Application of Non-Thermal Plasma for NOx Reduction in the Flue Gases
Abstract
:1. Introduction
2. Materials and Methods
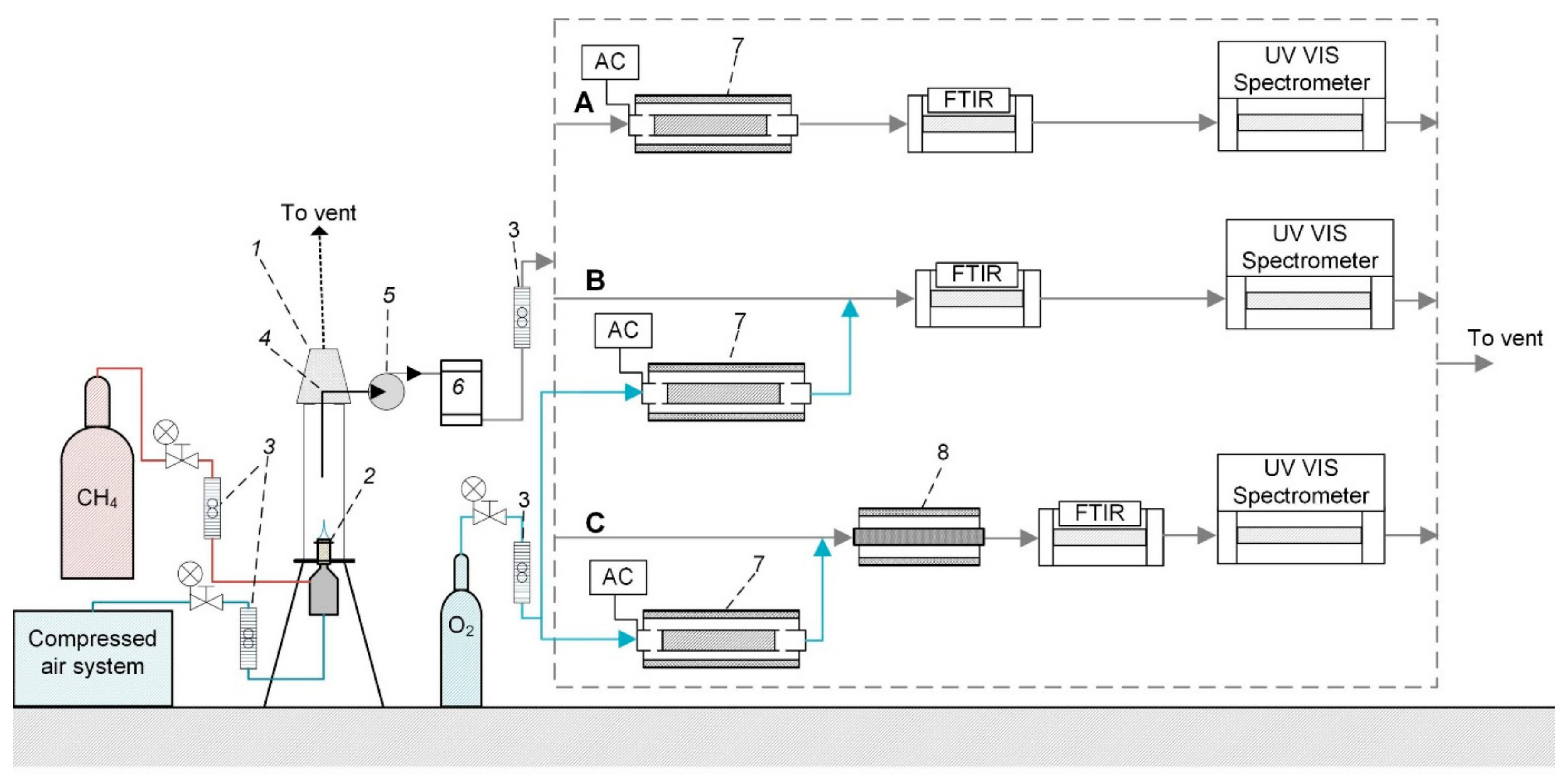
Description of Combustion and NOX Removal System
- In the case of direct plasma treatment, the exhaust gas flow was directed through a volume barrier discharge (VBD) device (8) of a coaxial design (see Figure 1 section A). The inner high-voltage electrode was a stainless steel tube with the outer diameter of 14 mm, the dielectric barrier was made from a quartz tube with the inner diameter of 16.3 mm and the outer grounded electrode was a steel mesh wrapped around the quartz tube. The length of the active zone was 8.5 cm. The high voltage was provided by a signal generator amplified by an Industrial Test Equipment Co power amplifier and a transformer. The frequency of the voltage varied from 80 Hz to 500 Hz. The specific input energy (SIE) of plasma was obtained by dividing the plasma input power determined by the method of Lissajous figures [45,46] by the flow rate of the gas. Besides, during the direct plasma treatment, the VBD device was heated by a wire heater to 60 °C to avoid the condensation of water vapor on dielectric surfaces. The condensed water could result in resistive losses which interferes with the input power measurement from the Lissajous figures. The temperature in the discharge reactor was measured by an Osensa Innovations fiber–optical sensor FTX-100-Gen.
- In the case of ozone treatment, ozone was produced from pure oxygen (99.999% of purity) with a flow rate of 0.5 L/min in the VBD device and the produced ozone flow was then mixed with the exhaust gas flow (see Figure 1 section B). Whereas, the flue gas was not supplied through the plasma, the VBD device was not heated in this experiment. The SIE was calculated by dividing the plasma input power by the flow rate of exhaust gas flow. The ozone concentration injected into the flue gas was obtained from the SIE by measuring the ozone production in absence of the flue gas.
- In the case of ozone treatment with a TiO2 catalyst, an additional reaction chamber was placed downstream from the mixing point of flue gas and ozone (see Figure 1 section C). The dimensions of the reaction chamber were similar to those of the VBD device. Degussa P25 TiO2 nanopowder with the mass of 0.5 g was pressed on the inner surface of the reactor chamber. The catalytic reactor was placed into the electrically heated oven and the reactor temperature was kept at 100 °C during the experiments. The temperature in the reactor was measured by the Osensa Innovations fiber–optical sensor FTX-100-gen. Additional ozone treatment experiments without the presence of catalyst were also carried out in this configuration for direct comparison of the effect of catalyst.
3. Results and Discussions
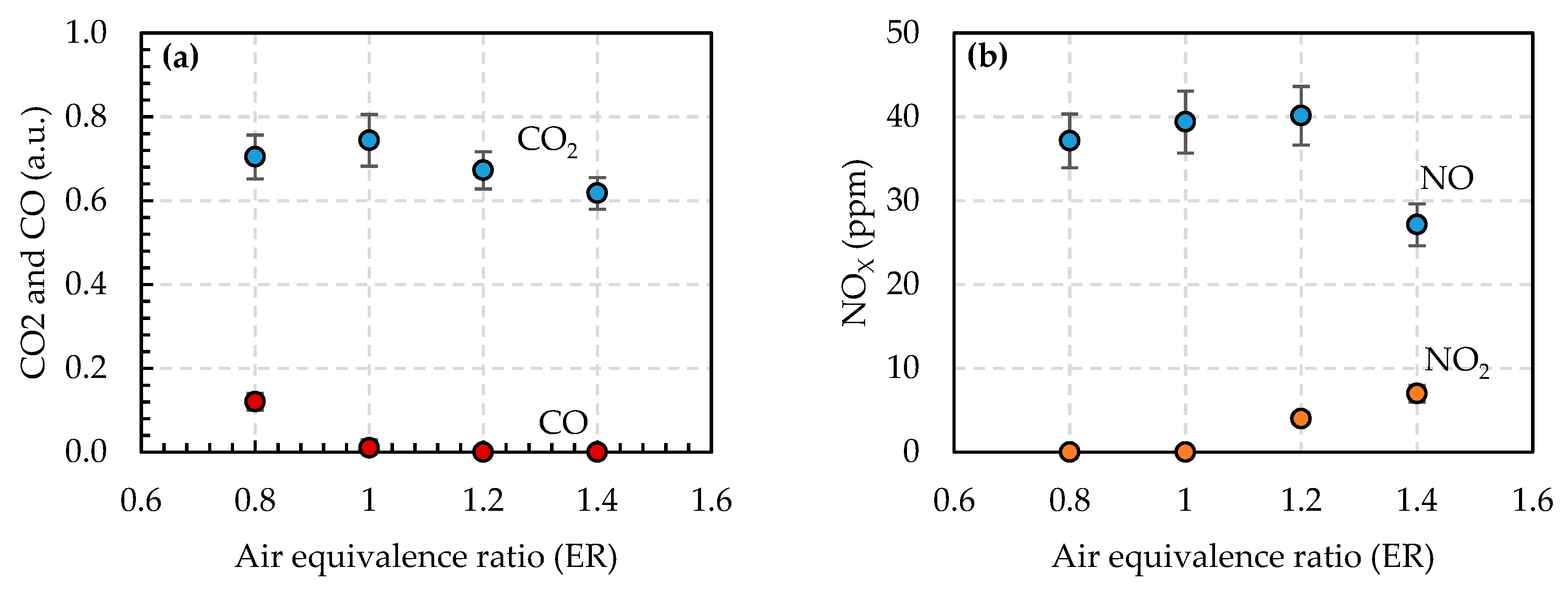
3.1. Emissions from Methane Combustion
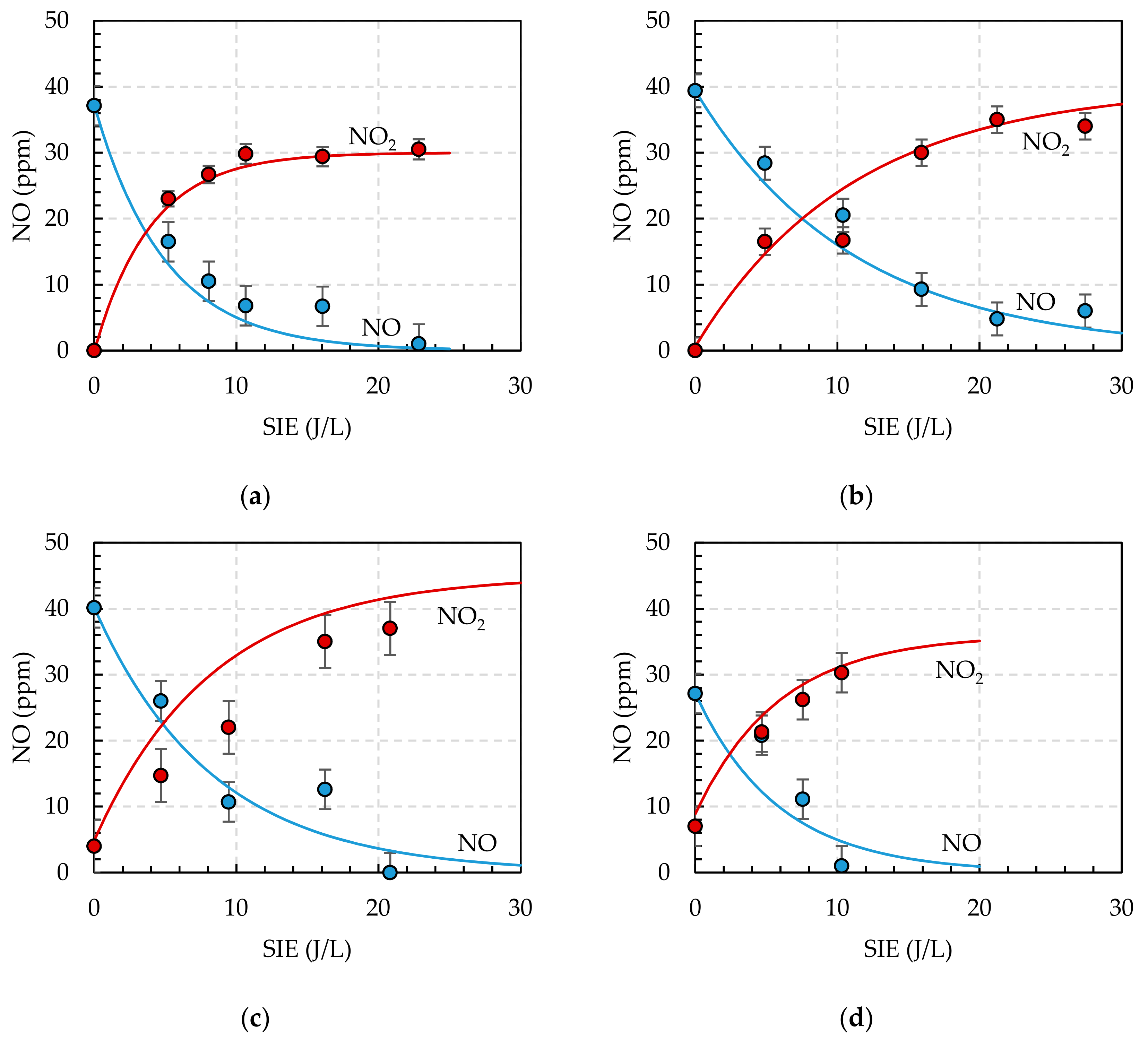
3.2. Direct Plasma Treatment
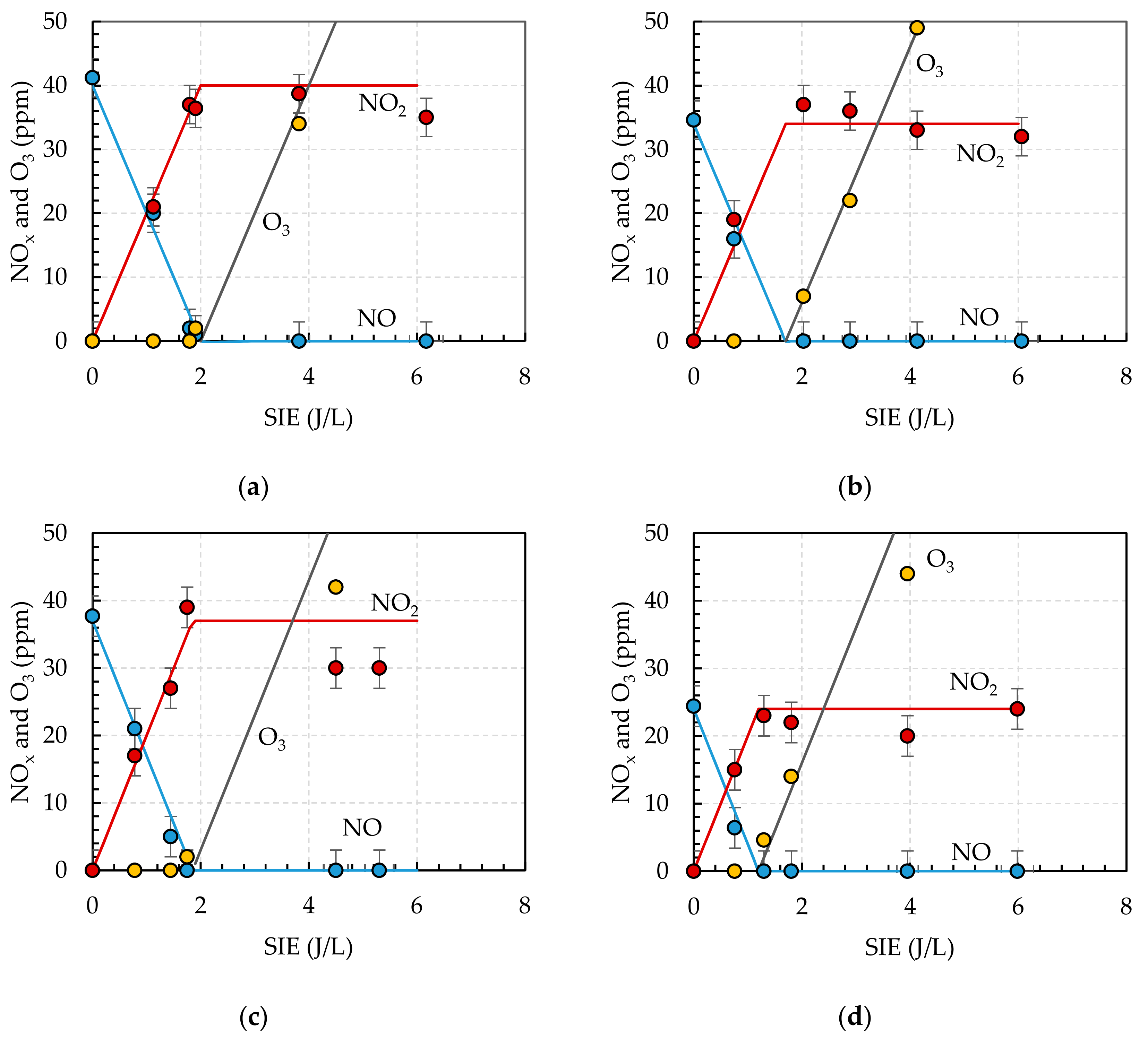
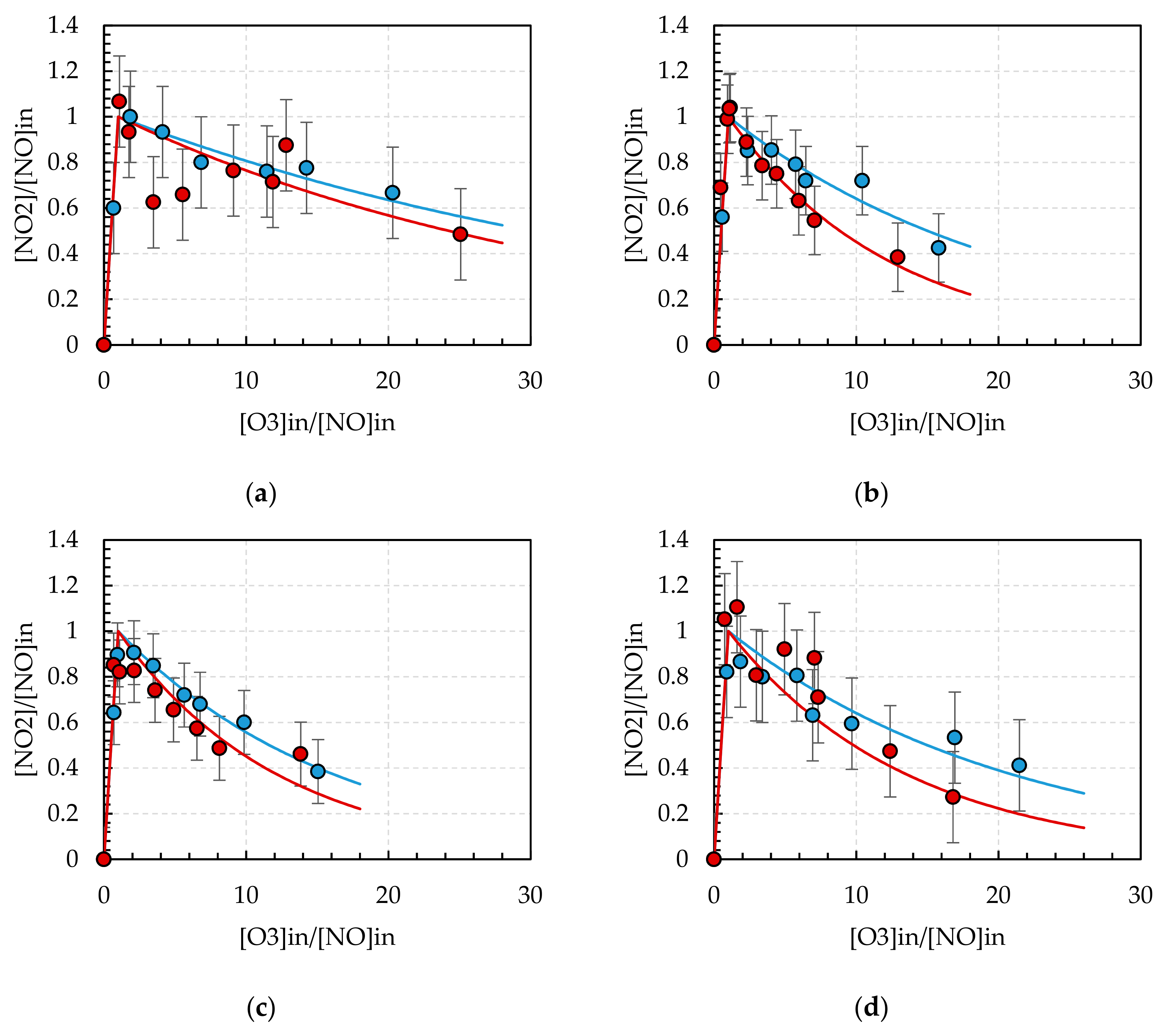
3.3. Ozone Treatment
3.4. Ozone Treatment with TiO2 Catalyst
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Market Observatory for Energy. Quarterly Report Energy on European Gas Markets Market Observatory for Energy DG Energy; European Commission: Brussels, Belgium, 2017; Volume 10. [Google Scholar]
- Aydin, M. Natural gas consumption and economic growth nexus for top 10 natural Gas-Consuming countries: A granger causality analysis in the frequency domain. Energy 2018, 165, 179–186. [Google Scholar] [CrossRef]
- Hill, S.; Douglas Smoot, L. Modeling of nitrogen oxides formation and destruction in combustion systems. Prog. Energy Combust. Sci. 2000, 26, 417–458. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOxabatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Pillier, L.; Idir, M.; Molet, J.; Matynia, A.; De Persis, S. Experimental study and modelling of NOx formation in high pressure counter-flow premixed CH4/air flames. Fuel 2015, 150, 394–407. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. International Energy Outlook 2016; U.S. Energy Information Administration: Washington, DC, USA, 2016; Volume 0484(2016), ISBN 2025866135.
- McDonell, V. Lean Combustion in Gas Turbines. Lean Combust. 2008, 121-IV. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, V. Dynamics and stability of lean-premixed swirl-stabilized combustion. Prog. Energy Combust. Sci. 2009, 35, 293–364. [Google Scholar] [CrossRef]
- Nair, S.; Lieuwen, T. Acoustic Detection of Blowout in Premixed Flames. J. Propuls. Power 2005, 21, 32–39. [Google Scholar] [CrossRef]
- Abbott, D.J.; Bowers, J.P.; James, S.R. The Impact of Natural Gas Composition Variations on the Operation of Gas Turbines for Power Generation. In Proceedings of the 6th International Conference on the Future of Gas Turbine Technology, Brussels, Belgium, 17–18 October 2012. [Google Scholar]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOxabatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Zhou, H.; Su, Y.; Liao, W.; Deng, W.; Zhong, F. NO reduction by propane over monolithic cordierite-based Fe/Al2O3catalyst: Reaction mechanism and effect of H2O/SO2. Fuel 2016, 182, 352–360. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Mok, Y.S.; Koh, D.J.; Shin, D.N.; Kim, K.T. Reduction of nitrogen oxides from simulated exhaust gas by using plasma–catalytic process. Fuel Process. Technol. 2004, 86, 303–317. [Google Scholar] [CrossRef]
- Mok, Y.S.; Koh, D.J.; Kim, K.T.; Nam, I.-S. Nonthermal Plasma-Enhanced Catalytic Removal of Nitrogen Oxides over V2O5/TiO2 and Cr2O3/TiO2. Ind. Eng. Chem. Res. 2003, 42, 2960–2967. [Google Scholar] [CrossRef]
- Sun Mok, Y.; Young Yoon, E. Effect of ozone injection on the catalytic reduction of nitrogen oxides. Ozone Sci. Eng. 2006, 28, 105–110. [Google Scholar] [CrossRef]
- Stamate, E.; Chen, W.; Jørgensen, L.; Jensen, T.K.; Fateev, A.; Michelsen, P. IR and UV gas absorption measurements during NOx reduction on an industrial natural gas fired power plant. Fuel 2010, 89, 978–985. [Google Scholar] [CrossRef]
- Jakubiak, M.P.; Kordylewski, W.K. Pilot-Scale studies on nox removal from flue gas via no ozonation and absorption into naoh solution. Chem. Process Eng. 2012, 33, 345–358. [Google Scholar] [CrossRef]
- Talebizadeh, P.; Babaie, M.; Brown, R.; Rahimzadeh, H.; Ristovski, Z.; Arai, M. The role of non-thermal plasma technique in NOxtreatment: A review. Renew. Sustain. Energy Rev. 2014, 40, 886–901. [Google Scholar] [CrossRef]
- Jõgi, I.; Erme, K.; Levoll, E.; Raud, J.; Stamate, E. Plasma and catalyst for the oxidation of NOx. Plasma Sources Sci. Technol. 2018, 27, 035001. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Belgacem, Z.B.; Carre, G.; Charpentier, E.; Le-Bras, F.; Maho, T.; Robert, E.; Pouvesle, J.M.; Polidor, F.; Gangloff, S.C.; Boudifa, M.; et al. Innovative non-thermal plasma disinfection process inside sealed bags: Assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms. PLoS ONE 2017, 12, e0180183. [Google Scholar] [CrossRef]
- Krugly, E.; Martuzevicius, D.; Tichonovas, M.; Jankunaite, D.; Rumskaite, I.; Sedlina, J.; Racys, V.; Baltrusaitis, J. Decomposition of 2-naphthol in water using a non-thermal plasma reactor. Chem. Eng. J. 2015, 260, 188–198. [Google Scholar] [CrossRef]
- Holzer, F.; Kopinke, F.-D.; Roland, U. Non-thermal plasma treatment for the elimination of odorous compounds from exhaust air from cooking processes. Chem. Eng. J. 2018, 334, 1988–1995. [Google Scholar] [CrossRef]
- Mitrović, T.; Lazović, S.; Nastasijević, B.; Pašti, I.A.; Vasić, V.; Lazarević-Pašti, T. Non-thermal plasma needle as an effective tool in dimethoate removal from water. J. Environ. Manage. 2019, 246, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bunoiu, M.; Jugunaru, I.; Bica, I.; Balasoiu, M. Nonthermal Argon Plasma Generator and Some Potential Applications. Ann. West Univ. Timis. Phys. 2015, 58, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Yamamoto, T.; Kuroki, T.; Okubo, M. Pilot-scale experiment for simultaneous dioxin and no x removal from garbage incinerator emissions using the pulse corona induced plasma chemical process. Plasma Chem. Plasma Process. 2009, 29, 373–386. [Google Scholar] [CrossRef]
- Schmidt, M.; Basner, R.; Brandenburg, R. Hydrocarbon assisted NO oxidation with non-thermal plasma in simulated marine diesel exhaust gases. Plasma Chem. Plasma Process. 2013, 33, 323–335. [Google Scholar] [CrossRef]
- Orlandini, I.; Riedel, U. Chemical kinetics of NO removal by pulsed corona discharges. J. Phys. D Appl. Phys 2000, 33, 2467–2474. [Google Scholar] [CrossRef]
- Penetrante, B.M.; Brusasco, R.M.; Merritt, B.T.; Pitz, W.J.; Vogtlin, G.E.; Kung, M.C.; Kung, H.H.; Wan, C.Z.; Voss, K.E. Plasma-Assisted Catalytic Reduction of NOx. In Proceedings of the 1998 Society of Automotive Engineers Fall Fuels and Lubricants Meeting, San Francisco, CA, USA, 19–22 October 1998. [Google Scholar]
- Khacef, A.; Cormier, J.M.; Pouvesle, J.M. NOx remediation in oxygen-rich exhaust gas using atmospheric pressure non-thermal plasma generated by a pulsed nanosecond dielectric barrier discharge. J. Phys. D Appl. Phys. 2002, 35, 1491–1498. [Google Scholar] [CrossRef]
- McLarnon, C.R.; Penetrante, B.M. Effect of Gas Composition on the NOx Conversion Chemistry in a Plasma. In Proceedings of the Society of Automotive Engineers Fall Fuels and Lubricants Meeting, San Francisco, CA, USA, 19–22 October 1998. [Google Scholar]
- Leipold, F.; Fateev, A.; Kusano, Y.; Stenum, B.; Bindslev, H. Reduction of NO in the exhaust gas by reaction with N radicals. Fuel 2006, 85, 1383–1388. [Google Scholar] [CrossRef]
- Zhao, G.-B.; Garikipati, S.V.B.J.; Hu, X.; Argyle, M.D.; Radosz, M. Effect of oxygen on nonthermal plasma reactions of nitrogen oxides in nitrogen. AIChE J. 2005, 51, 1800–1812. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tang, X.; Yi, H.; Yu, Q.; Wang, J.; Gao, F.; Gao, Y.; Li, D.; Cao, Y. The byproduct generation analysis of the NOx conversion process in dielectric barrier discharge plasma. RSC Adv. 2016, 6, 63946–63953. [Google Scholar] [CrossRef]
- Yan, K.; Kanazawa, S.; Ohkubo, T.; Nomoto, Y. Oxidation and Reduction Process During NOx Removal and Corona-Induced Nonthermal Plasma. Plasma Chem. Plasma Process. 1999, 19, 421–443. [Google Scholar] [CrossRef]
- Lin, H.; Gao, X.; Luo, Z.; Cen, K.; Pei, M.; Huang, Z. Removal of NOxfrom wet flue gas by corona discharge. Fuel 2004, 83, 1251–1255. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. In situ and ex situ NO oxidation assisted by submicrosecond pulsed multi-pin-to-plane corona discharge: The effect of pin density. RSC Adv. 2016, 6, 29983–29995. [Google Scholar] [CrossRef]
- Jogi, I.; Stamate, E.; Irimiea, C.; Schmidt, M.; Brandenburg, R.; Holub, M.; Bonislawski, M.; Jakubowski, T.; Kaariainen, M.-L.; Cameron, D.C. Comparison of direct and indirect plasma oxidation of NO combined with oxidation by catalyst. Fuel 2015, 144, 137–144. [Google Scholar] [CrossRef]
- Shang, S.; Li, X.; Chen, W.; Wang, B.; Shi, W. A total heat recovery system between the flue gas and oxidizing air of a gas-fired boiler using a non-contact total heat exchanger. Appl. Energy 2017, 207, 613–623. [Google Scholar] [CrossRef]
- Hinrichs, J.; Felsmann, D.; Schweitzer-De Bortoli, S.; Tomczak, H.-J.; Pitsch, H. Numerical and experimental investigation of pollutant formation and emissions in a full-scale cylindrical heating unit of a condensing gas boiler. Appl. Energy 2018, 229, 977–989. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Lee, S.; Kum, S.-M.; Lee, C.-E. An experimental study of a cylindrical multi-hole premixed burner for the development of a condensing gas boiler. Energy 2011, 36, 4150–4157. [Google Scholar] [CrossRef]
- Ding, Y.; Durox, D.; Darabiha, N.; Schuller, T. Chemiluminescence based operating point control of domestic gas boilers with variable natural gas composition. Appl. Therm. Eng. 2019, 149, 1052–1060. [Google Scholar] [CrossRef]
- Manley, T.C. The Electric Characteristics of the Ozonator Discharge. J. Electrochem. Soc. 1943, 84, 83–96. [Google Scholar] [CrossRef]
- Jõgi, I.; Bichevin, V.; Laan, M.; Haljaste, A.; Käämbre, H. NO Conversion by Dielectric Barrier Discharge and TiO2 Catalyst: Effect of Oxygen. Plasma Chem. Plasma Process. 2009, 29, 205–215. [Google Scholar] [CrossRef]
- Jõgi, I.; Erme, K.; Haljaste, A.; Laan, M. Oxidation of nitrogen oxide in hybrid plasma-catalytic reactors based on DBD and Fe2O3. EPJ Appl. Phys. 2013, 61. [Google Scholar] [CrossRef]
- Jõgi, I.; Haljaste, A.; Laan, M. Hybrid TiO2 based plasma-catalytic reactors for the removal of hazardous gasses. Surf. Coat. Technol. 2014, 242, 195–199. [Google Scholar] [CrossRef]
- Baukal, C.E. The John Zink Combustion Handbook, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9781420038699. [Google Scholar]
- Munir, S.; Nimmo, W. Nox Formation during Combustion Process and in-Furnace Control Technologies. J. Pak. Inst. Chem. Eng. 2009, 37, 13–21. [Google Scholar]
- Wiberg, E.; Wiberg, N.; Holleman, A.F. Inorganic Chemistry; Academic Press: Cambridge, MA, USA; De Gruyter: San Diego, CA, USA; Berlin, Germany; New York, NY, USA, 2001; ISBN1 0123526515. ISBN2 9780123526519. [Google Scholar]
- Jõgi, I.; Erme, K.; Levoll, E.; Stamate, E. Radical production efficiency and electrical characteristics of a coplanar barrier discharge built by multilayer ceramic technology. J. Phys. D Appl. Phys. 2017, 50, 465201. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Van Heesch, E.J.M.; Pemen, A.J.M.; Huijbrechts, P.A.H.J. From chemical kinetics to streamer corona reactor and voltage pulse generator. Plasma Chem. Plasma Process. 2001, 21, 107–137. [Google Scholar] [CrossRef]
- Rosocha, L.A. Nonthermal plasma applications to the environment: Gaseous electronics and power conditioning. IEEE Trans. Plasma Sci. 2005, 33, 129–137. [Google Scholar] [CrossRef]
- Malik, M.A.; Kolb, J.F.; Sun, Y.; Schoenbach, K.H. Comparative study of NO removal in surface-plasma and volume-plasma reactors based on pulsed corona discharges. J. Hazard. Mater. 2011, 197, 220–228. [Google Scholar] [CrossRef]
- Eliasson, B.; Kogelschatz, U.; Baessler, P. Dissociation of O2 in N2/O2 mixtures. J. Phys. B Mol. Phys. 1984, 17, L797–L801. [Google Scholar] [CrossRef]
- Jõgi, I.; Levoll, E.; Raud, J. Plasma oxidation of NO in O2:N2 mixtures: The importance of back-reaction. Chem. Eng. J. 2016, 301, 149–157. [Google Scholar] [CrossRef]
- Penetrante, B.M.; Bardsley, N.; Hsiao, M.C. Kinetic Analysis of Non-Thermal Plasmas Used for Pollution Control. Jpn. J. Appl. Phys. 1997, 36, 5007–5017. [Google Scholar] [CrossRef]
- Jõgi, I.; Erme, K.; Raud, J.; Laan, M. Oxidation of NO by ozone in the presence of TiO2 catalyst. Fuel 2016, 173, 45–54. [Google Scholar] [CrossRef]


| Air Equivalence Ratio | CH4, L/min | Air, L/min | Overall flow, L/min | Generated Power, kW |
|---|---|---|---|---|
| 0.8 | 1 | 7.6 | 8.6 | 0.56 |
| 1.0 | 9.5 | 10.5 | ||
| 1.2 | 11.4 | 12.4 | ||
| 1.4 | 13.3 | 14.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulauskas, R.; Jõgi, I.; Striūgas, N.; Martuzevičius, D.; Erme, K.; Raud, J.; Tichonovas, M. Application of Non-Thermal Plasma for NOx Reduction in the Flue Gases. Energies 2019, 12, 3955. https://doi.org/10.3390/en12203955
Paulauskas R, Jõgi I, Striūgas N, Martuzevičius D, Erme K, Raud J, Tichonovas M. Application of Non-Thermal Plasma for NOx Reduction in the Flue Gases. Energies. 2019; 12(20):3955. https://doi.org/10.3390/en12203955
Chicago/Turabian StylePaulauskas, Rolandas, Indrek Jõgi, Nerijus Striūgas, Dainius Martuzevičius, Kalev Erme, Jüri Raud, and Martynas Tichonovas. 2019. "Application of Non-Thermal Plasma for NOx Reduction in the Flue Gases" Energies 12, no. 20: 3955. https://doi.org/10.3390/en12203955





