Novel Yeast Strains for the Efficient Saccharification and Fermentation of Starchy By-Products to Bioethanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation, Genetic Characterization and Fermentative Abilities of Novel Yeast Strains in Glucose
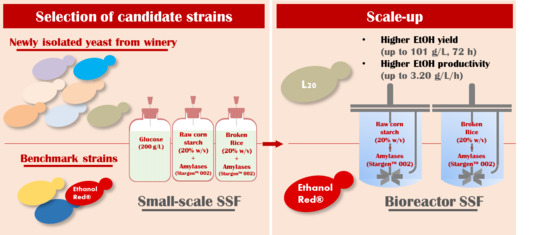
2.2. Fermentative Abilities under SSF Setting on Starchy Materials
2.3. Scale-up in 1-L Bench Fermenter
3. Materials and Methods
3.1. Feedstocks and Commercial Enzymes
3.2. Yeast Strains, Isolation and Genetic Identification
3.3 Fermentative Abilities of S. cerevisiae Strains in MNS Broth Supplemented with 200 g/L Glucose
3.4. Fermentative Abilities of S. cerevisiae Strains on Starchy Materials
3.5. Scale-up of SSF in 1-L Bench Fermenter
3.6. Analytical Methods and Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2016, 69, 627–642. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.; Silva, J.P.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef] [Green Version]
- Ishizaki, H.; Hasumi, K. Ethanol production from biomass. In Research Approaches to Sustainable Biomass Systems; Tojo, S., Hirasawa, T., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 243–258. ISBN 9780124046092. [Google Scholar]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Combined biogas and bioethanol production: Opportunities and challenges for industrial application. Energies 2015, 8, 8121–8144. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.H.; Mahlia, T.M.I. A review of bioethanol production from plant-based waste biomass by yeast fermentation. Int. J. Technol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Bentivoglio, D.; Finco, A.; Bacchi, M.R.P. Interdependencies between biofuel, fuel and food prices: The case of the brazilian ethanol market. Energies 2016, 9, 464. [Google Scholar] [CrossRef]
- Balat, M.; Ayar, G. Biomass energy in the world, use of biomass and potential trends. Energy Sources 2005, 27, 931–940. [Google Scholar] [CrossRef]
- Alvira, P.; Tomàs-Pejò, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Romanelli, M.G.; Povolo, S.; Favaro, L.; Fontana, F.; Basaglia, M.; Casella, S. Engineering Delftia acidovorans DSM39 to produce polyhydroxyalkanoates from slaughterhouse waste. Int. J. Biol. Macromol. 2014, 71, 21–27. [Google Scholar] [CrossRef]
- Alibardi, L.; Green, K.; Favaro, L.; Vale, P.; Soares, A.; Cartmell, E.; Fernández, Y.B. Performance and stability of sewage sludge digestion under CO2 enrichment: A pilot study. Bioresour. Technol. 2017, 245, 581–589. [Google Scholar] [CrossRef]
- Favaro, L.; Todorov, S.D. Bacteriocinogenic LAB strains for fermented meat preservation: Perspectives, challenges, and limitations. Probiot. Antimicrob. Proteins 2017, 9, 444–458. [Google Scholar] [CrossRef]
- Campanaro, S.; Treu, L.; Kougias, P.G.; Luo, G.; Angelidaki, I. Metagenomic binning reveals the functional roles of core abundant microorganisms in twelve full-scale biogas plants. Water Res. 2018, 140, 123–134. [Google Scholar] [CrossRef]
- Duan, N.; Ran, X.; Li, R.; Kougias, P.; Zhang, Y.; Lin, C.; Liu, H. Performance evaluation of mesophilic anaerobic digestion of chicken manure with algal digestate. Energies 2018, 11, 1829. [Google Scholar] [CrossRef]
- Lantz, M.; Prade, T.; Ahlgren, S.; Björnsson, L. Biogas and Ethanol from wheat grain or straw: Is there a trade-off between climate impact, avoidance of iLUC and production cost? Energies 2018, 11, 2633. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Casella, S. Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: A review. Biofuels Bioprod. Biorefining 2019, 13, 208–227. [Google Scholar] [CrossRef]
- Solomon, B.D.; Barnes, J.R.; Halvorsen, K.E. Grain and cellulosic ethanol: History, economics, and energy policy. Biomass Bioenergy 2007, 31, 416–425. [Google Scholar] [CrossRef]
- Nitsos, C.; Rova, U.; Christakopoulos, P. Organosolv fractionation of softwood biomass for biofuel and biorefinery applications. Energies 2017, 11, 50. [Google Scholar] [CrossRef]
- Cripwell, R.; Favaro, L.; Rose, S.H.; Basaglia, M.; Cagnin, L.; Casella, S.; van Zyl, W.H. Utilization of wheat bran as a substrate for bioethanol production using recombinant cellulases and amylolytic yeast. Appl. Energy 2015, 160, 610–617. [Google Scholar] [CrossRef]
- Favaro, L.; Cagnin, L.; Basaglia, M.; Pizzocchero, V.; van Zyl, W.H.; Casella, S. Production of bioethanol from multiple waste streams of rice milling. Bioresour. Technol. 2017, 244, 151–159. [Google Scholar] [CrossRef]
- Olguin-Maciel, E.; Larqué-Saavedra, A.; Pérez-Brito, D.; Barahona-Pérez, L.F.; Alzate-Gaviria, L.; Toledano-Thompson, T.; Lappe-Oliveras, P.E.; Huchin-Poot, E.G.; Tapia-Tussell, R. Brosimum alicastrum as a novel starch source for bioethanol production. Energies 2017, 10, 1574. [Google Scholar] [CrossRef]
- Pradyawong, S.; Juneja, A.; Sadiq, M.; Noomhorm, A.; Singh, V. Comparison of cassava starch with corn as a feedstock for bioethanol production. Energies 2018, 11, 3476. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Nigam, P.; Singh, D. Enzyme and microbial systems involved in starch processing. Enzyme Microb. Technol. 1995, 17, 770–778. [Google Scholar] [CrossRef]
- Castro, A.M.; Castilho, L.R.; Freire, D.M.G. An overview on advances of amylases production and their use in the production of bioethanol by conventional and non-conventional processes. Biomass Convers. Biorefinery 2011, 1, 245–255. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Favaro, L.; Jooste, T.; Basaglia, M.; Rose, S.H.; Saayman, M.; Görgens, J.F.; Casella, S.; van Zyl, W.H. Designing industrial yeasts for the consolidated bioprocessing of starchy biomass to ethanol. Bioengineered 2013, 4, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Favaro, L.; Viktor, M.J.; Rose, M.H.; Bloom, M.V.; van Zyl, W.H.; Basaglia, M.; Cagnin, L.; Casella, S. Consolidated bioprocessing of starchy substrates into ethanol by industrial Saccharomyces cerevisiae strains secreting fungal amylases. Biotechnol. Bioeng. 2015, 112, 1751–1760. [Google Scholar] [CrossRef]
- Lynd, L.R.; van Zyl, W.H.; McBride, J.E.; Laser, M. Consolidated bioprocessing of cellulosic biomass: An update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef]
- Favaro, L.; Jooste, T.; Basaglia, M.; Rose, S.H.; Saayman, M.; Görgens, J.F.; Casella, S.; van Zyl, W.H. Codon-optimized glucoamylase sGAI of Aspergillus awamori improves starch utilization in an industrial yeast. Appl. Microbiol. Biotechnol. 2012, 95, 957–968. [Google Scholar] [CrossRef]
- van Zyl, W.H.; Bloom, M.; Viktor, M.J. Engineering yeasts for raw starch conversion. Appl. Microbiol. Biotechnol. 2012, 95, 1377–1388. [Google Scholar] [CrossRef]
- Cripwell, R.A.; Rose, S.H.; van Zyl, W.H. Expression and comparison of codon optimised Aspergillus tubingensis amylase variants in Saccharomyces cerevisiae. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef]
- Walker, G.M.; Walker, R.S.K. Enhancing yeast alcoholic fermentations. Adv. Appl. Microbiol. 2018, 105, 87–129. [Google Scholar] [CrossRef]
- Bothast, R.J.; Schlicher, M.A. Biotechnological processes for conversion of corn into ethanol. Appl. Microbiol. Biotechnol. 2005, 67, 19–25. [Google Scholar] [CrossRef]
- Görgens, J.F.; Bressler, D.C.; van Rensburg, E. Engineering Saccharomyces cerevisiae for direct conversion of raw, uncooked or granular starch to ethanol. Crit. Rev. Biotechnol. 2014, 8551, 1–23. [Google Scholar] [CrossRef]
- Mohd Esa, N.; Ling, T.B. By-products of rice processing: An overview of health benefits and applications. Rice Res. Open Access 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Trento, A.; Van Rensburg, E.; García-Aparicio, M.; van Zyl, W.H.; Casella, S. Exploring grape marc as trove for new thermotolerant and inhibitor-tolerant Saccharomyces cerevisiae strains for second-generation bioethanol production. Biotechnol. Biofuels 2013, 6, 168. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; van Zyl, W.H.; Casella, S. Using an efficient fermenting yeast enhances ethanol production from unfiltered wheat bran hydrolysates. Appl. Energy 2013, 102, 170–178. [Google Scholar] [CrossRef]
- Jansen, T.; Hoff, J.W.; Jolly, N.; van Zyl, W.H. Mating of natural Saccharomyces cerevisiae strains for improved glucose fermentation and lignocellulosic inhibitor tolerance. Folia Microbiol. 2018, 63, 55–68. [Google Scholar] [CrossRef]
- He, L.; Zhao, X.; Bai, F. Engineering industrial Saccharomyces cerevisiae strain with the FLO1-derivative gene isolated from the flocculating yeast SPSC01 for constitutive flocculation and fuel ethanol production. Appl. Energy 2012, 100, 33–40. [Google Scholar] [CrossRef]
- Ortiz-Muñiz, B.; Carvajal-Zarrabal, O.; Torrestiana-Sanchez, B.; Aguilar-Uscanga, M.G. Kinetic study on ethanol production using Saccharomyces cerevisiae ITV-01 yeast isolated from sugar cane molasses. J. Chem. Technol. Biotechnol. 2010, 85, 1361–1367. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Karhumaa, K.; Larsson, C.U.; Gorwa-Grauslund, M.; Görgens, J.; van Zyl, W.H. Role of cultivation media in the development of yeast strains for large scale industrial use. Microb. Cell Fact. 2005, 4, 31. [Google Scholar] [CrossRef]
- Dahod, S.K. Raw material selection and medium development for industrial fermentation processes. In Manual of Industrial Microbiology and Biotechnology, 2nd ed.; Demain, A.L., Davies, J.E., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 213–220. ISBN 9781555815127. [Google Scholar]
- Borglum, G.B. Starch hydrolysis for ethanol production. Am. Chem. Soc. Div. Fuel Chem. 1980, 25, 264–269. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 9780470010365. [Google Scholar]
- Zhang, L.; Zhao, H.; Gan, M.; Jin, Y.; Gao, X.; Chen, Q.; Guan, J.; Wang, Z. Application of simultaneous saccharification and fermentation (SSF) from viscosity reducing of raw sweet potato for bioethanol production at laboratory, pilot and industrial scales. Bioresour. Technol. 2011, 102, 4573–4579. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, F.R. Optimization and scale up of industrial fermentation processes. Appl. Microbiol. Biotechnol. 2005, 68, 425–435. [Google Scholar] [CrossRef]
- Chu-Ky, S.; Pham, T.H.; Bui, K.L.T.; Nguyen, T.T.; Pham, K.D.; Nguyen, H.D.T.; Luong, H.N.; Tu, V.D.; Nguyen, T.H.; Ho, P.H.; et al. Simultaneous liquefaction, saccharification and fermentation at very high gravity of rice at pilot scale for potable ethanol production and distillers dried grains composition. Food Bioprod. Process. 2016, 98, 79–85. [Google Scholar] [CrossRef]
- Horwitz, W.; Senzel, A.; Reynolds, H.; Park, D.L. Official Methods of Analysis of the Association of Official Analytical Chemists, 12th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1975. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Int. J. Gen. Mol. Microb. 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Delfini, C. Scienza e Tecnica di Microbiologia Enologica; Edizione: Il lievito, Asti, 1995. [Google Scholar]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef]
- Hamelinck, C.; Van Hooijdonk, G.; Faaij, A. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Cagnin, L.; Favaro, L.; Gronchi, N.; Rose, S.H.; Basaglia, M.; van Zyl, W.H.; Casella, S. Comparing laboratory and industrial yeast platforms for the direct conversion of cellobiose into ethanol under simulated industrial conditions. FEMS Yeast Res. 2019, foz018. [Google Scholar] [CrossRef]



| S. cerevisiae Strains | Relevant Phenotype and Origin | Source/Reference |
|---|---|---|
| L1–L21 | wild-type | This study |
| Ethanol Red™ | industrial strain | Fermentis |
| Fm17 | wild-type strain with high lignocellulosic inhibitors tolerance | [41] |
| M2n | distillery strain | [32] |
| MEL2 | wild type strain from grape marcs | [42] |
| HR4 | wild-type strain from wine fermentations | [43] |
| WL3 | wild-type strain from wine fermentations | [43] |
| YI30 | wild-type strain from wine fermentations | [43] |
| Broken Rice | Raw Corn Starch | |||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Residual Glucose (g/L) | Glycerol (g/L) | Ethanol Concentration (g/L) | YE/S | Residual Glucose (g/L) | Glycerol (g/L) | Ethanol Concentration (g/L) | YE/S |
| Ethanol Red™ | - | 8.83 ± 0.12 | 101.05 ± 0.54 | 91 | 0.30 ± 0.06 | 10.05 ± 0.17 | 109.36 ± 0.33 | 86 |
| L11 | - | 8.75 ± 0.02 | 107.70 ± 0.44 | 97 | 1.68 ± 0.28 | 9.19 ± 0.20 | 116.07 ± 0.06 | 91 |
| L12 | 0.58 ± 0.14 | 9.03 ± 0.03 | 108.39 ± 1.22 | 98 | 0.82 ± 0.65 | 9.21 ± 0.19 | 116.22 ± 1.97 | 91 |
| L13 | 0.62 ± 0.01 | 9.45 ± 0.18 | 107.15 ± 0.28 | 97 | 1.55 ± 0.76 | 9.63 ± 0.04 | 116.12 ± 0.96 | 91 |
| L15 | - | 8.90 ± 0.06 | 107.43 ± 0.16 | 97 | - | 8.24 ± 0.08 | 117.17 ± 0.08 | 92 |
| L16 | - | 8.93 ± 0.04 | 107.77 ± 0.21 | 97 | - | 9.25 ± 0.15 | 117.93 ± 0.14 | 92 |
| L17 | - | 8.36 ± 0.02 | 107.16 ± 0.66 | 97 | - | 8.53 ± 0.01 | 116.78 ± 0.42 | 92 |
| L18 | - | 7.79 ± 0.05 | 106.73 ± 0.34 | 96 | - | 8.45 ± 0.09 | 117.35 ± 1.06 | 92 |
| L19 | - | 8.25 ± 0.08 | 107.32 ± 0.25 | 97 | - | 8.60 ± 0.11 | 116.44 ± 0.21 | 91 |
| L20 | - | 8.17 ± 0.14 | 107.19 ± 0.15 | 97 | - | 7.97 ± 0.09 | 116.98 ± 1.73 | 92 |
| WL3 | 0.30±0.01 | 9.16±0.12 | 106.17 ± 0.36 | 96 | 0.29 ± 0.04 | 9.60 ± 0.07 | 115.05 ± 0.24 | 90 |
| Feedstock | S. cerevisiae L20 | S. cerevisiae Ethanol Red™ | ||
|---|---|---|---|---|
| Broken rice = a glucose equivalent of 198.55 g/L and a total carbon available (mol C) of 6.62 | ||||
| Product (g/L) | 24 h | 72 h | 24 h | 72 h |
| Glucose | nd | nd | nd | nd |
| Glycerol | 8.40 | 8.70 | 7.54 | 8.12 |
| Ethanol | 74.44 | 87.01 | 67.86 | 85.46 |
| CO2 | 71.20 | 83.23 | 64.91 | 81.74 |
| Total carbon | 5.13 | 5.96 | 4.67 | 5.84 |
| Carbon conversion (mol C) | 77% | 90% | 71% | 88% |
| YE/S(% of theoretical) | 73% | 86% | 67% | 83% |
| Q (g/L/h) | 3.10 | 1.21 | 2.83 | 1.19 |
| Qmax (g/L/h) | 3.10 after 24 h | 2.83 after 24 h | ||
| Raw corn starch = a glucose equivalent of 238.82 g/L and a total carbon available (mol C) of 7.93 | ||||
| Product (g/L) | 24 h | 72 h | 24 h | 72 h |
| Glucose | 1.04 | nd | 1.14 | nd |
| Glycerol | 7.92 | 8.86 | 6.10 | 8.84 |
| Ethanol | 73.10 | 100.84 | 56.02 | 94.20 |
| CO2 | 69.92 | 96.46 | 53.58 | 90.10 |
| Total carbon | 5.06 | 6.87 | 3.89 | 6.43 |
| Carbon conversion (mol C) | 64% | 86% | 49% | 81% |
| YE/S(% of theoretical) | 60% | 83% | 46% | 77% |
| Q (g/L/h) | 3.05 | 1.40 | 2.33 | 1.31 |
| Qmax (g/L/h) | 3.20 after 18 h | 2.41 after 18 h | ||
| Feedstock | Dry Matter, DM (%) | Protein (% DM) | Starch (% DM) |
|---|---|---|---|
| Broken rice | 96.0 | 8.5 | 84.0 |
| Raw corn starch | 90.3 | 0.3 | 95.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gronchi, N.; Favaro, L.; Cagnin, L.; Brojanigo, S.; Pizzocchero, V.; Basaglia, M.; Casella, S. Novel Yeast Strains for the Efficient Saccharification and Fermentation of Starchy By-Products to Bioethanol. Energies 2019, 12, 714. https://doi.org/10.3390/en12040714
Gronchi N, Favaro L, Cagnin L, Brojanigo S, Pizzocchero V, Basaglia M, Casella S. Novel Yeast Strains for the Efficient Saccharification and Fermentation of Starchy By-Products to Bioethanol. Energies. 2019; 12(4):714. https://doi.org/10.3390/en12040714
Chicago/Turabian StyleGronchi, Nicoletta, Lorenzo Favaro, Lorenzo Cagnin, Silvia Brojanigo, Valentino Pizzocchero, Marina Basaglia, and Sergio Casella. 2019. "Novel Yeast Strains for the Efficient Saccharification and Fermentation of Starchy By-Products to Bioethanol" Energies 12, no. 4: 714. https://doi.org/10.3390/en12040714