Enhanced Degradation of Pharmaceutical Compounds by a Microbubble Ozonation Process: Effects of Temperature, pH, and Humic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pharmaceutical Compounds and Reagents
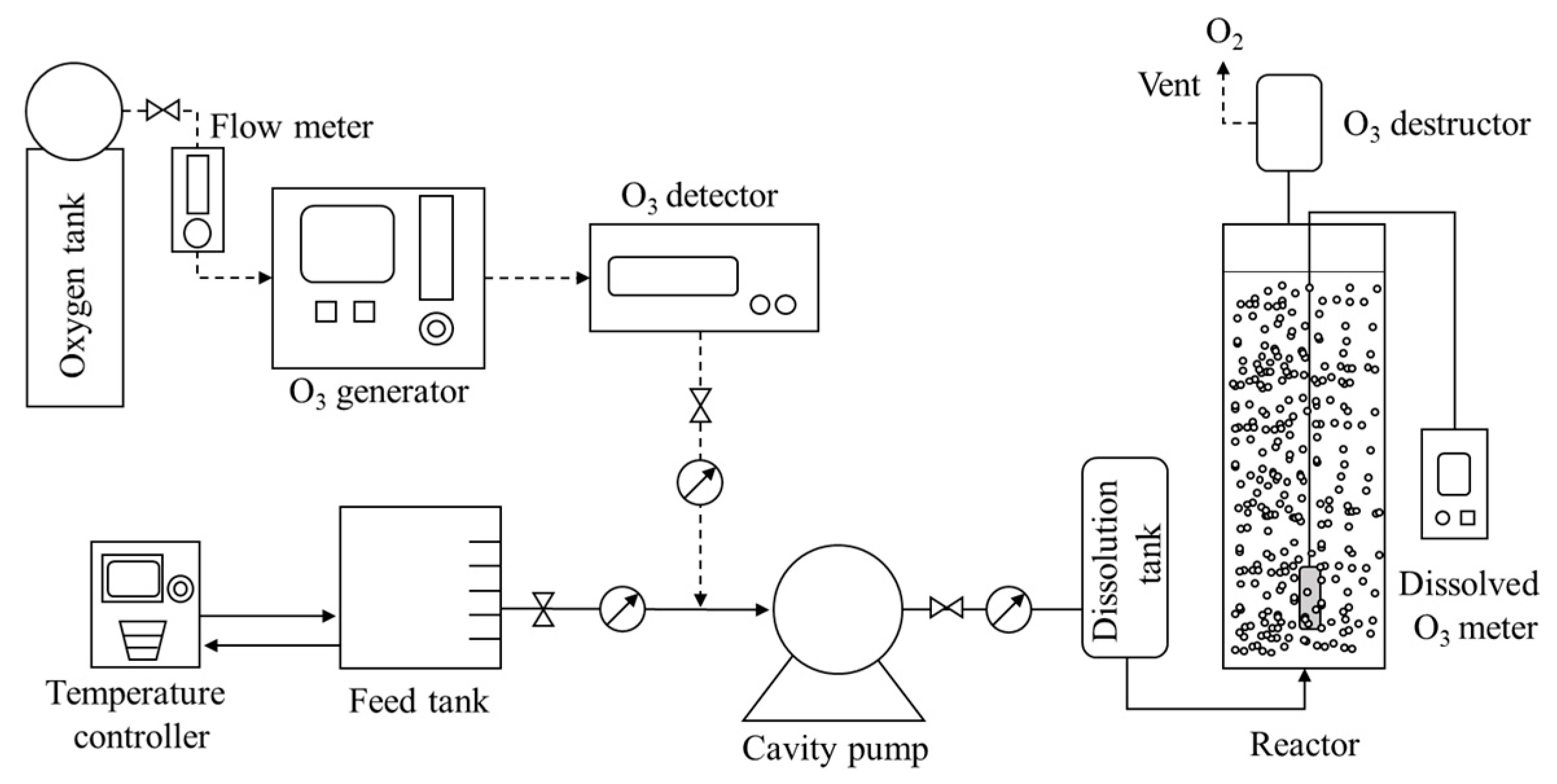
2.2. System Description
2.3. Microbubble Ozonation
2.4. Analytical Methods
3. Results and Discussion
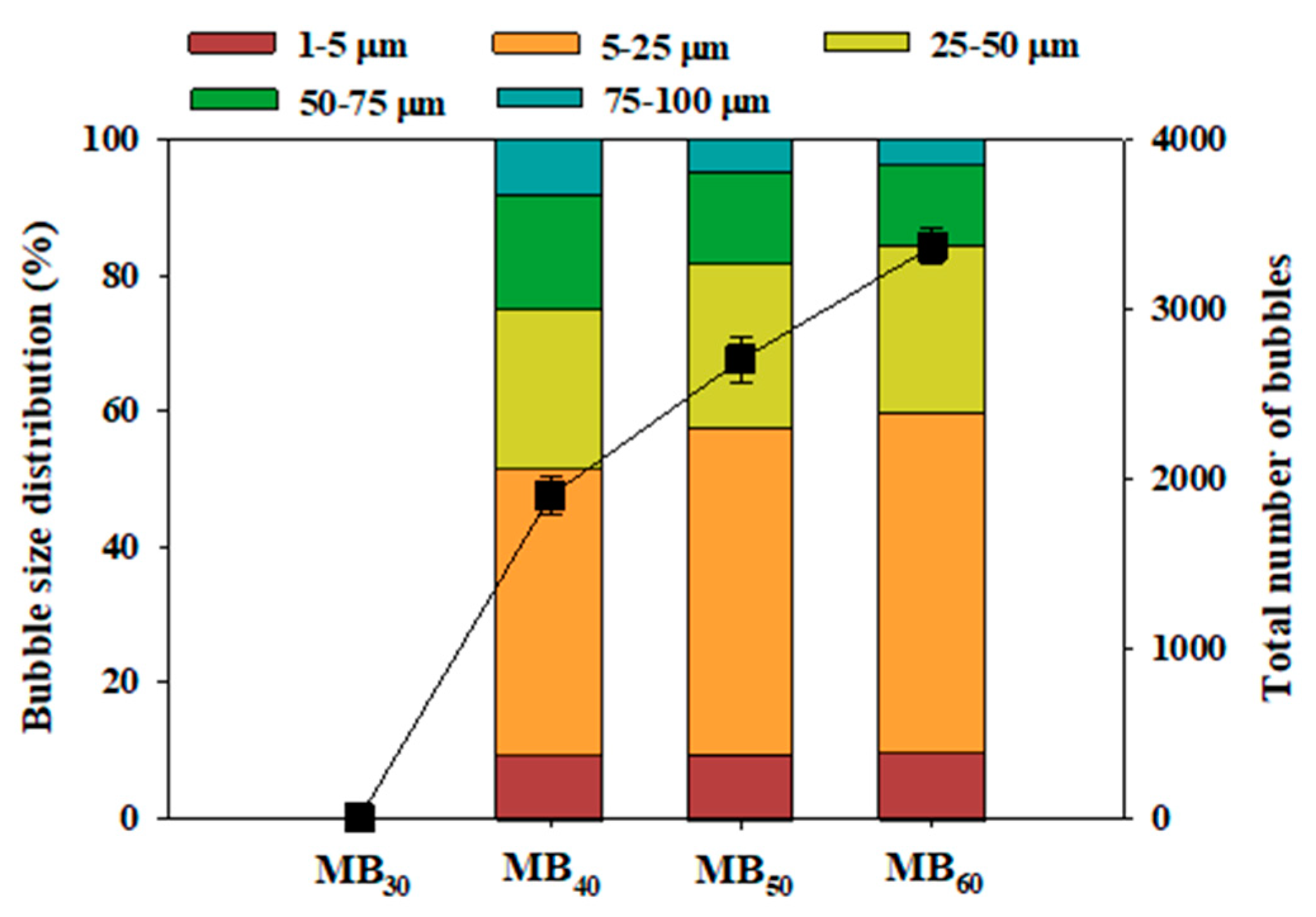
3.1. Size Distribution of Microbubbles
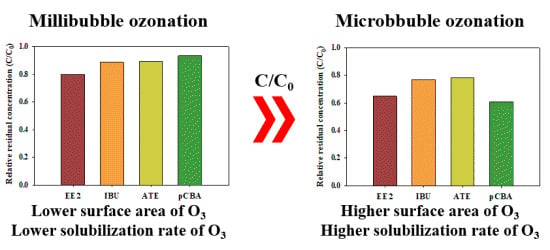
3.2. Bubble Size Effects on Solubilization Rate of O3 and OH Radicals
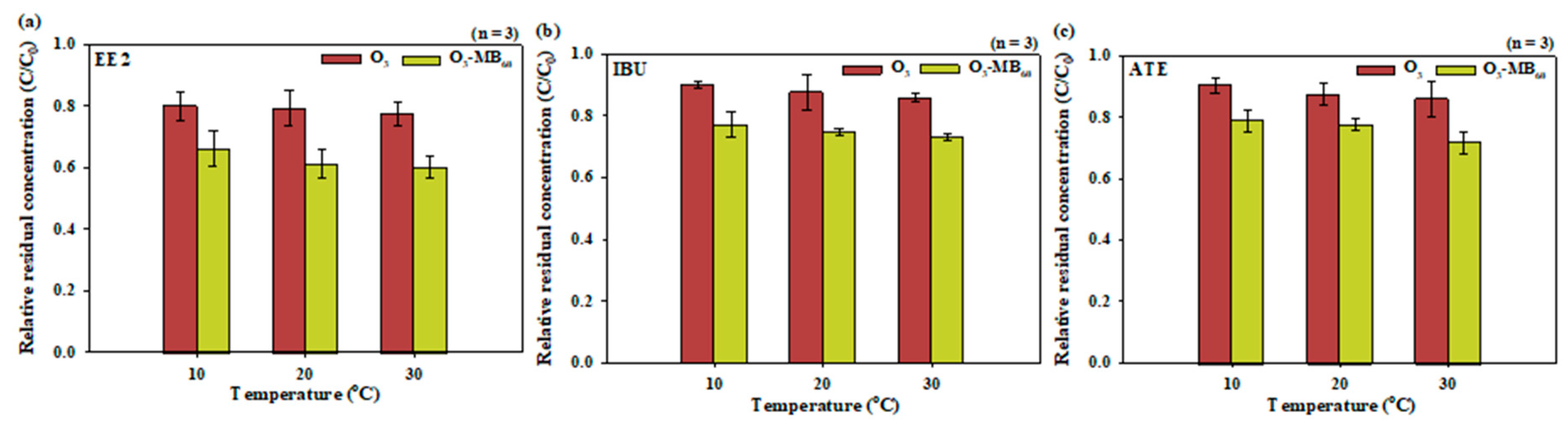
3.3. Effects of Temperature on Degradation of the Pharmaceutical Compounds
3.4. Effects of pH on the Degradation of Pharmaceutical Compounds
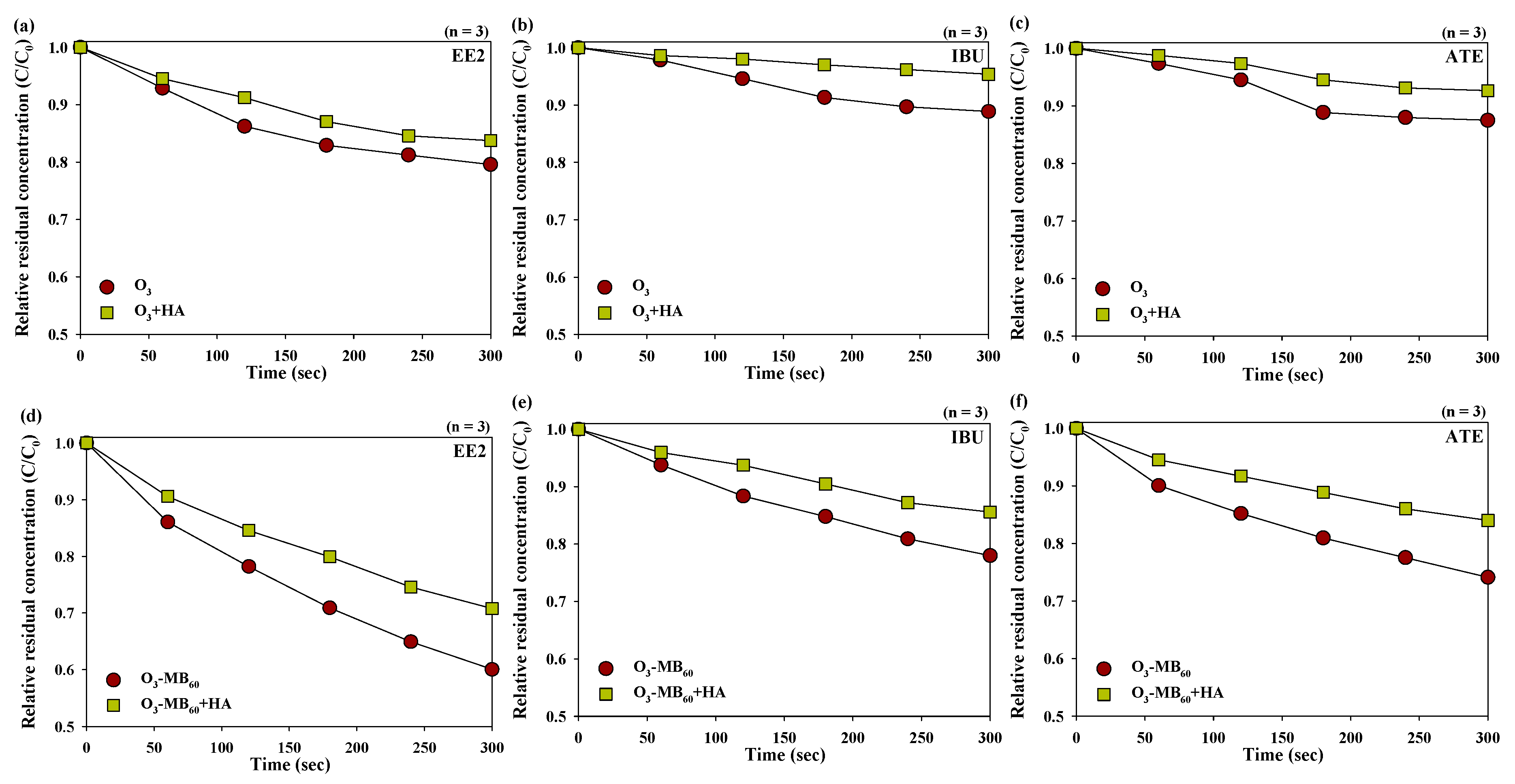
3.5. Effects of Humic Acids on the Degradation of Pharmaceutical Compounds
4. Conclusions
- The fractions of microbubbles whose diameters ranged from 1–25 μm gradually increased with increasing the cavity pump frequency (40 Hz: 51.4%; 50 Hz: 57.5%; 60 Hz: 59.9%).
- The increase of the solubilization rate of O3 and OH radicals induced by their elevated mass transfer at the gas–water interface through the destruction of O3 microbubbles with the higher specific area than O3 millibubbles might substantially improve the degradation of the pharmaceutical compounds.
- Both the solubilization rate and the reactivity of O3 and OH radicals provided strong effects on the degradation of the pharmaceutical compounds during the millibubble and microbubble ozonation processes over the temperature range of 10–30 °C.
- The changes in the degradation of pharmaceutical compounds depending on the pH of the solution and the presence of humic acids were much more pronounced for the microbubble ozonated waters than the millibubble ozonated waters.
- The facilitatory and inhibitory effects of humic acids on the degradation of the pharmaceutical compounds were more significant in the microbubble ozonated waters compared to the millibubble ozonated waters.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Zoh, K.D. Occurrence and removals of micropollutants in water environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef]
- Onesios, K.M.; Jim, T.Y.; Bouwer, E.J. Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: A review. Biodegradation 2009, 20, 441–466. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of eu decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Chon, K.; Salhi, E.; von Gunten, U. Combination of uv absorbance and electron donating capacity to assess degradation of micropollutants and formation of bromate during ozonation of wastewater effluents. Water Res. 2015, 81, 388–397. [Google Scholar] [CrossRef]
- Wang, K.; Liu, S.; Zhang, Q.; He, Y. Pharmaceutical wastewater treatment by internal micro-electrolysis-coagulation, biological treatment and activated carbon adsorption. Environ. Technol. 2009, 30, 1469–1474. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A review. Ozone: Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Ozonation of drinking water: Part ii. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Miyamoto, H.; Yampolski, Y.; Young, C.L. Iupac-nist solubility data series. 103. Oxygen and ozone in water, aqueous solutions, and organic liquids (supplement to solubility data series volume 7). J. Phys. Chem. Ref. Data 2014, 43, 033102. [Google Scholar] [CrossRef]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y.; Hayashi, T. Removal of pharmaceuticals in water by introduction of ozonated microbubbles. Sep. Purif. Technol. 2019, 212, 483–489. [Google Scholar] [CrossRef]
- ISO 20480–1:2017 Fine Bubble Technology-General principles for usage and measurement of fine bubbles- Part 1: Terminology 2017. Available online: https://www.iso.org/obp/ui/#iso:std:iso:20480:-1:ed-1:v1:en (accessed on 5 November 2019).
- Jabesa, A.; Ghosh, P. Removal of diethyl phthalate from water by ozone microbubbles in a pilot plant. J. Environ. Manag. 2016, 180, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, o3/h2o2 and uv/h2o2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef]
- Chu, L.B.; Xing, X.H.; Yu, A.F.; Sun, X.L.; Jurcik, B. Enhanced treatment of practical textile wastewater by microbubble ozonation. Process. Saf. Environ. Prot. 2008, 86, 389–393. [Google Scholar] [CrossRef]
- Li, P.; Takahashi, M.; Chiba, K. Degradation of phenol by the collapse of microbubbles. Chemosphere 2009, 75, 1371–1375. [Google Scholar] [CrossRef]
- Oppenländer, T. Photochemical Purification of Water and Air: Advanced Oxidation Processes (Aops)-Principles, Reaction Mechanisms Reactor Concepts; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Chu, L.B.; Yan, S.T.; Xing, X.H.; Yu, A.F.; Sun, X.L.; Jurcik, B. Enhanced sludge solubilization by microbubble ozonation. Chemosphere 2008, 72, 205–212. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Tamaki, M. Removal of residual pesticides in vegetables using ozone microbubbles. J. Hazard. Mater. 2011, 186, 956–959. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferratevi, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Tabei, K.; Haruyama, S.; Yamaguchi, S.; Shirai, H.; Takakusagi, F. Study of micro bubble generation by a swirl jet. J. Environ. Eng. 2007, 2, 172–182. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A: Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Formation of hydroxyl radicals by collapsing ozone microbubbles under strongly acidic conditions. J. Phys. Chem. B 2007, 111, 11443–11446. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Quantitative prediction of generation of hydroxyl radicals from ozone microbubbles. Chem. Eng. Res. Des. 2015, 98, 231–239. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O.; Onubogu, J.; Joyce, E.; Mason, T. Sonochemical degradation of endocrine disrupting chemicals 17β-estradiol and 17α-ethinylestradiol in water and wastewater. Int. J. Environ. Sci. Technol. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef]
- Huber, M.M.; Ternes, T.A.; von Gunten, U. Removal of estrogenic activity and formation of oxidation products during ozonation of 17α-ethinylestradiol. Environ. Sci. Technol. 2004, 38, 5177–5186. [Google Scholar] [CrossRef]
- Benner, J.; Salhi, E.; Ternes, T.; von Gunten, U. Ozonation of reverse osmosis concentrate: Kinetics and efficiency of beta blocker oxidation. Water Res. 2008, 42, 3003–3012. [Google Scholar] [CrossRef]
- Bahr, C.; Schumacher, J.; Ernst, M.; Luck, F.; Heinzmann, B.; Jekel, M. Suva as control parameter for the effective ozonation of organic pollutants in secondary effluent. Water Sci. Technol. 2007, 55, 267–274. [Google Scholar] [CrossRef]
- Buffle, M.O.; Schumacher, J.; Meylan, S.; Jekel, M.; von Gunten, U. Ozonation and advanced oxidation of wastewater: Effect of o3 dose, ph, dom and ho -scavengers on ozone decomposition and ho generation. Ozone: Sci. Eng. 2006, 28, 247–259. [Google Scholar] [CrossRef]
- Elovitz, M.S.; von Gunten, U. Hydroxyl radical/ozone ratios during ozonation processes. I. The rct concept. Ozone: Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Lou, Z.; Zhu, N.; Yuan, H. The degradation processes of refractory substances in nanofiltration concentrated leachate using micro-ozonation. Waste Manag. 2017, 69, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Duan, Y.; Fan, W.; Guo, T.; Huo, M.; Yang, W.; Zhu, S.; An, W. Intensifying ozonation treatment of municipal secondary effluent using a combination of microbubbles and ultraviolet irradiation. Environ. Science Pollut. Res. 2019, 26, 21915–21924. [Google Scholar] [CrossRef] [PubMed]
- Wenk, J.; Aeschbacher, M.; Salhi, E.; Canonica, S.; von Gunten, U.; Sander, M. Chemical oxidation of dissolved organic matter by chlorine dioxide, chlorine, and ozone: Effects on its optical and antioxidant properties. Environ. Sci. Technol. 2013, 47, 11147–11156. [Google Scholar] [CrossRef] [Green Version]
- Buffle, M.O.; von Gunten, U. Phenols and amine induced HO generation during the initial phase of natural water ozonation. Environ. Sci. Technol. 2006, 40, 3057–3063. [Google Scholar] [CrossRef]



| Compounds (Abbreviation, Molar Mass (g mol−1)) | Use | Structure | Electron-Rich Organic Moiety | pKa | at pH 7 (M−1 s−1) | at pH 7 (M−1 s−1) | UVA Detection (nm) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 17α -Ethinylestradiol (EE2, 296.40) | Ovulation inhibitor | 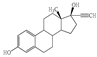 | Phenol | 10.4 | 3.0 × 106 | 9.8 × 109 | 230 | [23,24] |
| Ibuprofen (IBU, 206.28) | Analgesic | 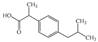 | Alkyl aromatic | 4.9 | 9.6 | 7.4 × 109 | 210 | [25] |
| Atenolol (ATE, 266.34) | β-blocker |  | Secondary amine | 9.6 | 1.7 × 104 | 8.0 × 109 | 251 | [26] |
| Para-Chlorobenzoic acid (pCBA, 156.57) | OH radical probe compound |  | Chloro-aromatic | 4.0 | <0.2 | 5.0 × 109 | 240 | [8] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-G.; Park, Y.; Lee, G.; Kim, Y.; Chon, K. Enhanced Degradation of Pharmaceutical Compounds by a Microbubble Ozonation Process: Effects of Temperature, pH, and Humic Acids. Energies 2019, 12, 4373. https://doi.org/10.3390/en12224373
Lee Y-G, Park Y, Lee G, Kim Y, Chon K. Enhanced Degradation of Pharmaceutical Compounds by a Microbubble Ozonation Process: Effects of Temperature, pH, and Humic Acids. Energies. 2019; 12(22):4373. https://doi.org/10.3390/en12224373
Chicago/Turabian StyleLee, Yong-Gu, Yongeun Park, Gwanghee Lee, Yeongkwan Kim, and Kangmin Chon. 2019. "Enhanced Degradation of Pharmaceutical Compounds by a Microbubble Ozonation Process: Effects of Temperature, pH, and Humic Acids" Energies 12, no. 22: 4373. https://doi.org/10.3390/en12224373