Study of Conventional Sintered Cu2Se Thermoelectric Material
Abstract
:1. Introduction
2. Experimental procedures
2.1. Preparation of Materials
2.2. Characterizations
2.2.1. Powder X-ray Diffraction (XRD)
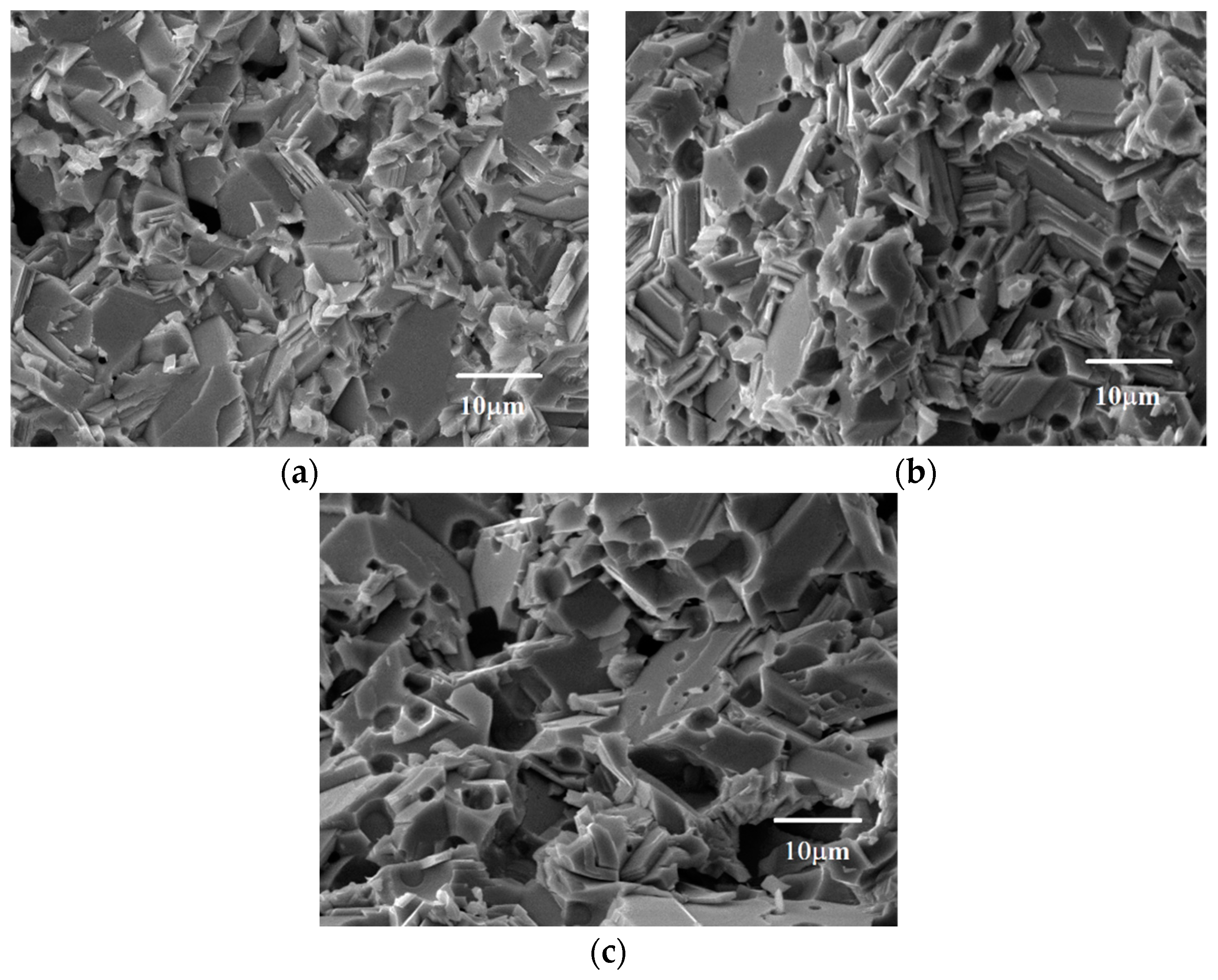
2.2.2. Scanning Electron Microscopy (SEM)
2.3. Thermoelectric Characterization
2.3.1. Seebeck Coefficient and Electrical Conductivity Measurements
2.3.2. Thermal Conductivity Measurement
3. Results and Discussion
3.1. Microstructural Properties
3.2. Thermoelectric Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tritt, T.M.; Subramanian, M.A. Thermoelectric materials, phenomena, and applications: A bird’s eye view. MRS Bull. 2006, 31, 188–198. [Google Scholar] [CrossRef]
- Han, C.; Sun, Q.; Zhen, L.; Shi, X.D. Thermoelectric Enhancement of Different kinds of metal chalcogenides. Adv. Energy Mater. 2016, 6, 1600498. [Google Scholar] [CrossRef]
- Zhao, L.D.; Tan, G.J.; Hao, S.Q.; He, J.Q.; Pei, Y.L.; Chi, H.; Wang, H.; Gong, S.K.; Xu, H.B.; Dravid, V.P.; et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 2016, 351, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Shi, X.; Xu, F.F.; Zhang, L.L.; Zhang, W.Q.; Chen, L.D.; Li, Q.; Uher, C.; Day, T.; Snyder, G.J. Copper ion liquid-like thermoelectrics. Nat. Mater. 2012, 11, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qin, X.Y.; Liu, Y.F.; Song, C.J.; Wang, L.; Zhang, J.; Xin, H.X.; Guo, G.L.; Zou, T.H.; Sun, G.L.; et al. Chemical synthesis of nanostructured Cu2Se with high thermoelectric performance. RSC Adv. 2014, 4, 8638. [Google Scholar] [CrossRef]
- Zhao, L.L.; Wang, X.L.; Wang, J.Y.; Cheng, Z.X.; Dou, S.X.; Wang, J.; Liu, L.Q. Superior intrinsic thermoelectric performance with zT of 1.8 in single-crystal and melt-quenched highly dense Cu2−xSe bulks. Sci. Rep. 2015, 5, 7671. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.G.; Han, G.; Hong, M.; Zou, Y.C.; Zou, J. High-performance thermoelectric Cu2Se nanoplates through nanostructure engineering. Nano Energy 2015, 16, 367–374. [Google Scholar] [CrossRef]
- Day, T.W.; Borup, K.A.; Zhang, T.S.; Drymiotis, F.; Brown, D.R.; Shi, X.; Chen, L.D.; Iversen, B.B.; Snyder, G.J. High temperature thermoelectric properties of Cu1.97Ag0.03Se1+y. Mater. Renew. Sustain. Energy 2014, 3, 26. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.Q.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Bohra, A.; Bhatt, R.; Bhattacharya, S.; Basu, R.; Ahmad, S.; Singh, A.; Aswal, D.K.; Gupta, S.K. Study of thermal stability of Cu2Se thermoelectric material. J. Appl. Phys. 2016, 1731, 110010. [Google Scholar]
- Olvera, A.A.; Moroz, N.A.; Sahoo, P.; Ren, P.; Bailey, T.P.; Page, A.A.; Uher, C.; Poudeu, P.F. Partial indium solubility induced chemical stability and colossal thermoelectric figure of merit in Cu2Se. Energy Environ. Sci. 2017, 10, 1668–1676. [Google Scholar] [CrossRef]
- Kang, S.D.; Pohls, J.H.; Aydemir, U.; Qiu, P.F.; Stoumpos, C.C.; Hanus, R.; White, M.A.; Shi, X.; Chen, L.D.; Kanatzidis, M.G.; et al. Enhanced stability and thermoelectric figure-of-merit in copper selenide by lithium doping. Mater. Today Phys. 2017, 1, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.P.; Hui, S.; Xie, H.Y.; Olvera, A.; Poudeu, P.F.P.; Tang, X.F.; Uher, C. Enhanced zT and attempts to chemically stabilize Cu2Se via Sn doping. J. Mater. Chem. A 2016, 4, 17225–17235. [Google Scholar] [CrossRef]
- Nunna, R.; Qiu, P.F.; Yin, M.J.; Chen, H.Y.; Hanus, R.; Song, Q.F.; Zhang, T.S.; Chou, M.Y.; Agne, M.T.; He, J.Q.; et al. Ultrahigh thermoelectric performance in Cu2Se-based hybrid materials with highly dispersed molecular CNTs. Energy Environ. Sci. 2017, 10, 1928. [Google Scholar] [CrossRef]
- Dennler, G.; Chmielowski, R.; Jacob, S.; Capet, F.; Roussel, P.; Zastrow, S.; Nielsch, K.; Opahle, I.; Madsen, G.K.H. Are binary copper sulfides/selenides really new and promising thermoelectric materials? Anv. Energy Mater. 2014, 4, 1301581. [Google Scholar] [CrossRef]
- Liu, F.S.; Huang, M.J.; Gong, Z.N.; Ao, W.Q.; Li, Y.; Li, J.Q. Enhancing the thermoelectric performance of β-Cu2Se by incorporating SnSe. J. Alloy Compd. 2015, 651, 648–654. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.G.; Han, G.; Hong, M.; Huang, L.Q.; Zou, J. Te-Doped Cu2Se nanoplates with a high average thermoelectric figure of merit. J. Mater. Chem. A 2016, 4, 9213. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General structure analysis system (GSAS). Los Alamos Natl. Lab. Rep. LAUR 2000, 86–748. [Google Scholar]
- Sun, Y.X.; Xi, L.L.; Yang, J.; Wu, L.H.; Shi, X.; Chen, L.D.; Snyder, J.; Yang, J.H.; Zhang, W.Q. The “electron crystal” behaviour in copper chalcogenides Cu2X (X = Se, S). J. Mater. Chem. A 2017, 5, 5098. [Google Scholar] [CrossRef]
- Mansour, B.A.; Demian, S.E. Determination of the effective mass for highly degenerate copper selenide from reflectivity measurements. J. Mater. Sci. Mater. Electron. 1992, 3, 249–252. [Google Scholar] [CrossRef]
- Zhang, P.J.; Meng, Y.; Liu, Z.Y.; Li, Y.D.; Su, T.; Meng, Q.Y.; Mao, Q.; Pan, X.Y.; Chen, D.M.; Zhao, H.W. Impact of interfacial resistance switching on thermoelectric effect of Nb-doped SrTiO3 single crystalline. J. Appl. Phys. 2012, 111, 063702. [Google Scholar] [CrossRef]
- Solannki, G.K.; Gujarathi, D.N.; Deshpande, M.P.; Lakshminarayana, F.; Agarwal, M.K. Transport property measurements in tungsten sulphoselenide single crystals frown by a CVT technique. Cryst. Res. Technol. 2008, 43, 179–185. [Google Scholar] [CrossRef]
- Suh, D.; Lee, D.; Kang, C.Y.; Shon, I.J.; Kim, W.; Baik, S. Enhanced thermoelectric properties of tungsten disulfide-multiwalled carbon nanotube composites. J. Mater. Chem. 2012, 22, 21376–21381. [Google Scholar] [CrossRef]
- Liu, F.S.; Gong, Z.N.; Huang, M.J.; Ao, W.Q.; Li, Y.; Li, Q.J. Enhanced thermoelectric properties of β-Cu2Se by incorporating CuGaSe2. J. Alloys Compd. 2016, 688, 521–526. [Google Scholar] [CrossRef]
- Peng, P.; Gong, Z.N.; Liu, F.S.; Huang, M.J.; Ao, W.Q.; Li, Y.; Li, J.Q. Structure and thermoelectric performance of beta-Cu2Se doped with Fe, Ni, Mn, In, Zn or Sm. Intermetallics 2016, 75, 72–78. [Google Scholar] [CrossRef]
- Wu, L.C.; Su, X.L.; Yan, Y.G.; Uher, C.; Tang, X.F. Ultra-Fast One-Step Fabrication of Cu2Se Thermoelectric Legs with Ni-Al electrodes by plasma-activated reactive sintering technique. Adv. Eng. Mater. 2016, 7, 1181–1188. [Google Scholar] [CrossRef]
- Cao, F.; Du, X.H.; Wu, F.; Li, X.J.; Hu, X.; Song, H.Z. Thermoelectric properties of Cu2Se/xNi0.85Se hot-pressed from hydrothermal synthesis nanopowders. Mod. Phys. Lett. B 2017, 31, 1750093. [Google Scholar]

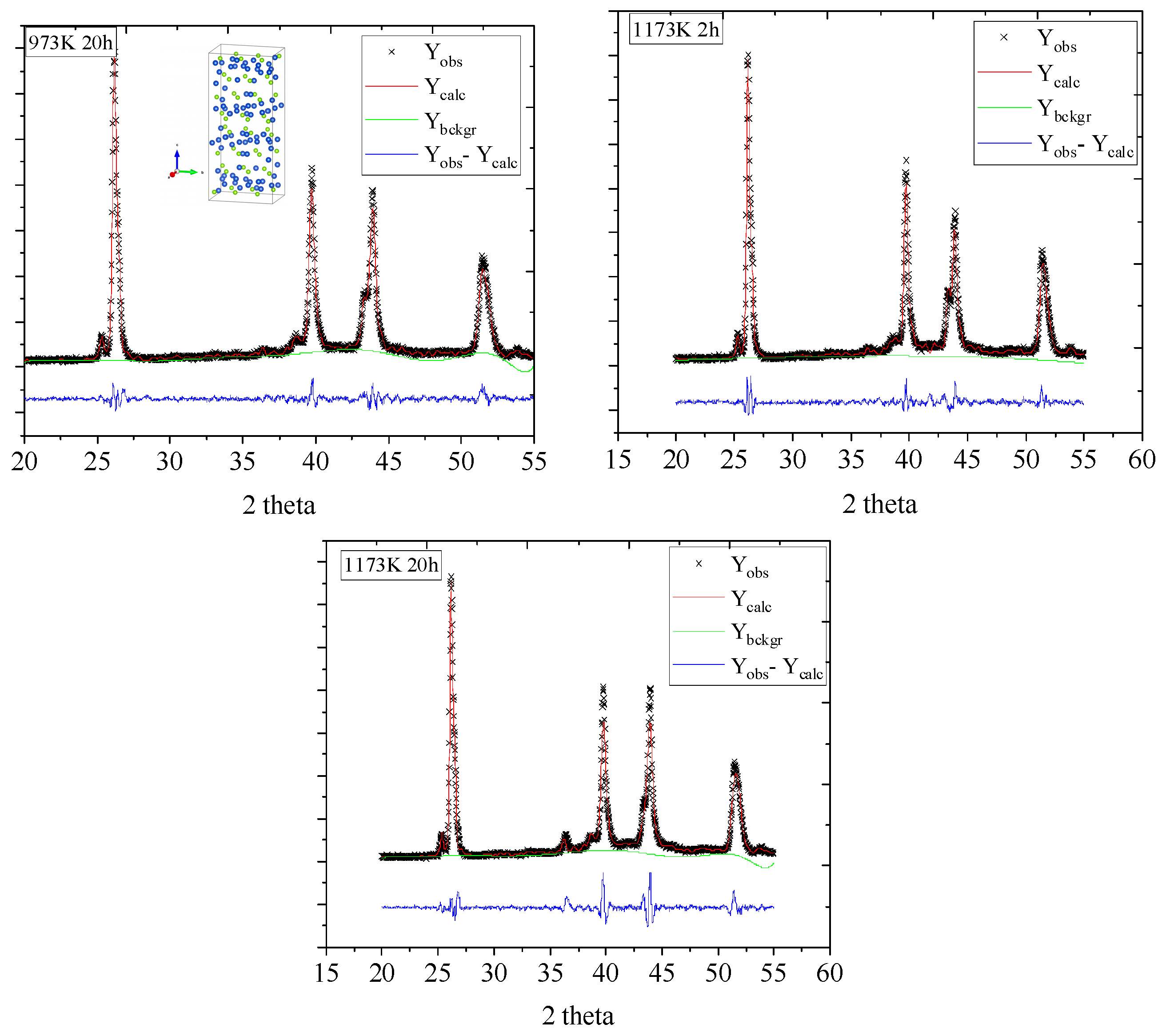


| Samples | Lattice Parameters | R-Factors | ||||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | beta | Vol (Å3) | χ2 | Rp (%) | Rwp (%) | |
| Cu2Se-973 K 20 h | 7.158 | 12.470 | 27.574 | 94.669 | 2452.977 | 2.535 | 7.09 | 9.34 |
| Cu2Se-1173 K 2 h | 7.110 | 12.505 | 27.391 | 95.165 | 2425.473 | 2.58 | 7.17 | 9.39 |
| Cu2Se-1173 K 20 h | 7.164 | 12.376 | 27.432 | 94.408 | 2424.877 | 4.459 | 9.74 | 12.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Geng, Z.; Lam, K.H. Study of Conventional Sintered Cu2Se Thermoelectric Material. Energies 2019, 12, 401. https://doi.org/10.3390/en12030401
Shi D, Geng Z, Lam KH. Study of Conventional Sintered Cu2Se Thermoelectric Material. Energies. 2019; 12(3):401. https://doi.org/10.3390/en12030401
Chicago/Turabian StyleShi, Dongliang, Zhiming Geng, and Kwok Ho Lam. 2019. "Study of Conventional Sintered Cu2Se Thermoelectric Material" Energies 12, no. 3: 401. https://doi.org/10.3390/en12030401





