Improving Anaerobic Digestion of Sewage Sludge by Hydrogen Addition: Analysis of Microbial Populations and Process Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
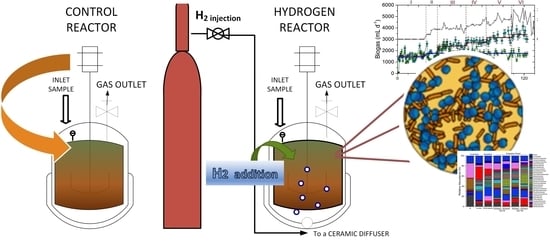
2.2. Experimental Set-Up: Semi-Continuous Digestion
2.3. Analytical Techniques
2.4. High-Throughput Sequencing of Massive 16S rRNA Gene Libraries
2.5. Energy Balance
3. Results and Discussion
3.1. Analysis of Methane Production
3.2. Effect of Hydrogen Addition on Microbial Communities
3.3. Energy Balance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Silvestri, D.; Padil, V.V.; Wacławek, M.; Černík, M.; Varma, S.R. Disintegration of Wastewater Activated Sludge (WAS) for Improved Biogas Production. Energies 2018, 12, 21. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Johnson, T.; Eskicioglu, C. Comparison of Different Electricity-Based Thermal Pretreatment Methods for Enhanced Bioenergy Production from Municipal Sludge. Molecules 2018, 23, 2006. [Google Scholar] [CrossRef]
- Martínez, E.J.; Rosas, J.G.; Morán, A.; Gómez, X. Effect of ultrasound pretreatment on sludge digestion and dewatering characteristics: Application of particle size analysis. Water 2015, 7, 6483–6495. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, C.; Zhang, T. Microbial effects of part-stream low-frequency ultrasonic pretreatment on sludge anaerobic digestion as revealed by high-throughput sequencing-based metagenomics and metatranscriptomics. Biotechnol. Biofuels 2018, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Sánchez, E.M.; Gómez, X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C 2018, 4, 59. [Google Scholar] [CrossRef]
- Keucken, A.; Habagil, M.; Batstone, D.; Jeppsson, U.; Arnell, M. Anaerobic Co-Digestion of Sludge and Organic Food Waste—Performance, Inhibition, and Impact on the Microbial Community. Energies 2018, 11, 2325. [Google Scholar] [CrossRef]
- Martínez, E.J.; Gil, M.V.; Fernandez, C.; Rosas, J.G.; Gómez, X. Anaerobic codigestion of sludge: Addition of butcher’s fat waste as a cosubstrate for increasing biogas production. PLoS ONE 2016, 11, e0353139. [Google Scholar] [CrossRef]
- Al bkoor Alrawashdeh, K.; Pugliese, A.; Slopiecka, K.; Pistolesi, V.; Massoli, S.; Bartocci, P.; Bidini, G.; Fantozzi, F. Codigestion of Untreated and Treated Sewage Sludge with the Organic Fraction of Municipal Solid Wastes. Fermentation 2017, 3, 35. [Google Scholar] [CrossRef]
- Zinder, S.H. Microbiology of anaerobic conversion of organic wastes to methane: Recent developments. Am. Soc. Microbiol. News 1984, 50, 294–298. [Google Scholar]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Bio/Technology 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Smith, J.A. Biologically Produced Methane as a Renewable Energy Source. Adv. Appl. Microbiol. 2016, 97, 1–61. [Google Scholar] [PubMed]
- Cayol, J.-L.; Fardeau, M.-L.; Garcia, J.-L.; Ollivier, B. Evidence of interspecies hydrogen transfer from glycerol in saline environments. Extremophiles 2002, 6, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Bagi, Z.; Ács, N.; Bálint, B.; Horváth, L.; Dobó, K.; Perei, K.R.; Rákhely, G.; Kovács, K.L. Biotechnological intensification of biogas production. Appl. Microbiol. Biotechnol. 2007, 76, 473–482. [Google Scholar] [CrossRef]
- Nzila, A. Mini review: Update on bioaugmentation in anaerobic processes for biogas production. Anaerobe 2017, 46, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Co-digestion of manure and whey for in situ biogas upgrading by the addition of H2: Process performance and microbial insights. Appl. Microbiol. Biotechnol. 2013, 97, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Hollow fiber membrane based H2 diffusion for efficient in situ biogas upgrading in an anaerobic reactor. Appl. Microbiol. Biotechnol. 2013, 97, 3739–3744. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer—Can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. from laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Stoll, A.Z.; Dolfing, J.; Xu, P. Minimum Performance Requirements for Microbial Fuel Cells to Achieve Energy-Neutral Wastewater Treatment. Water 2018, 10, 243. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Irving Olsen, S.; Singh Nigam, P.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2005; ISBN 9780875530475. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gomez, X. Co-Digestion of Swine Manure and Crude Glycerine: Increasing Glycerine Ratio Results in Preferential Degradation of Labile Compounds. Water. Air. Soil Pollut. 2016, 227. [Google Scholar] [CrossRef]
- Callaway, T.R.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McReynolds, J.L.; Edrington, T.S.; Byrd, J.A.; Anderson, R.C.; Krueger, N.; Nisbet, D.J. Evaluation of the bacterial diversity in cecal contents of laying hens fed various molting diets by using bacterial tag-encoded FLX amplicon pyrosequencing. Poult. Sci. 2009, 88, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Takai, K.E.N. Rapid Detection and Quantification of Members of the Archaeal Community by Quantitative PCR Using Fluorogenic Probes. Appl. Environ. Microbiol. 2000, 66, 5066–5072. [Google Scholar] [CrossRef] [Green Version]
- Bensmann, A.; Hanke-Rauschenbach, R.; Heyer, R.; Kohrs, F.; Benndorf, D.; Reichl, U.; Sundmacher, K. Biological methanation of hydrogen within biogas plants: A model-based feasibility study. Appl. Energy 2014, 134, 413–425. [Google Scholar] [CrossRef]
- Demirel, B. Major pathway of methane formation from energy crops in agricultural biogas digesters. Crit. Rev. Environ. Sci. Technol. 2014, 44, 199–222. [Google Scholar] [CrossRef]
- Karakashev, D.; Batstone, D.J.; Trably, E.; Angelidaki, I. Acetate Oxidation Is the Dominant Methanogenic Pathway from Acetate in the Absence of Methanosaetaceae. Appl. Environ. Microbiol. 2006, 72, 5138–5141. [Google Scholar] [CrossRef]
- Ács, N.; Bagi, Z.; Rákhely, G.; Minárovics, J.; Nagy, K.; Kovács, K.L. Bioaugmentation of biogas production by a hydrogen-producing bacterium. Bioresour. Technol. 2015, 186, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Li, Y.; Li, X.; Luo, P.; Wang, H.; Wang, X.; Wu, J.; Li, F. Energy Self-sufficient Wastewater Treatment Plants: Feasibilities and Challenges. Energy Procedia 2017, 105, 3741–3751. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Escapa, A.; Carracedo, B.; Morán, A.; Gómez, X. Performance of a semi-pilot tubular microbial electrolysis cell (MEC) under several hydraulic retention times and applied voltages. Bioresour. Technol. 2013, 146, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef] [PubMed]
- Selembo, P.A.; Perez, J.M.; Lloyd, W.A.; Logan, B.E. High hydrogen production from glycerol or glucose by electrohydrogenesis using microbial electrolysis cells. Int. J. Hydrogen Energy 2009, 34, 5373–5381. [Google Scholar] [CrossRef]
- Houillon, G.; Jolliet, O. Life cycle assessment of processes for the treatment of wastewater urban sludge: Energy and global warming analysis. J. Clean. Prod. 2005, 13, 287–299. [Google Scholar] [CrossRef]
- Mizuta, K.; Shimada, M. Benchmarking energy consumption in municipal wastewater treatment plants in Japan. Water Sci. Technol. 2010, 62, 2256–2262. [Google Scholar] [CrossRef]
- Hernández-Sancho, F.; Molinos-Senante, M.; Sala-Garrido, R. Energy efficiency in Spanish wastewater treatment plants: A non-radial DEA approach. Sci. Total Environ. 2011, 409, 2693–2699. [Google Scholar] [CrossRef]
- Pöschl, M.; Ward, S.; Owende, P. Evaluation of energy efficiency of various biogas production and utilization pathways. Appl. Energy 2010, 87, 3305–3321. [Google Scholar] [CrossRef]
- Brown, R.K.; Harnisch, F.; Dockhorn, T.; Schröder, U. Examining sludge production in bioelectrochemical systems treating domestic wastewater. Bioresour. Technol. 2015, 198, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Ren, F.; Wang, J.Y.; Giannis, A. Effect of pretreatment techniques on food waste solubilization and biogas production during thermophilic batch anaerobic digestion. J. Mater. Cycles Waste Manag. 2016, 18, 222–230. [Google Scholar] [CrossRef]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Smith, R.; Blanco, D.; Fierro, J.; Gómez, X. Application of thermal analysis for evaluating the effect of glycerine addition on the digestion of swine manure. J. Therm. Anal. Calorim. 2019, 135, 2277–2286. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L. Codigestion of manure and organic wastes in centralized biogas plants. Appl. Microbiol. Biotechnol. 2003, 109, 95–105. [Google Scholar]
- Escapa, A.; Gómez, X.; Tartakovsky, B.; Morán, A. Estimating microbial electrolysis cell (MEC) investment costs in wastewater treatment plants: Case study. Int. J. Hydrogen Energy 2012, 37, 18641–18653. [Google Scholar] [CrossRef]





| Chemical Parameters | Inoculum | PS | WAS | Mixture |
|---|---|---|---|---|
| Total solids (g kg−1) | 30.8 ± 0.9 | 31.5 ± 0.9 | 27.0 ± 0.8 | 28.2 ± 0.7 |
| Volatile solids (g kg−1) | 18.2 ± 0.5 | 26.4 ± 0.8 | 21.4 ± 0.6 | 22.9 ± 0.7 |
| Total soluble organic carbon (g L−1) | 2.97 ± 0.14 | 3.45 ± 0.17 | 1.06 ± 0.05 | 1.41 ± 0.08 |
| Ammonia (mg L−1) | 742 ± 29 | 968 ± 38 | 487 ± 19 | 655 ± 21 |
| pH | 7.10 ± 0.01 | 5.20 ± 0.01 | 6.13 ± 0.01 | 5.62 ± 0.01 |
| Chemical oxygen demand (mg L−1) | 2178 ± 109 | 3172 ± 159 | 2569 ± 128 | 2744 ± 137 |
| Organic matter (%)* | 42.43 ± 0.14 | 53.85 ± 1.06 | 59.21 ± 0.09 | 56.40 ± 0.49 |
| Kjeldahl nitrogen (%)* | 4.87 ± 0.19 | 5.10 ± 0.15 | 9.43 ± 0.28 | 7.86 ± 0.23 |
| C/N ratio | 5.04 | 6.16 | 3.65 | 4.21 |
| Acetic acid (mg L−1) | n.d. | 2279 ± 114 | 397 ± 16 | 1007 ± 40 |
| Propionic acid (mg L−1) | n.d. | 1581 ± 79 | 228 ± 14 | 682 ± 26 |
| Isobutyric acid (mg L−1) | n.d. | 199 ± 10 | 49.42 ± 2.52 | 89.33 ± 4.46 |
| Butyric acid (mg L−1) | n.d. | 1471 ± 59 | 51.71 ± 1.55 | 517 ± 16 |
| Isovaleric acid (mg L−1) | n.d. | 208 ± 26 | 51.23 ± 1.20 | 132 ± 21 |
| Valeric acid (mg L−1) | n.d. | 449 ± 13 | 44.70 ± 1.10 | 209 ± 15 |
| Volatile fatty acids, total (mg L−1) | n.d. | 5083 ± 254 | 658 ± 18 | 2639 ± 132 |
| Cadmium (mg kg−1) | 0.030 ± 0.001 | 0.020 ± 0.001 | 0.030 ± 0.001 | 0.020 ± 0.001 |
| Chromium (mg kg−1) | 1.10 ± 0.01 | 0.68 ± 0.01 | 0.73 ± 0.01 | 0.75 ± 0.01 |
| Copper (mg kg−1) | 3.97 ± 0.20 | 2.67 ± 0.13 | 3.75 ± 0.15 | 3.32 ± 0.12 |
| Phosphorus (mg kg−1) | 447 ± 22 | 336 ± 17 | 862 ± 43 | 681 ± 34 |
| Nickel (mg kg−1) | 0.66 ± 0.01 | 0.37 ± 0.01 | 0.34 ± 0.01 | 0.33 ± 0.01 |
| Lead (mg kg−1) | 2.47 ± 0.12 | 1.87 ± 0.10 | 1.23 ± 0.10 | 1.39 ± 0.11 |
| Zinc (mg kg−1) | 32.55 ± 0.98 | 18.17 ± 0.75 | 22.03 ± 0.83 | 19.61 ± 0.75 |
| Control Reactor (CR) | Hydrogen Reactor (HR) | |||||||
|---|---|---|---|---|---|---|---|---|
| Periods | Adaptation | Evaluation | Adaptation | Evaluation | ||||
| I | (II-VI) | I | II | III | IV | V | VI | |
| Days evaluated | 28 | 105 | 28 | 11 | 27 | 20 | 24 | 23 |
| HRT (d) | 25 | 20 | 25 | 20 | 20 | 20 | 20 | 20 |
| OLR ([kg VS] L−1 d−1) | 0.99 | 1.08 | 0.99 | 1.14 | 1.04 | 1.1 | 0.97 | 1.17 |
| H2 injection flow (mL d−1) | n.a. | n.a. | 0 | 780 ± 269 | 1125 ± 207 | 1588 ± 260 | 1938 ± 330 | 2004 ± 350 |
| H2 Transported (mL d−1) | n.a. | n.a. | 0 | 617 ± 291 | 683 ± 286 | 709 ± 166 | 768 ± 196 | 750 ± 206 |
| Volatile solids (g L−1) | 13.2 ± 0.1 | 10.52 | 13.6 ± 0.1 | 12.5 ± 0.1 | 8.2 ± 0.1 | 8.0 ± 0.1 | 8.1 ± 0.1 | 8.3 ± 0.1 |
| Volatile solids removal (%) | 46.8 | 49.46 | 45.1 | 49.5 | 59.7 | 55.4 | 57.1 | 60.6 |
| Biogas (mL d−1) | 1434 ± 436 | 1762 ± 235 | 1524 ± 338 | 1779 ± 368 | 2398 ± 273 | 2736 ± 249 | 3146 ± 364 | 3417 ± 390 |
| CH4 (mL d−1) | 857 ± 25 | 1113.23 ± 55 | 927 ± 15 | 1030 ± 20 | 1318 ± 26 | 1266 ± 25 | 1353 ± 27 | 1222 ± 24 |
| Biogas without H2 (mL d−1) | -- | -- | 1524 ± 338 | 1523 ± 237 | 1955 ± 258 | 1904 ± 270 | 1976 ± 271 | 1842 ± 450 |
| %CH4 | 59.79 ± 1.43 | 63.18 | 60.86 ± 1.71 | 59.39 ± 3.19 | 55.81 ± 6.91 | 44.41 ± 8.07 | 41.61 ± 6.50 | 41.78 ± 6.5 |
| %CH4 normalised* | -- | -- | 60.86 ± 1.71 | 67.67 ± 1.63 | 67.42 ± 5.52 | 66.51 ± 3.60 | 68.51 ± 2.70 | 66.39 ± 4.5 |
| Parameter | Value | Unit |
| WWTP capacity [38] | 150 000 | Eq. Inh. |
| Wastewater quantity | 350 | L (Eq. Inh.)−1 d−1 |
| Characteristics of conventional WWTP (base scenario) | ||
| Energy demand WAS [39] | 1.1 | kW h m−3 |
| WWTP energy consumption [40] | 1.68 | kW h (kg COD removed)−1 |
| Sludge flow | 8.0 | m3 h−1 |
| TS | 53.0 | g L−1 |
| %VS | 73 | % |
| Digester volume | 4000 | m3 |
| Electric efficiency CHP unit [41] | 33 | % |
| Energy production from biogas valorisation by CHP | 342 | kW |
| BES and H2 conversion during anaerobic digestion | ||
| Energy consumption | 0.38 | kW h (kg COD removed)−1 |
| Secondary sludge production | 2.7 | m3 h−1 |
| Sludge flow | 3.8 | m3 h−1 |
| Biogas production from H2 conversion (from experimental results) | 0.33 | (m3 biogas) (m3 H2)−1 |
| Energy production from biogas valorisation by CHP | 318 | kW |
| Addition of co-substrate: Total energy production from biogas valorisation by CHP | 554 | kW |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, E.J.; Sotres, A.; Arenas, C.B.; Blanco, D.; Martínez, O.; Gómez, X. Improving Anaerobic Digestion of Sewage Sludge by Hydrogen Addition: Analysis of Microbial Populations and Process Performance. Energies 2019, 12, 1228. https://doi.org/10.3390/en12071228
Martínez EJ, Sotres A, Arenas CB, Blanco D, Martínez O, Gómez X. Improving Anaerobic Digestion of Sewage Sludge by Hydrogen Addition: Analysis of Microbial Populations and Process Performance. Energies. 2019; 12(7):1228. https://doi.org/10.3390/en12071228
Chicago/Turabian StyleMartínez, Elia Judith, Ana Sotres, Cristián B. Arenas, Daniel Blanco, Olegario Martínez, and Xiomar Gómez. 2019. "Improving Anaerobic Digestion of Sewage Sludge by Hydrogen Addition: Analysis of Microbial Populations and Process Performance" Energies 12, no. 7: 1228. https://doi.org/10.3390/en12071228