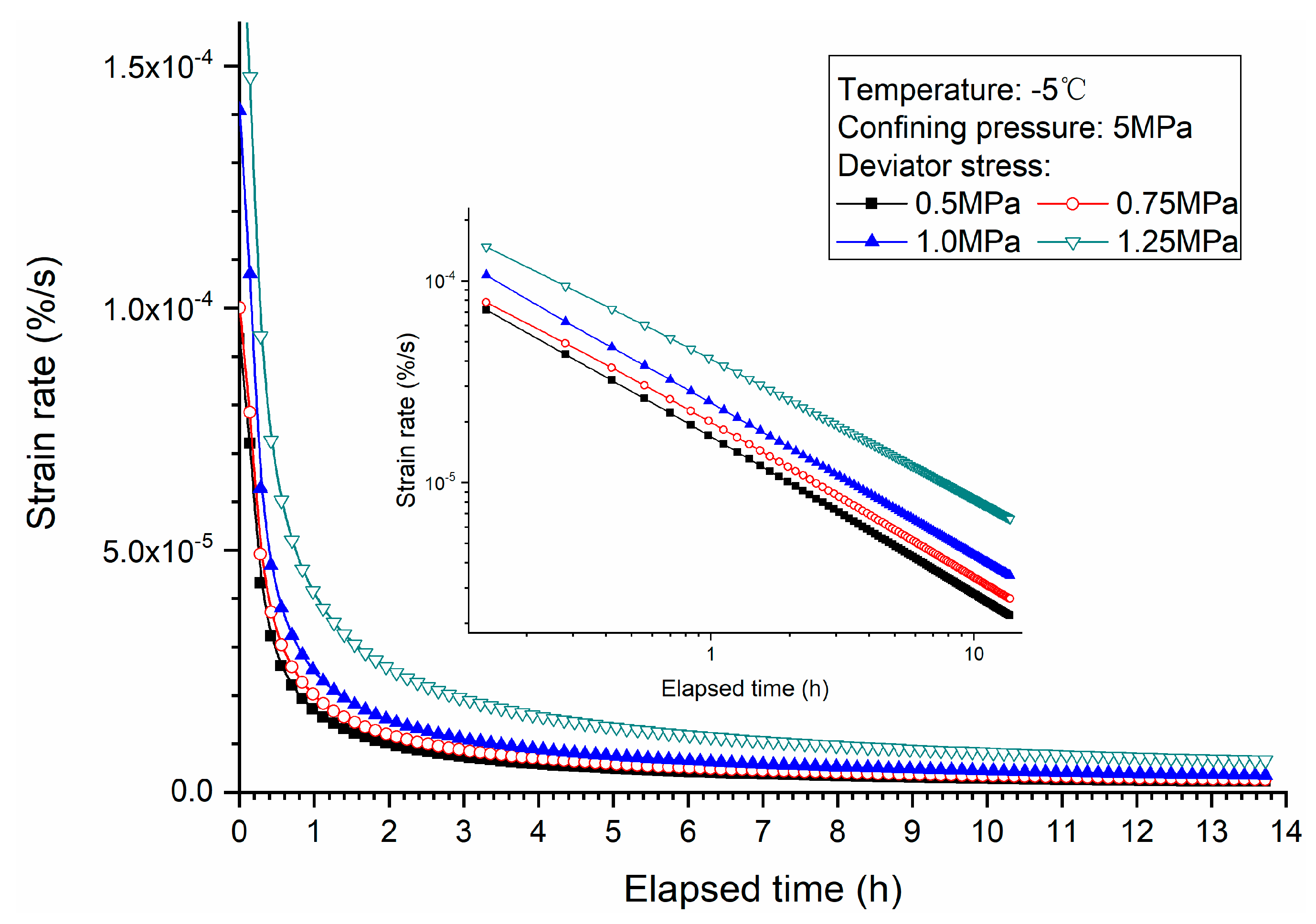
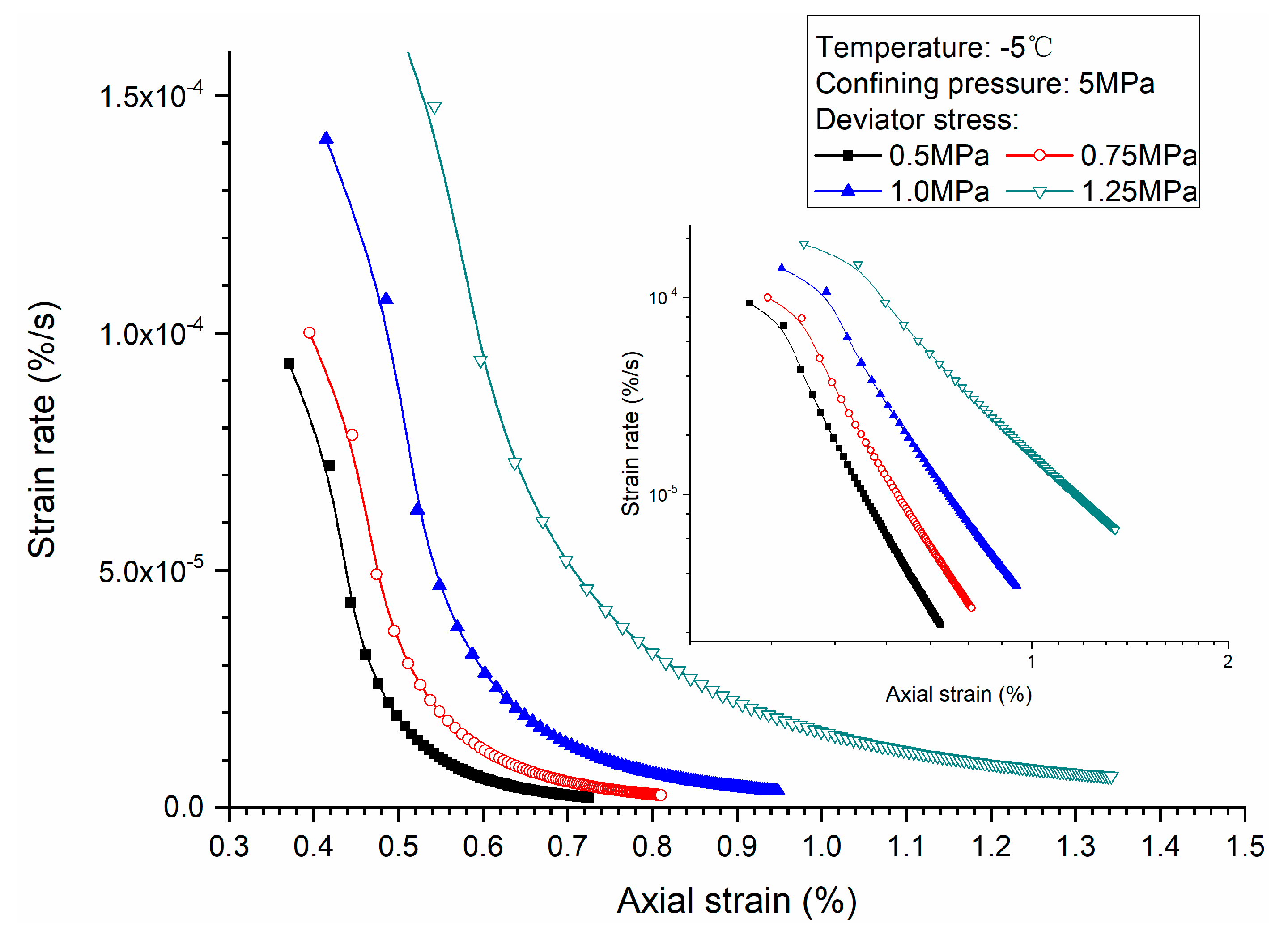
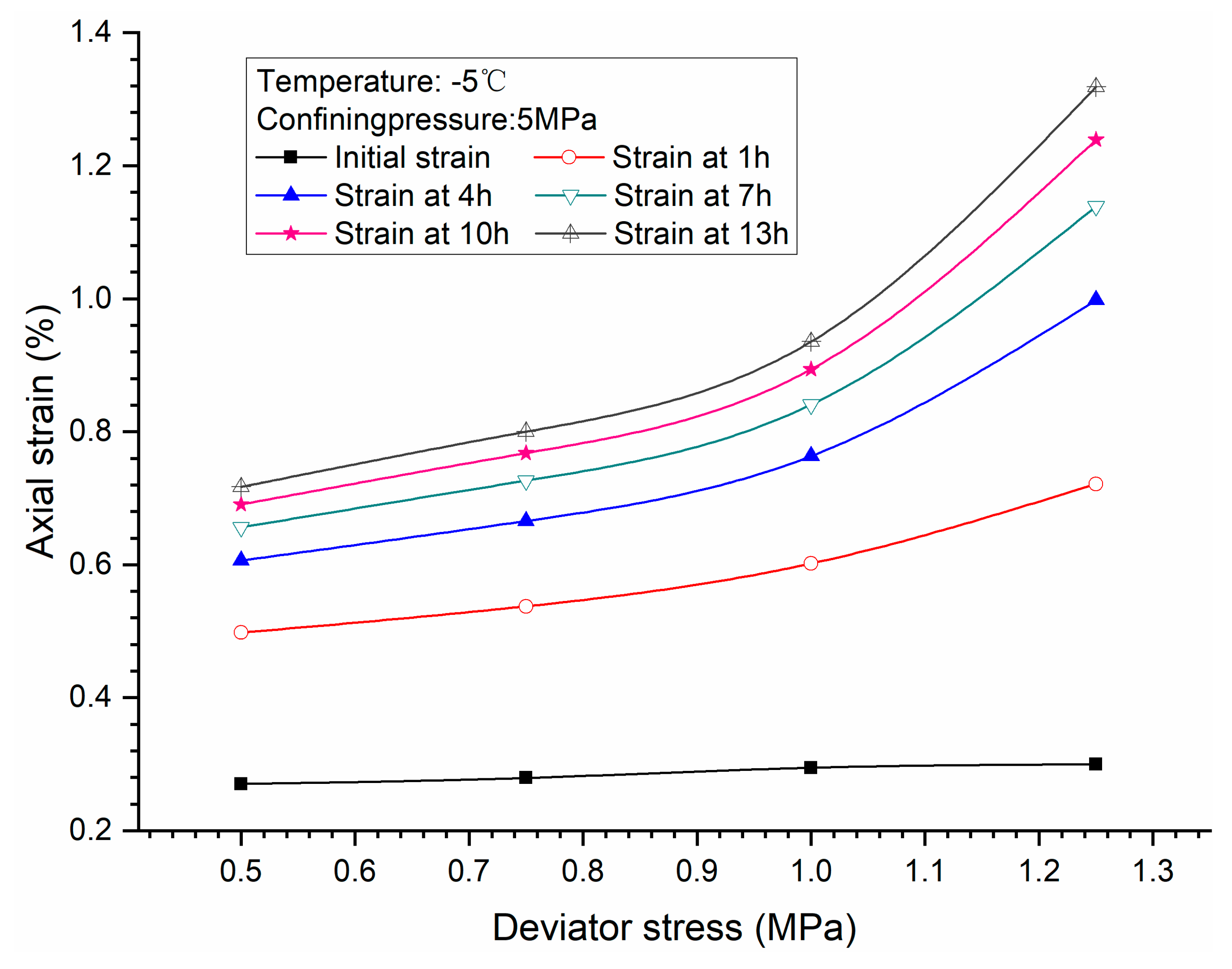
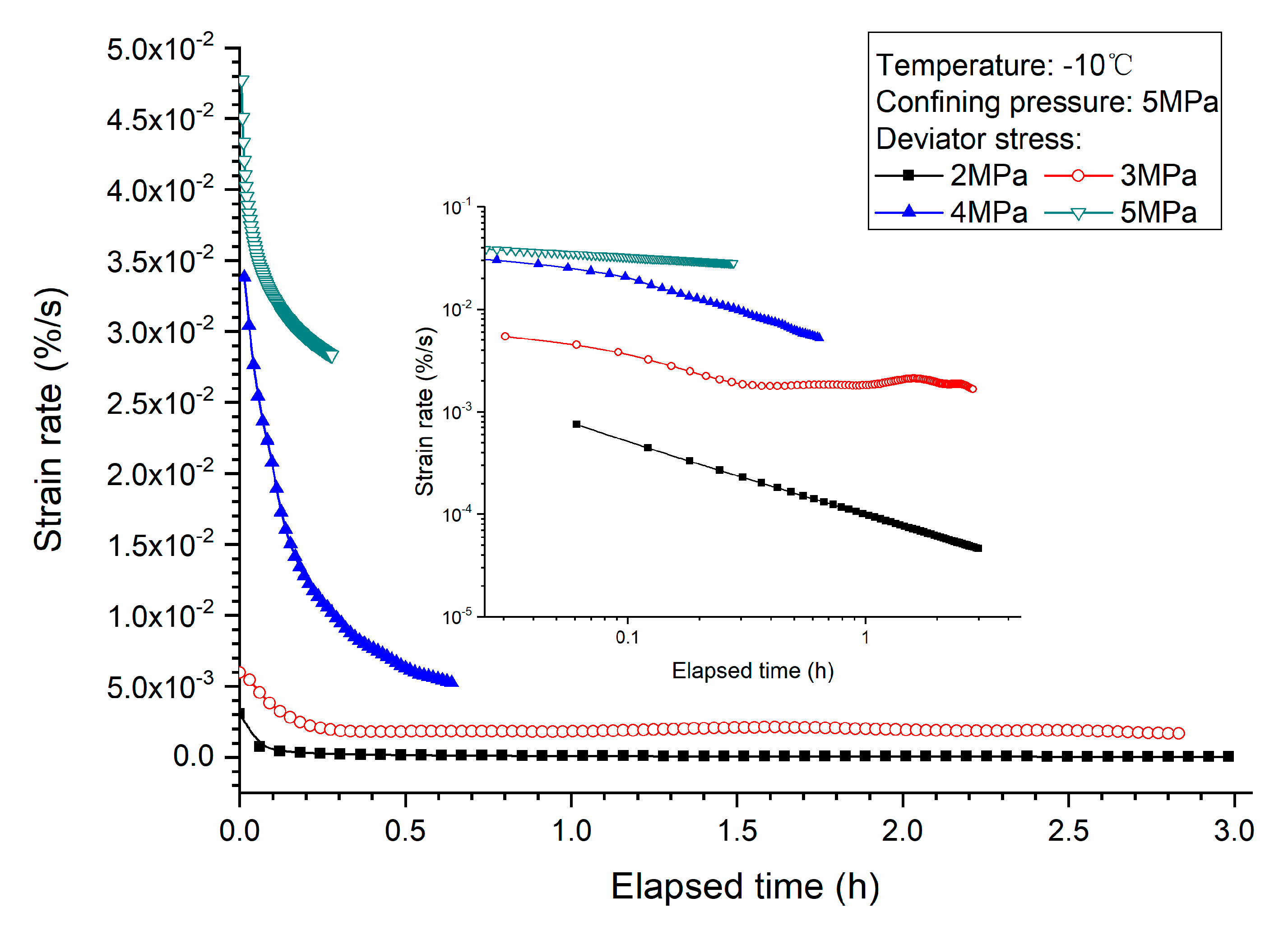
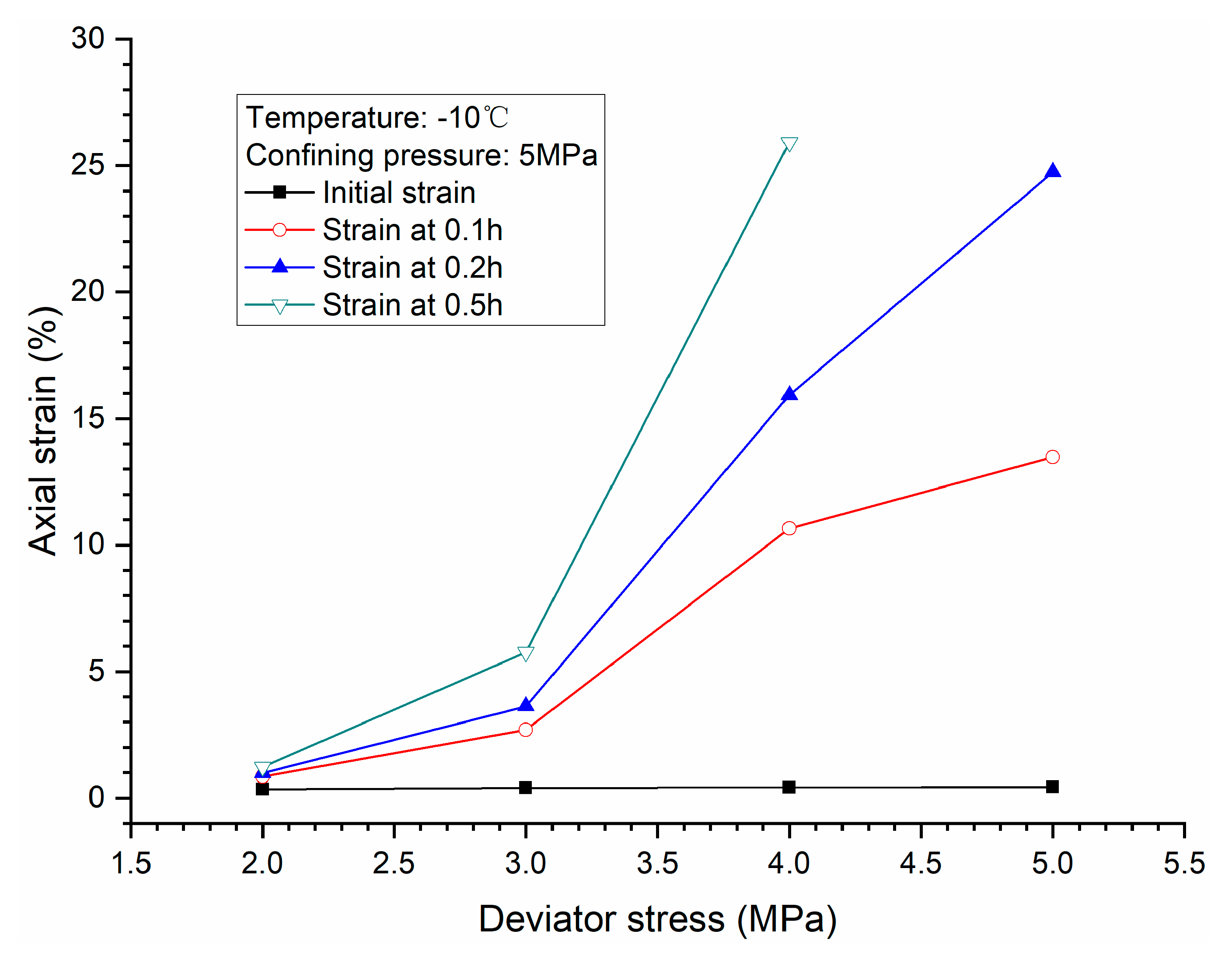
3.1. Effects of Deviator Stress
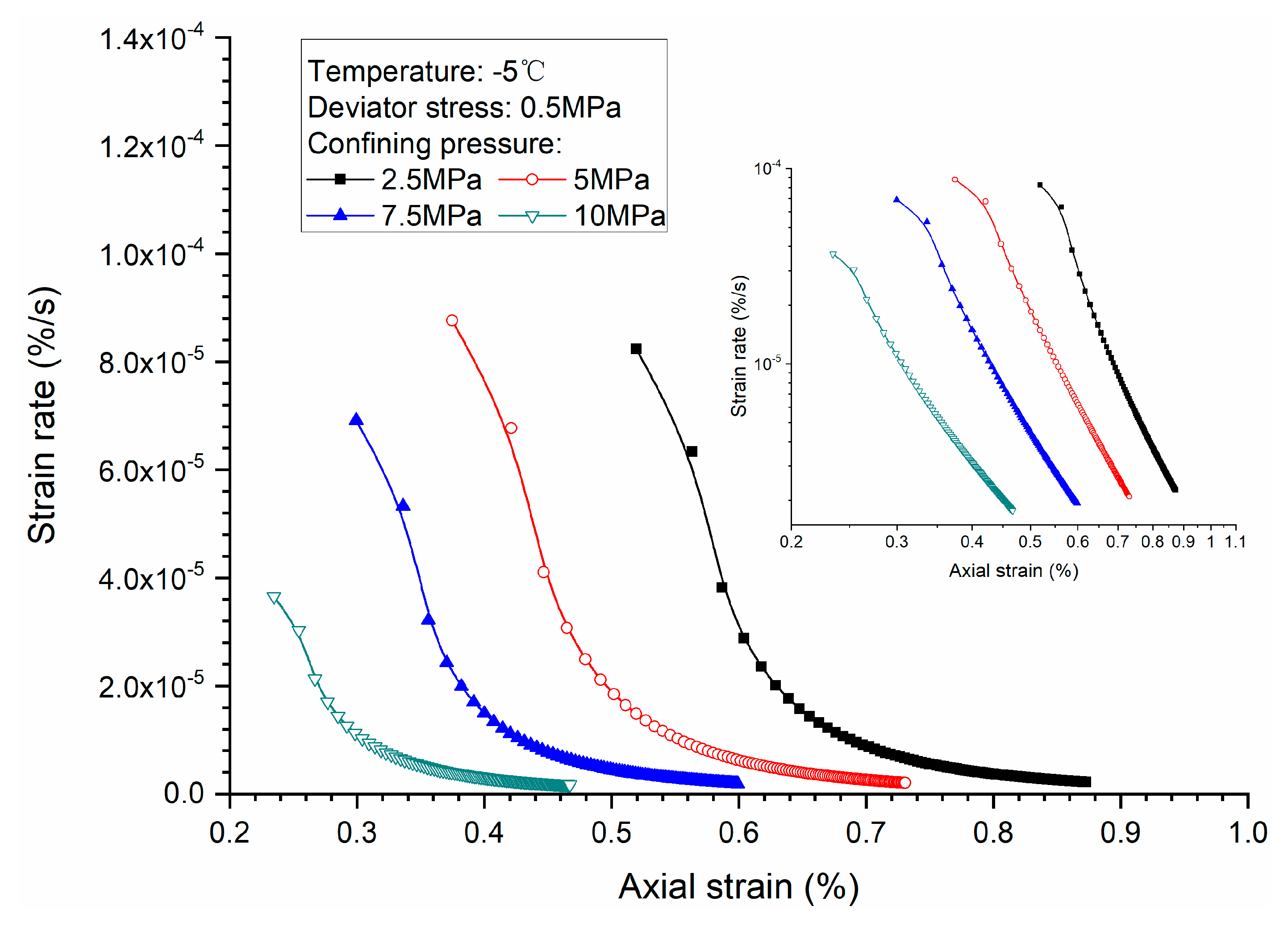
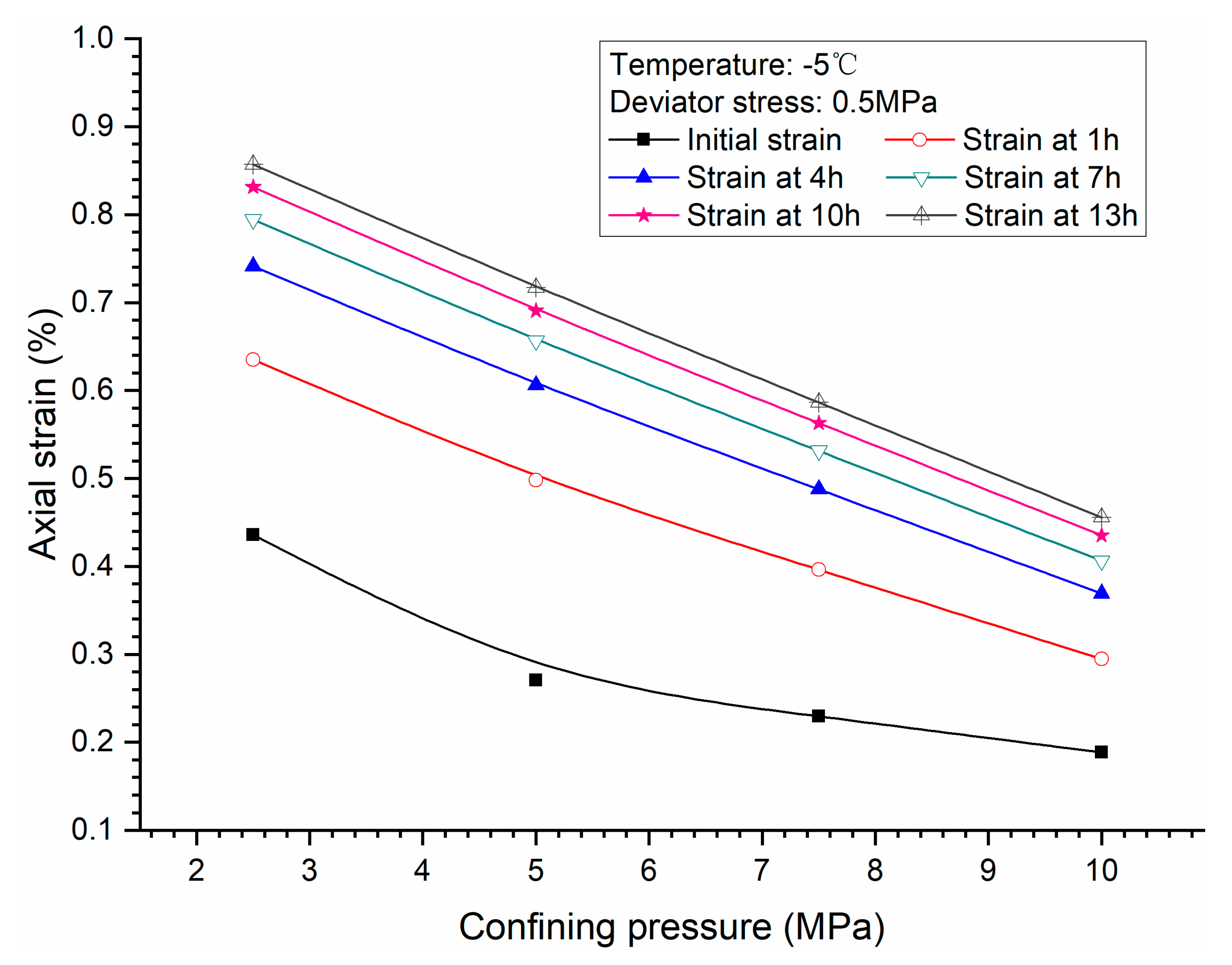
3.2. Effects of Confining Pressure
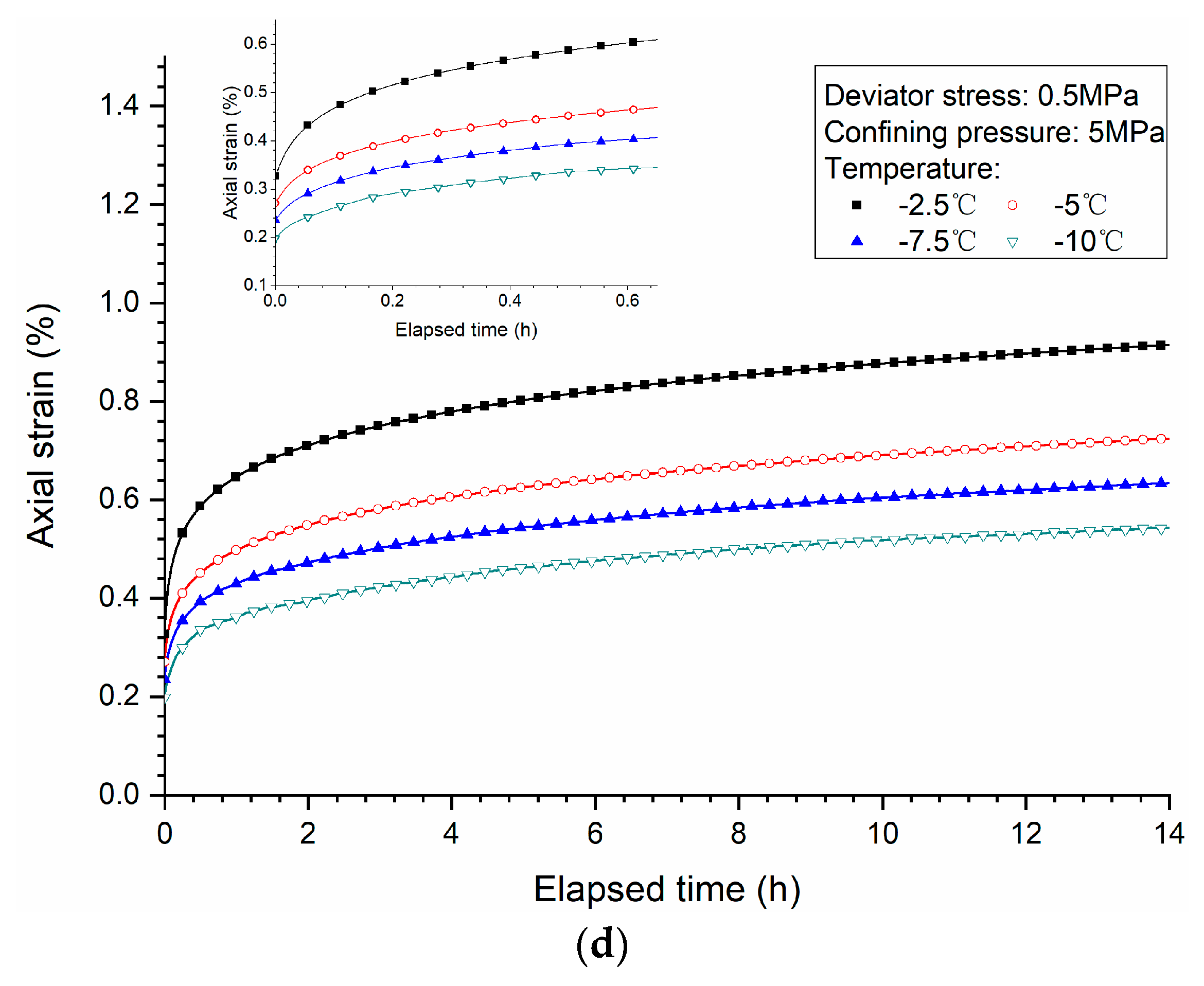
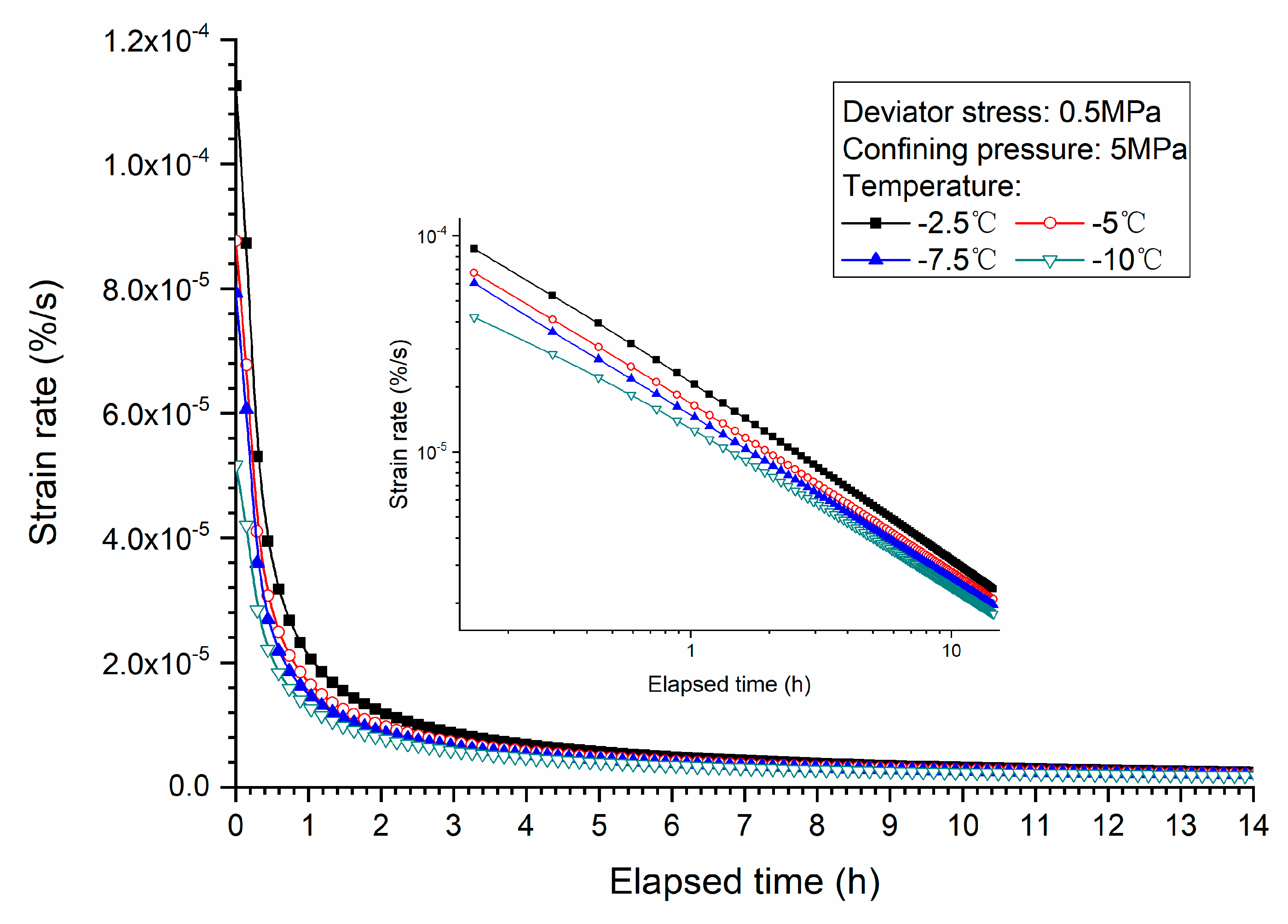
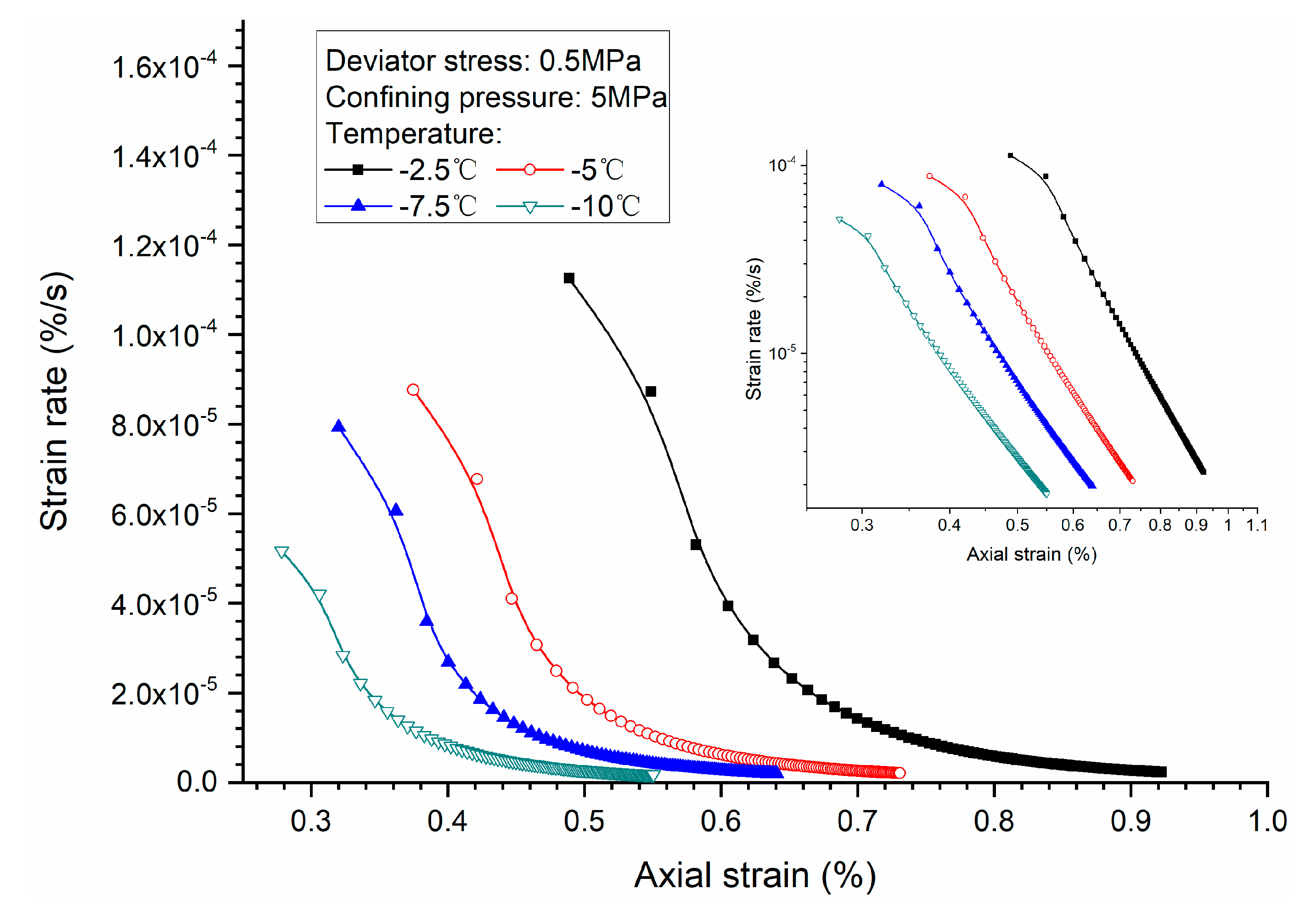
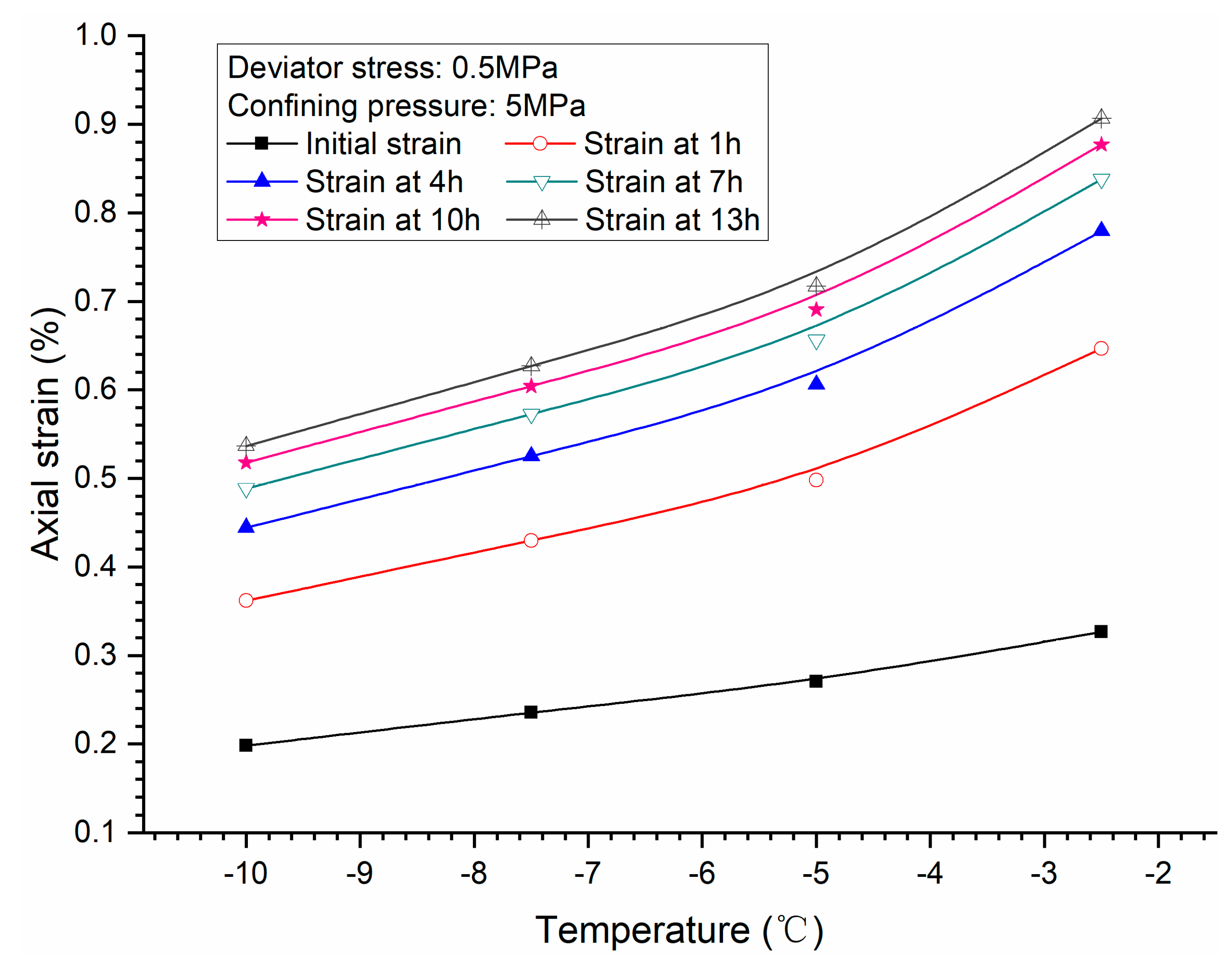
3.3. Effects of Temperature
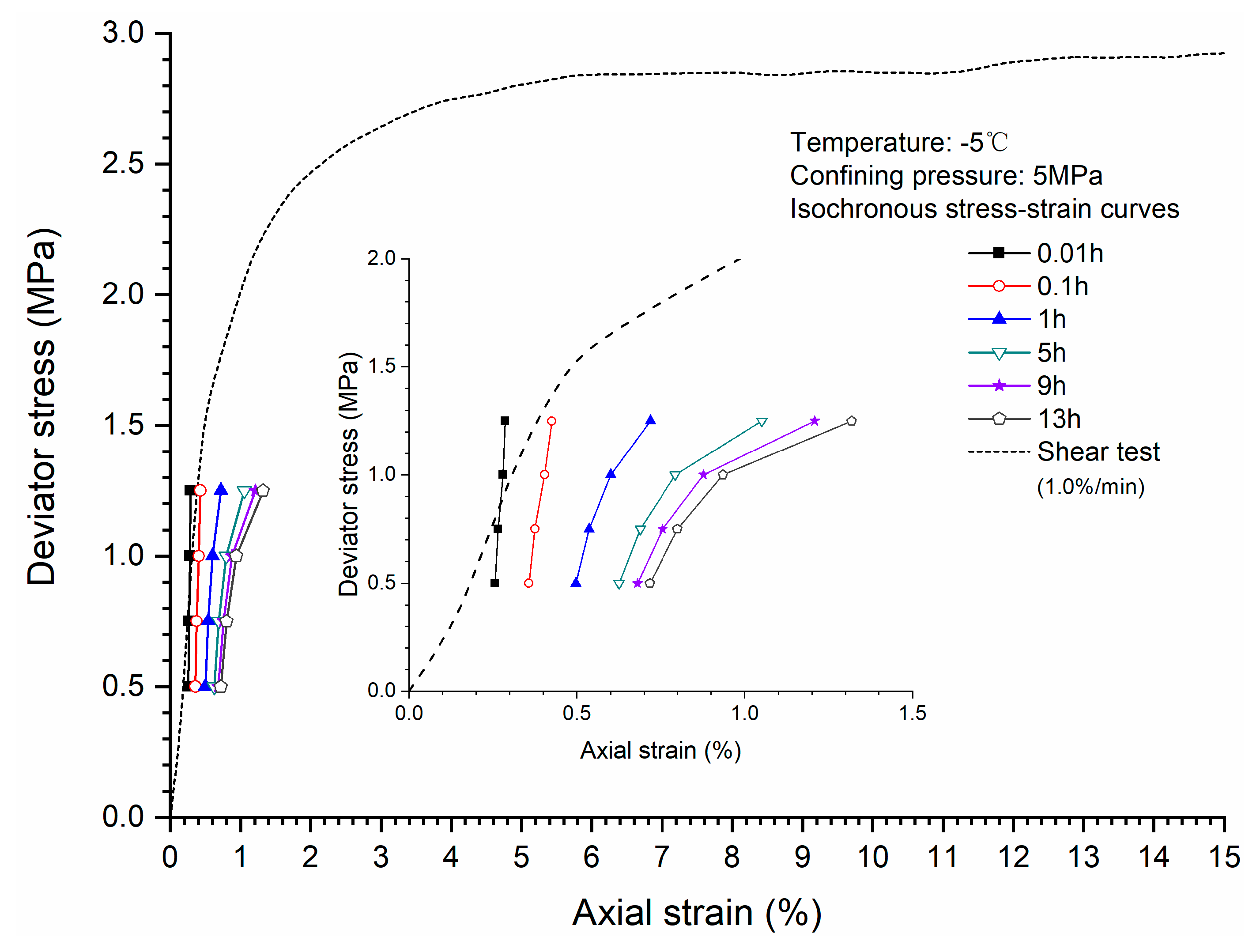
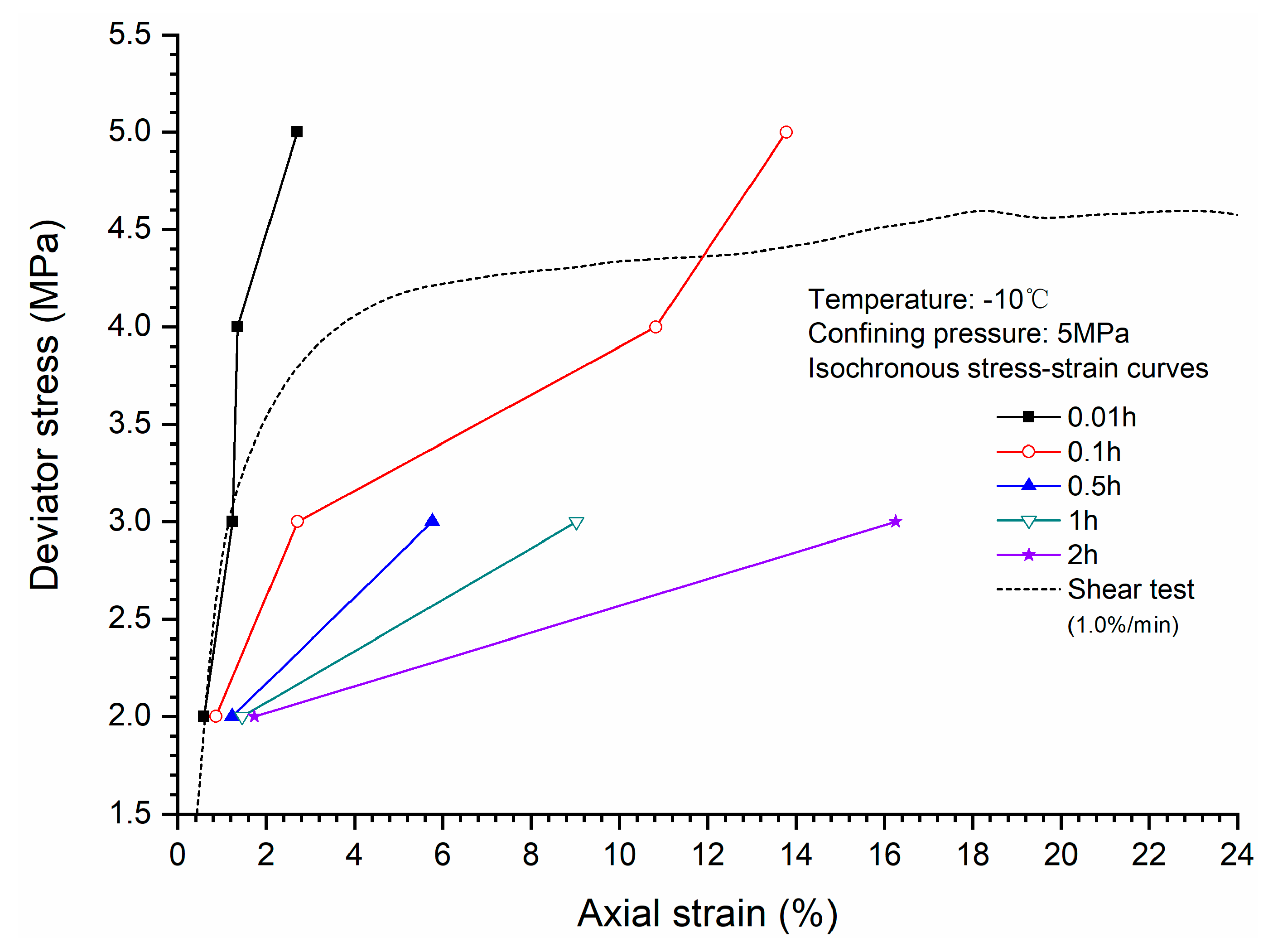
3.4. Stress-Strain Relationship
4. Conclusions
- Higher deviator stress results in a larger axial strain and strain rate at a specific elapsed time. Under the condition of low deviator stress, the axial strain is not large, and does not get into the tertiary creep stage. However, under the condition of high deviator stress, the axial strain is very large and causes failure.
- Both creep strain and strain rate of methane hydrate-bearing frozen specimens increase with the increase of deviator stress, and the decrease of confining pressure, and temperature.
- Comparison of the stress-strain relationship between creep test and quasi-static triaxial tests shows that when the deviator stress exceeds the quasi-static strength, the specimens will be damaged rather than in stable creep stage during the creeping of the specimens.
Author Contributions
Funding
Conflicts of Interest
References
- Schoderbek, D.; Farrell, H.; Hester, K.; Howard, J.; Raterman, K.; Silpngarmlert, S.; Martin, K.L.; Smith, B.; Klein, P. Oil & Natural Gas Technology, ConocoPhillips Gas Hydrate Production Test: Final Technical Report; National Energy Technology Laboratory: Albany, OR, USA, 2013. [Google Scholar]
- Yamamoto, K.; Dallimore, S. Aurora-JOGMEC-NRCan Mallik 2006–2008 Gas Hydrate Research Project Progress: Fire in the Ice, DOE/NETL Methane Hydrate Newsletter; National Energy Technology Laboratory: Albany, OR, USA, 2008; pp. 1–5. [Google Scholar]
- Yamamoto, K.; Terao, Y.; Fujii, T.; Ikawa, T.; Seki, M.; Matsuzawa, M.; Kanno, T. Operational overview of the first offshore production test of methane hydrates in the Eastern Nankai Trough. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 5–8 May 2014. [Google Scholar]
- Ning, F.; Yu, Y.; Kjelstrup, S.; Vlugt, T.J.H.; Glavatskiy, K. Mechanical properties of clathrate hydrates: Status and perspectives. Energy Environ. Sci. 2012, 5, 6779–6795. [Google Scholar] [CrossRef]
- Rutqvist, J.; Moridis, G.J.; Grover, T.; Collett, T. Geomechanical response of permafrost-associated hydrate deposits to depressurization-induced gas production. J. Pet. Sci. Eng. 2009, 67, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, Y.; Kakumoto, M.; Miyazaki, K.; Tenma, N.; Komai, T.; Yamaguchi, T.; Shimokawara, M. Numerical study on dissociation of methane hydrate and gas production behavior in laboratory-scale experiments for depressurization: Part 3—Numerical study on estimation of permeability in methane hydrate reservoir. Int. J. Offshore Polar Eng. 2009, 19, 124–134. [Google Scholar]
- Kato, A.; Nakata, Y.; Hyodo, M.; Yoshimoto, N. Macro and micro behaviour of methane hydrate-bearing sand subjected to plane strain compression. Soils Found. 2016, 56, 835–847. [Google Scholar] [CrossRef]
- Hyodo, M.; Li, Y.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Nishimura, A. Effects of dissociation on the shear strength and deformation behavior of methane hydrate-bearing sediments. Mar. Pet. Geol. 2014, 51, 52–62. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Zhu, Y.; Chen, Y.; Song, Y.; Li, Q. Mechanical behaviors of permafrost-associated methane hydrate-bearing sediments under different mining methods. Appl. Energy 2016, 162, 1627–1632. [Google Scholar] [CrossRef]
- Uchida, S.; Xie, X.G.; Leung, Y.F. Role of critical state framework in understanding geomechanical behavior of methane hydrate-bearing sediments. J. Geophys. Res. Solid Earth 2016, 121, 5580–5595. [Google Scholar] [CrossRef]
- Liu, L.; Lu, X.; Zhang, X.; Liu, C.; Du, B. Numerical simulations for analyzing deformation characteristics of hydrate-bearing sediments during depressurization. Adv. Geo-Energy Res. 2017, 1, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Deusner, C.; Haeckel, M.; Helmig, R.; Wohlmuth, B. Testing a coupled hydro-thermo-chemo-geomechanical model for gas hydrate bearing sediments using triaxial compression lab experiments. Geochem. Geophys. Geosyst. 2017, 18. [Google Scholar] [CrossRef]
- Mountjoy, J.; Pecher, I.; Henrys, S.; Barnes, P.; Plaza-Faverola, A. Creeping deformation mechanisms for mixed hydrate-sediment submarine landslides. In Proceedings of the EGU General Assembly 2013, Vienna, Austria, 7–12 April 2013. [Google Scholar]
- Miyazaki, K.; Yanaguchi, T.; Sakamoto, Y.; Aoki, K. Time-dependent behaviors of methane-hydrate bearing sedimentsin triaxial compression test. Int. J. JSRM 2011, 7, 43–48. [Google Scholar]
- Parameswaran, V.R.; Paradis, M.; Handa, Y.P. Strength of frozen sand containing tetrahydrofuran hydrate. Can. Geotech. J. 1989, 26, 479–483. [Google Scholar] [CrossRef]
- Cameron, I.; Handa, Y.P.; Baker, T.H.W. Compressive strength and creep behavior of hydrateo-consolidated sand. Can. Geotech. J. 1990, 27, 255–258. [Google Scholar] [CrossRef]
- Parameswaran, V.R. Deformation behaviour and strength of frozen sand. Can. Geotech. J. 1980, 17, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47, 1–38. [Google Scholar] [CrossRef]
- Durham, W.B.; Kirby, S.H.; Stern, L.A.; Zhang, W. The strength and rheology of methane clathrate hydrate. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, K.; Yamaguchi, T.; Sakamoto, Y.; Haneda, H.; Ogata, Y.; Aoki, K.; Okubo, S. Creep of sediment containing synthetic methane hydrate. J. MMIJ 2009, 125, 156–164. [Google Scholar] [CrossRef]
- Miyazaki, K.; Endo, Y.; Tenma, N.; Yamaguchi, T. Constitutive equation for triaxial compression creep of artificial methane-hydrate-bearing sand. J. MMIJ 2015, 131, 47–55. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Song, Y.; Yang, M.; Zhao, J. Creep behaviors of methane hydrate coexisting with ice. J. Nat. Gas Sci. Eng. 2016, 33, 347–354. [Google Scholar] [CrossRef]
- Yakushev, V.S.; Chuvilin, E.M. Natural gas and gas hydrate accumulations within permafrost in Russia. Cold Reg. Sci. Technol. 2000, 31, 189–197. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Collett, T.S. Gas hydrates associated with deep permafrost in the Mackenzie delta, N.W.T., Canada: Regional overview. In Proceedings of the Seventh International Permafrost Conference, Yellowknife, NT, Canada, 23–27 June 1998. [Google Scholar]
- Zhu, Y.; Zhang, Y.; Wen, H.; Lu, Z.; Jia, Z.; Li, Y.; Li, Q.; Liu, C.; Wang, P.; Guo, X. Gas hydrates in the Qilian mountain permafrost, Qinghai, northwest China. Acta Geol. Sin. 2009, 83, 1762–1771. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, G.; Zhang, P. Assessing the permafrost temperature and thickness conditions favorable for the occurrence of gas hydrate in the Qinghai-Tibet Plateau. Energy Convers. Manag. 2010, 51, 783–787. [Google Scholar]
- Miyazaki, K.; Tenma, N.; Yamaguchi, T. Relationship between creep property and loading-rate dependence of strength of artificial methane-hydrate-bearing Toyoura sand under triaxial compression. Energies 2017, 10, 1466. [Google Scholar] [CrossRef]
- Li, D.; Fan, J.; Wang, R. Research on visco-elastic-plastic creep model of artificially frozen soil under high confining pressures. Cold Reg. Sci. Technol. 2011, 65, 219–225. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, Y.; Chang, X. Experimental and theoretical studies on the creep behavior of warm ice-rich frozen sand. Cold Reg. Sci. Technol. 2010, 63, 61–67. [Google Scholar] [CrossRef]
- Jones, S.J.; Chew, H.A.M. Creep of ice as a function of hydrostatic pressure. J. Phys. Chem. 1983, 87, 4064–4066. [Google Scholar] [CrossRef]
- Hyodo, M.; Li, Y.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Kajiyama, S.; Nishimura, A.; Song, Y. A comparative analysis of mechanical behavior of carbon dioxide and methane hydrate-bearing sediments. Am. Mineral. 2014, 99, 178–183. [Google Scholar] [CrossRef]
- Hyodo, M.; Li, Y.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Nishimura, A.; Song, Y. Mechanical behavior of gas-saturated methane hydrate-bearing sediments. J. Geophys. Res. Solid Earth 2013, 118, 5185–5194. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, Y.; Yu, F.; Liu, W.; Zhao, J. Experimental study on mechanical properties of gas hydrate-bearing sediments using kaolin clay. China Ocean Eng. 2011, 25, 113–122. [Google Scholar] [CrossRef]
- Helgerud, M.B.; Waite, W.F.; Kirby, S.H.; Nur, A. Elastic wave speeds and moduli in polycrystalline ice Ih, sI methane hydrate, and sII methane-ethane hydrate. J. Geophys. Res. 2009, 114, B2212. [Google Scholar] [CrossRef]
- Hyodo, M.; Hyde, A.F.L.; Nakata, I.; Yoshimoto, N.; Fukunaga, M.; Kubo, K.; Nanjo, Y.; Matsuo, T.; Nakamura, K. Triaxial Compressive Strength of Methane Hydrate. In Proceedings of the Twelfth International Offshore and Polar Engineering Conference, Kitakyushu, Japan, 26–31 May 2002; pp. 422–428. [Google Scholar]
- Song, Y.; Yu, F.; Li, Y.; Liu, W.; Zhao, J. Mechanical property of artificial methane hydrate under triaxial compression. J. Nat. Gas Chem. 2010, 19, 246–250. [Google Scholar] [CrossRef]
- Czurda, K.A.; Hohmann, M. Freezing effect on shear strength of clayey soils. Appl. Clay Sci. 1997, 12, 165–187. [Google Scholar] [CrossRef]
- Chamberlain, E.; Groves, C.; Perham, R. The mechanical behaviour of frozen earth materials under high pressure triaxial test conditions. Géotechnique 1972, 22, 469–483. [Google Scholar] [CrossRef]
- Alkire, B.D.; Andersland, O.B. The Effect of Confining Pressure on the Mechanical Properties of Sand–Ice Materials. J. Glaciol. 1973, 12, 2747–2754. [Google Scholar] [CrossRef]


| Void Ratio | Hydrate Saturation (%) | Temperature (°C) | Deviator Stress (MPa) | Confining Pressure (MPa) |
|---|---|---|---|---|
| 1.5 | 26–33 | −2.5 | 0.5 | 5 |
| −5 | 0.5 | 2.5, 5, 7.5, 10 | ||
| 0.75, 1, 1.25 | 5 | |||
| −7.5 | 0.5 | 5 | ||
| −10 | 0.5, 2, 3, 4, 5 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, P.; Sun, X.; Liu, W.; Song, Y.; Zhao, J. Creep Behaviors of Methane Hydrate-Bearing Frozen Sediments. Energies 2019, 12, 251. https://doi.org/10.3390/en12020251
Li Y, Wu P, Sun X, Liu W, Song Y, Zhao J. Creep Behaviors of Methane Hydrate-Bearing Frozen Sediments. Energies. 2019; 12(2):251. https://doi.org/10.3390/en12020251
Chicago/Turabian StyleLi, Yanghui, Peng Wu, Xiang Sun, Weiguo Liu, Yongchen Song, and Jiafei Zhao. 2019. "Creep Behaviors of Methane Hydrate-Bearing Frozen Sediments" Energies 12, no. 2: 251. https://doi.org/10.3390/en12020251
APA StyleLi, Y., Wu, P., Sun, X., Liu, W., Song, Y., & Zhao, J. (2019). Creep Behaviors of Methane Hydrate-Bearing Frozen Sediments. Energies, 12(2), 251. https://doi.org/10.3390/en12020251






