Well-Dispersed ZnFe2O4 Nanoparticles onto Graphene as Superior Anode Materials for Lithium Ion Batteries
Abstract
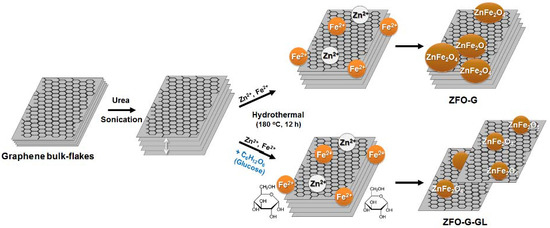
:1. Introduction
2. Experimental
2.1. Preparation of Electrodes
2.2. Characterization
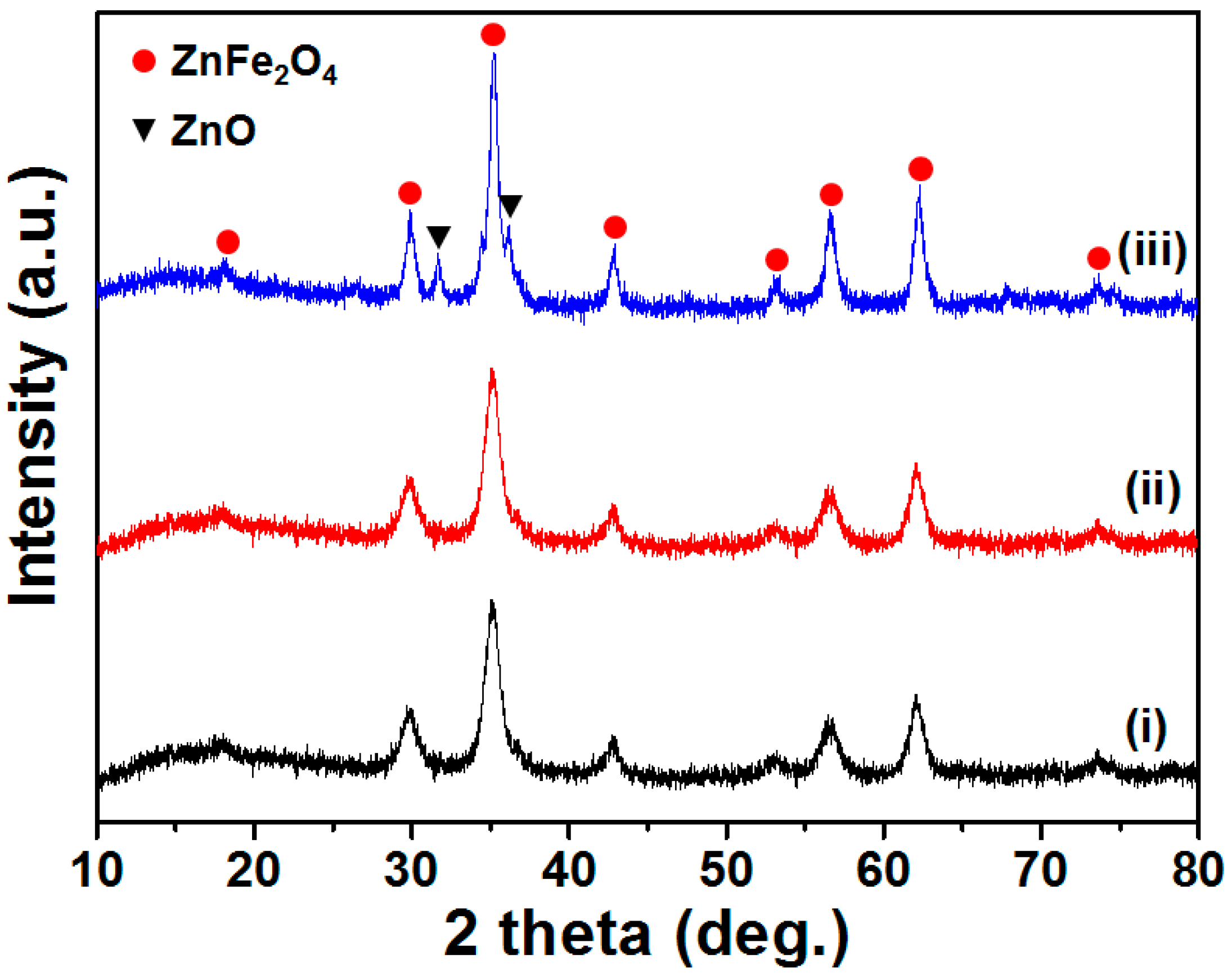
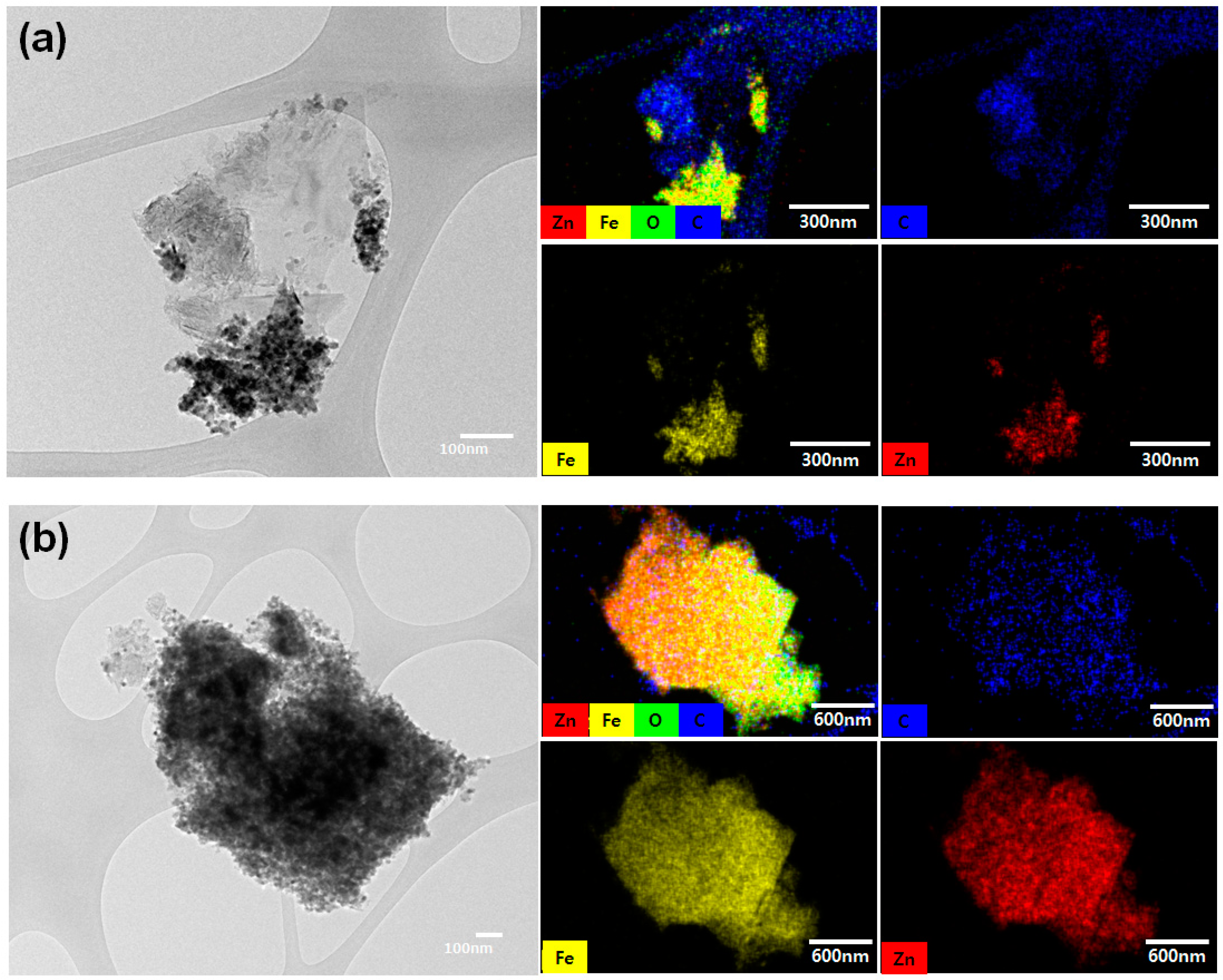
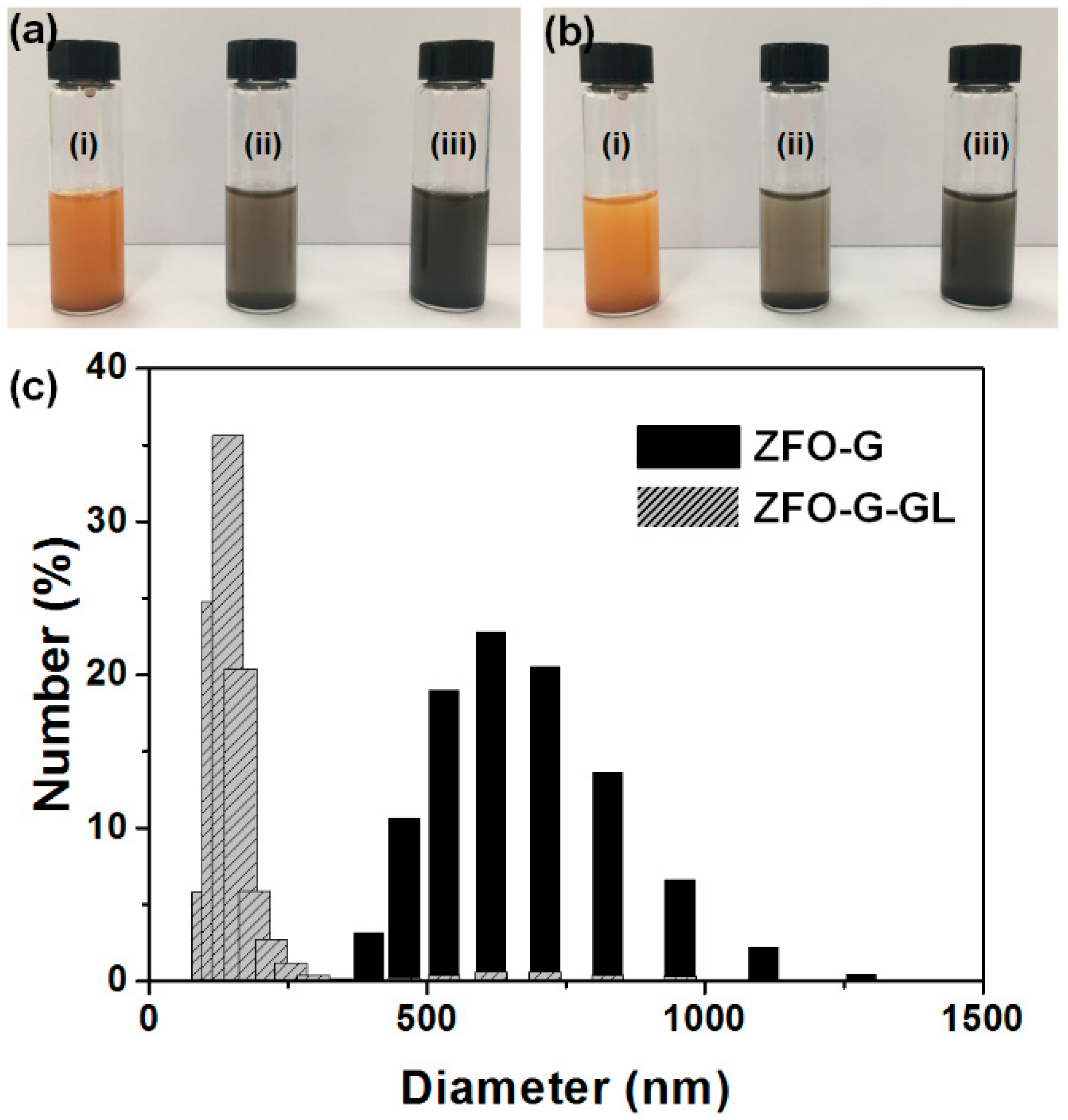
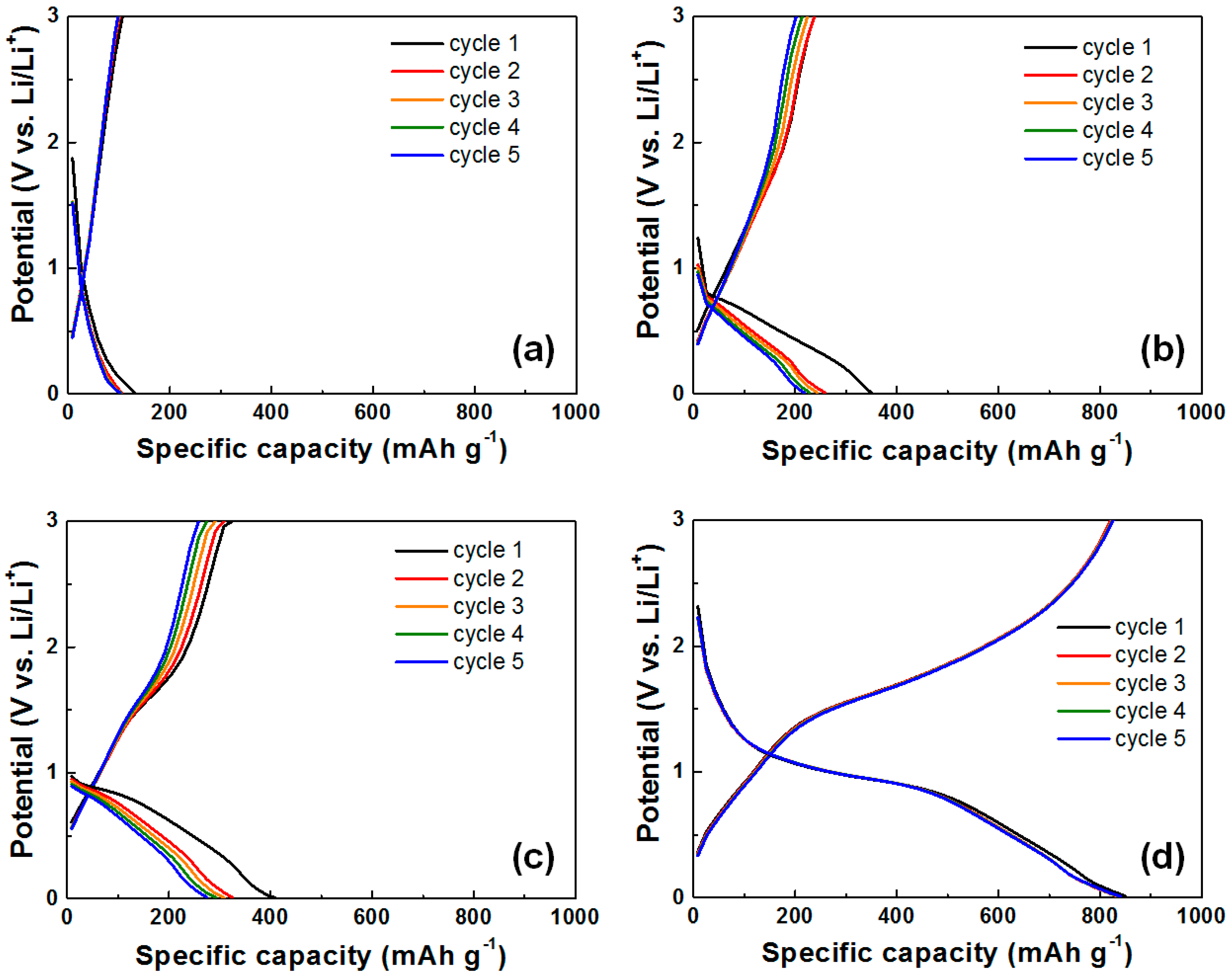
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.B.; Lou, X.W. Iron-oxide-based advanced anode materials for lithium-ion batteries. Adv. Energy Mater. 2014, 4, 1300958. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.; Shao, H. High capacity ZnFe2O4 anode material for lithium ion batteries. Electrochim. Acta 2011, 56, 9433–9438. [Google Scholar] [CrossRef]
- Guo, X.; Lu, X.; Fang, X.; Mao, Y.; Wang, Z.; Chen, L.; Xu, X.; Yang, H.; Liu, Y. Lithium storage in hollow spherical ZnFe2O4 as anode materials for lithium ion batteries. Electrochem. Commun. 2010, 12, 847–851. [Google Scholar] [CrossRef]
- Teh, P.F.; Sharm, Y.; Pramana, S.S.; Srinivasan, M. Nanoweb anodes composed of one-dimensional, high aspect ratio, size tunable electrospun ZnFe2O4 nanofibers for lithium ion batteries. J. Mater. Chem. 2011, 21, 14999–15008. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Chen, L.; Li, L.E.; Xu, L.; Yang, J.; Qian, Y. A comparative study of nanoparticles and nanospheres ZnFe2O4 as anode material for lithium ion batteries. Int. J. Electrochem. Sci. 2012, 7, 7976–7983. [Google Scholar]
- Deng, Y.; Zhang, Q.; Tang, S.; Zhang, L.; Deng, S.; Shi, Z.; Chen, G. One-pot synthesis of ZnFe2O4/C hollow spheres as superior anode materials for lithium ion batteries. Chem. Commun. 2011, 47, 6828–6830. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Zhang, C.; Hong, D.; Li, J.; Cheng, Q.; Li, Z.; Cai, W. Facile synthesis of MWCNT–ZnFe2O4 nanocomposites as anode materials for lithium ion batteries. J. Mater. Chem. 2012, 22, 13674–13681. [Google Scholar] [CrossRef]
- Varzi, A.; Bresser, D.; Zamory, J.V.; Müller, F.; Passerini, S. ZnFe2O4 -C/LiFePO4 -CNT: A novel high-power lithium-ion battery with excellent cycling performance. Adv. Energy Mater. 2014, 4, 1400054. [Google Scholar] [CrossRef] [PubMed]
- Bresser, D.; Paillard, E.; Kloepsch, R.; Krueger, S.; Fiedler, M.; Schmitz, R.; Baither, D.; Winter, M.; Passerini, S. Carbon coated ZnFe2O4 nanoparticles for advanced lithium-ion anodes. Adv. Energy Mater. 2013, 3, 513–523. [Google Scholar] [CrossRef]
- Yao, X.; Kong, J.; Zhou, D.; Zhao, C.; Zhou, R.; Lu, X. Mesoporous zinc ferrite/graphene composites: Towards ultra-fast and stable anode for lithium-ion batteries. Carbon 2014, 79, 493–499. [Google Scholar] [CrossRef]
- He, P.; Zhou, C.; Tian, S.; Sun, J.; Yang, S.; Ding, G.; Xie, X.; Jiang, M. Urea-assisted aqueous exfoliation of graphite for obtaining high-quality graphene. Chem. Commun. 2015, 51, 4651–4654. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Choi, K.-H.; Yoo, J.-T.; Kim, J.-H.; Chun, S.-J.; Park, S.-B.; Choi, D.-H.; Wu, Q.; Lee, S.-Y.; Lee, S.-Y. Hetero-nanonet rechargeable paper batteries: Toward ultrahigh energy density and origami foldability. Adv. Funct. Mater. 2015, 25, 6029–6040. [Google Scholar] [CrossRef]
- Hoe, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-sacrifice template fabrication of hierachical mesoporous bi-component-active ZnO/ZnFe2O4 sub-microcube as superior anode towards high-performance lithium-ion battery. Adv. Funct. Mater. 2015, 25, 238–246. [Google Scholar] [CrossRef]
- Zhong, X.-B.; Jin, B.; Yang, Z.-Z.; Wang, C.; Wang, H.-Y. Facile shape design and fabrication of ZnFe2O4 as an anode material for Li-ion batteries. RSC Adv. 2014, 4, 55173–55178. [Google Scholar] [CrossRef]
- Lee, E.; Park, J.S.; Wu, T.; Sun, C.-J.; Kim, H.; Stair, P.C.; Lu, J.; Zhou, D.; Johnson, C.S. Role of Cr3+/Cr6+ redox in chromium-substituted Li2MnO3 LiNi1/2Mn1/2O2 layered composite cathodes: Electrochemistry and voltage fade. J. Mater. Chem. A 2015, 3, 9915–9924. [Google Scholar] [CrossRef]
- Huang, Q.; Loveridge, M.J.; Genieser, R.; Lain, M.J.; Bhagat, R. Electrochemical evaluation and phase-related impedance studies on silicon–few layer graphene (FLG) composite electrode systems. Sci. Rep. 2018, 8, 1386. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Wang, T.; Chen, L.; Wang, F.; Yang, X.; Qin, C.; Ding, Y. Crystal-seeds induced construction of ZnO-ZnFe2O4 micro-cubic composites as excellent anode materials for lithium ion battery. RSC Adv. 2018, 8, 16187–16192. [Google Scholar] [CrossRef]
- Grugeon, S.; Laruelle, S.; Dupont, L.; Tarascon, J.-M. An updateon the reactivity of nanoparticles Co-based compounds towards Li. Solid State Sci. 2003, 5, 895–904. [Google Scholar] [CrossRef]
- Zou, F.; Hu, X.; Li, Z.; Qie, L.; Hu, C.; Zeng, R.; Jiang, Y.; Huang, Y. MOF-derived porous ZnO/ZnFe2O4/C octahedra with hollow interiors for high-rate lithium-ion batteries. Adv. Mater. 2014, 26, 6622–6628. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Oh, M.; Kim, J.H. Well-Dispersed ZnFe2O4 Nanoparticles onto Graphene as Superior Anode Materials for Lithium Ion Batteries. Energies 2019, 12, 304. https://doi.org/10.3390/en12020304
Park Y, Oh M, Kim JH. Well-Dispersed ZnFe2O4 Nanoparticles onto Graphene as Superior Anode Materials for Lithium Ion Batteries. Energies. 2019; 12(2):304. https://doi.org/10.3390/en12020304
Chicago/Turabian StylePark, Yiseul, Misol Oh, and Jae Hyun Kim. 2019. "Well-Dispersed ZnFe2O4 Nanoparticles onto Graphene as Superior Anode Materials for Lithium Ion Batteries" Energies 12, no. 2: 304. https://doi.org/10.3390/en12020304
APA StylePark, Y., Oh, M., & Kim, J. H. (2019). Well-Dispersed ZnFe2O4 Nanoparticles onto Graphene as Superior Anode Materials for Lithium Ion Batteries. Energies, 12(2), 304. https://doi.org/10.3390/en12020304