Investigation on Long Term Operation of Thermochemical Heat Storage with MgO-Based Composite Honeycombs
Abstract
:1. Introduction
2. Experiments
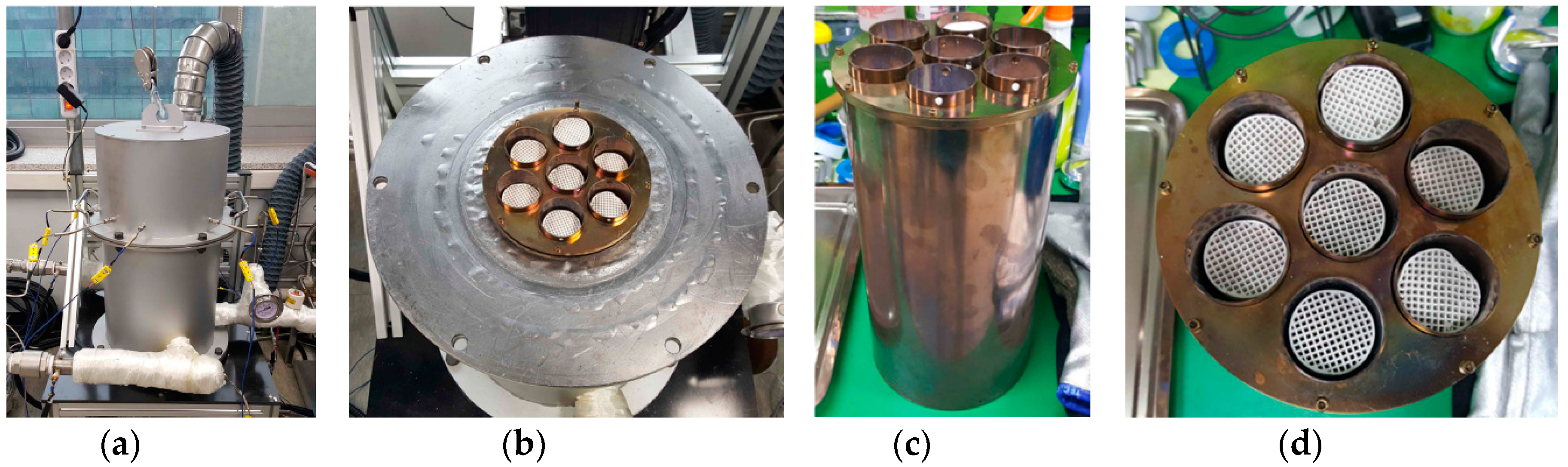
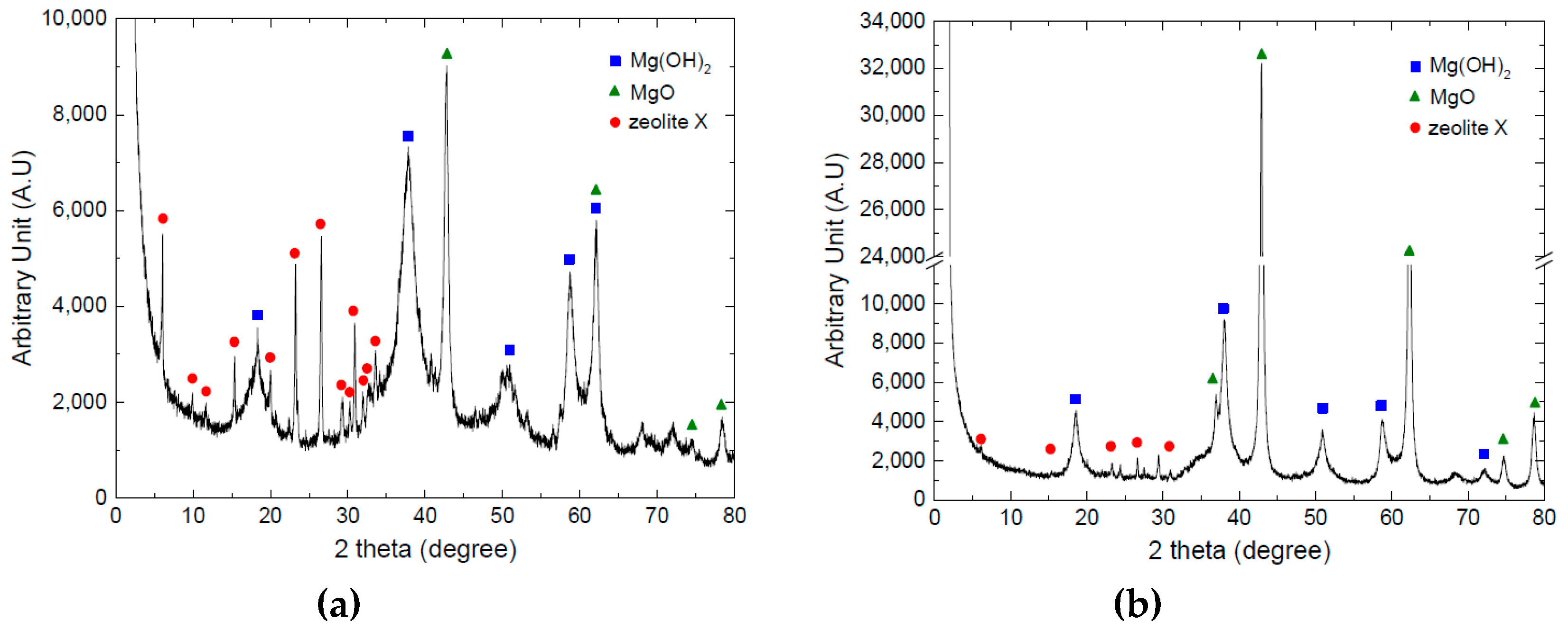
2.1. TCM Honeycomb Preparation
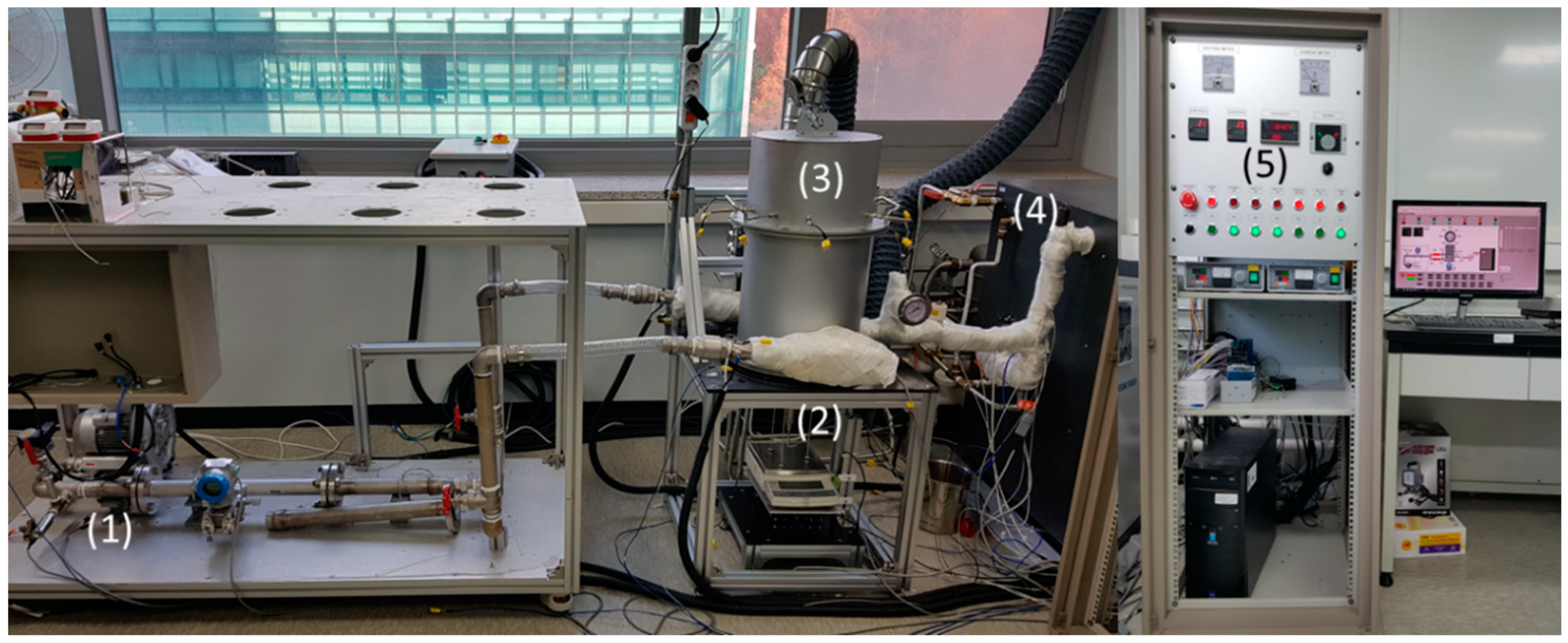
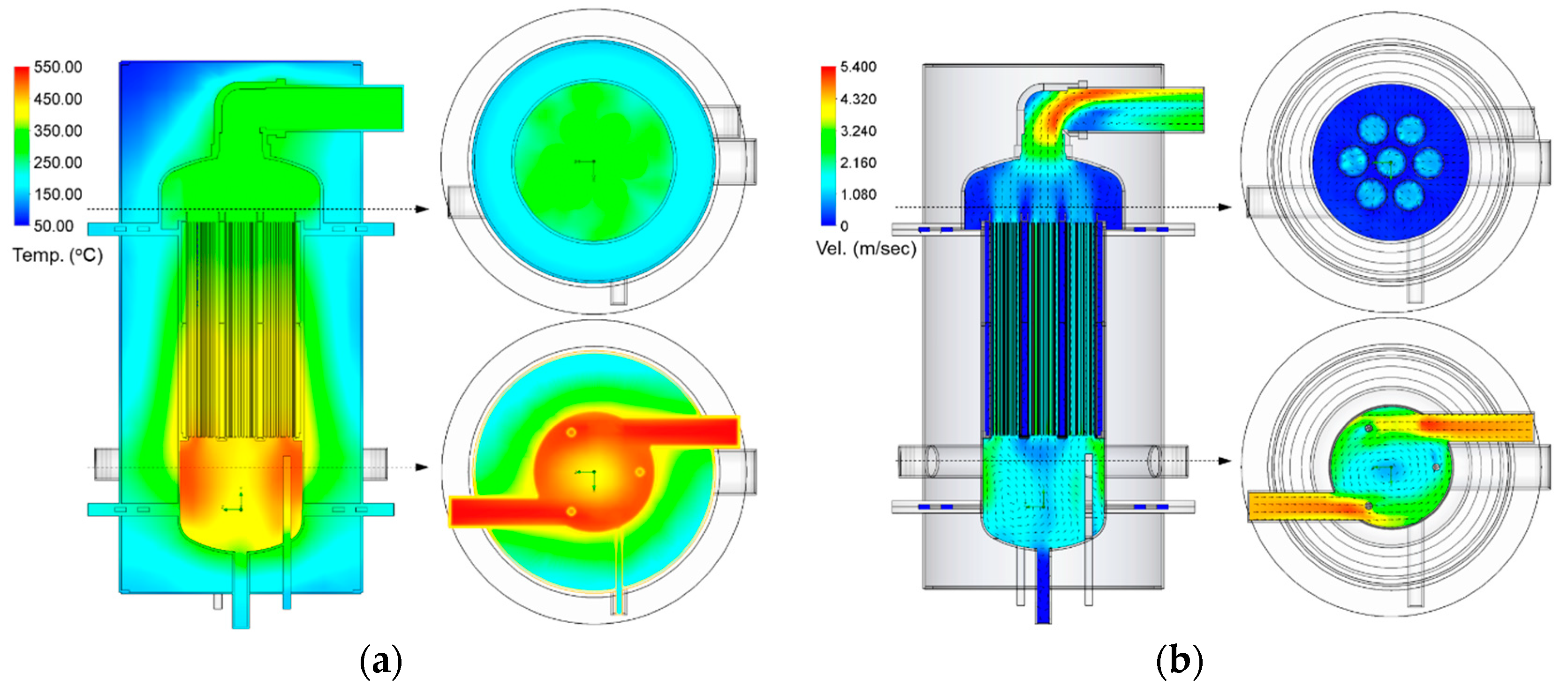
2.2. System Design
2.3. Experimental Procedures
- Step 1 (stabilizing the gas flow rate): Before the heat charging, the gas(air) flow rate is adjusted to be constant. While the digital inverter controls the air blower (Figure 2 (1)), the Proportional Integral Derivative (PID) controller finds the optimum duty to maintain the desired flow rate (0.5 Nm/m3). It has a feedback control with a digital flow meter (Figure 2 (2))
- Step 2 (heat charging—dehydration): After the air flow rate becomes constant, gas inline heaters (Figure 2 (3)) start to heat the air to 550 °C. After the ambient temperature of TCM honeycombs reached a turnover temperature, Mg(OH)2 is turned to MgO, which means the heat is charged. The rest of the hot exhaust gas goes into the gas cooler (Figure 2 (6)) to exchange heat with the city water line before exiting the fume hood.
- Step 3 (TCM cooling down): Immediately after the heat charging, the honeycombs are not ready for thermochemical reactions due to their high temperature. It is thus necessary to cool the honeycombs to a lower temperature than the reactants in order to evaluate the ‘no heat loss’ properties after the heat charging and to determine whether the heat charging has been successful. In this step, an air blower supplies ambient air into the cartridge without heating, making the honeycomb temperature lower than the steam temperature (100 °C).
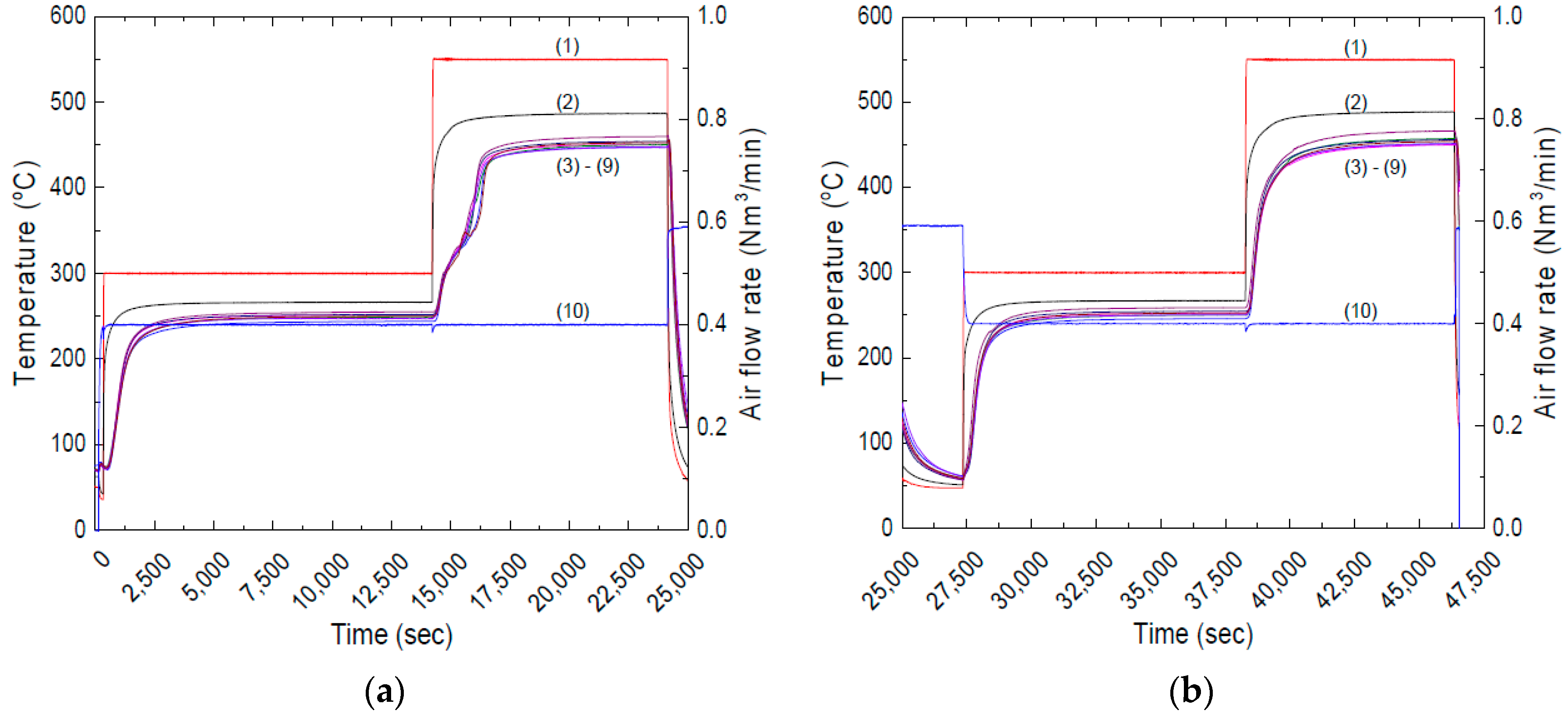
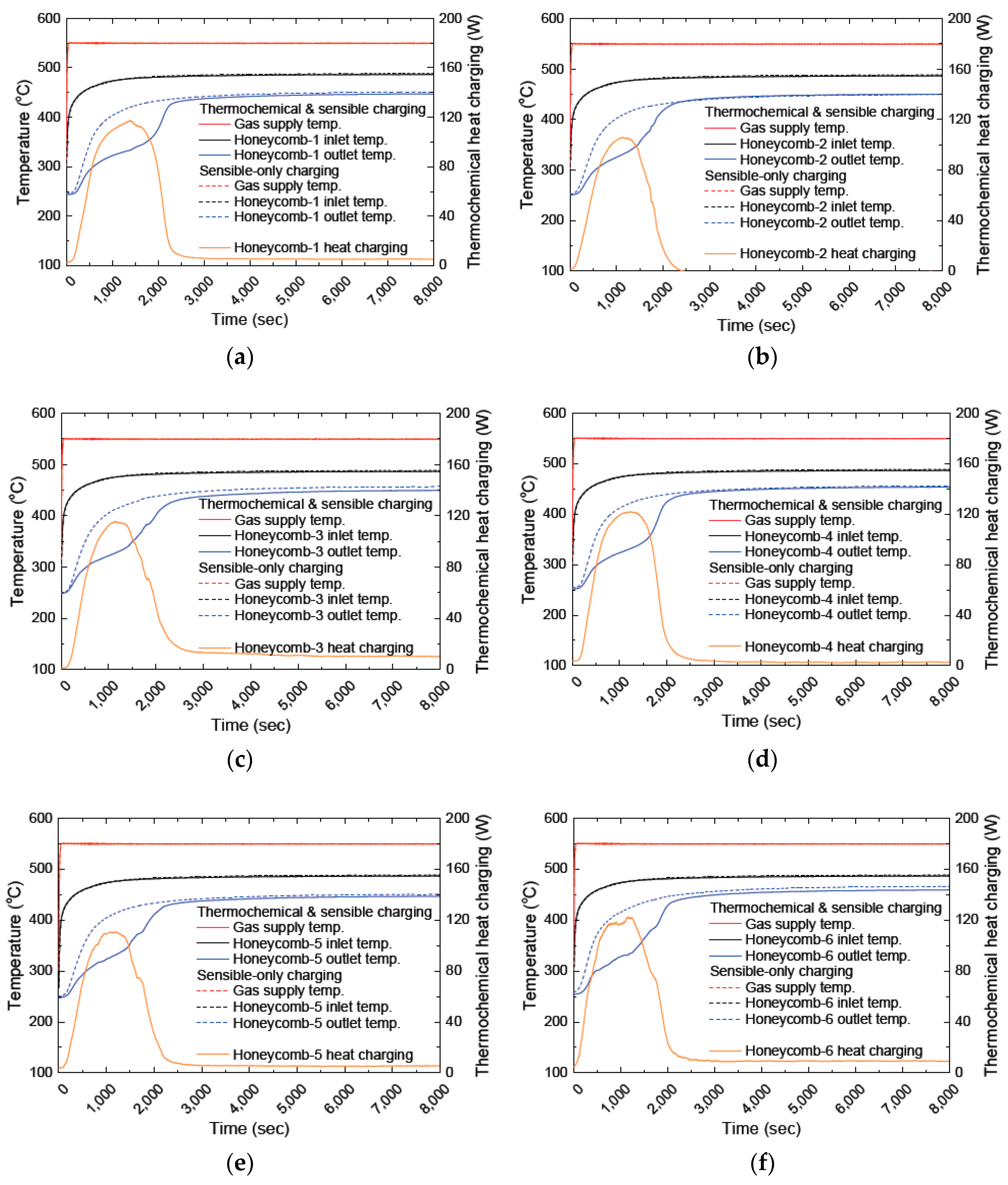
- Step 4 (optional process—measuring the amount of thermochemical heat charging): During the thermochemical heat charging process, the chemical reaction absorbs the additional heat with a sensible heat charging process. Therefore, the gas temperature decreases more than the sensible-only heat charging. Hence, it is expected to have a lower exhaust temperature, however, it is not easy to separately calculate the temperature decrease due to the thermochemical heat charging. Thus, there is an optional step to run the same operation parameter one more time in order to evaluate the amount of heat absorption during the thermochemical process, it is the so-called sensible-only heat charging. After finishing this step, overlapping the temperature behavior of the TCM honeycombs reveals the non-overlapping temperature section due to the different heat charging mechanism. When we calculate the temperature change between Step 3 and Step 2 with the air flow rate, we can calculate how much heat was charged during the thermochemical process. Because of the limited time, however, we conducted this optional step only for validating the thermochemical performances. The analysis of this process will be considered in the next chapter.
- Step 5 (heat discharging—hydration): After cooling down, the solenoid valve (Figure 2 (8)) opens to supply steam to the reactor (Figure 2 (5)). While steam enters the reactor, the steam temperature is maintained at a saturated temperature at ambient pressure due to heat loss (Figure 2 (7)). During the saturation, steam is flown through the cartridge and the reactant is combined with TCM honeycombs, resulting in producing dry, superheated steam. During the experiment, the gas cooler (Figure 2 (6)) cools down the steam before exiting the hood.
- Step 6 (post drying the peripheral devices after heat discharging): During hydration, steam and condensed water can exist somewhere inside the system, including the pipelines. To prevent an electrical shortage when the gas inline heater runs, hot gas (120 °C) is supplied into the system for enough time to dry all the equipment for the next heat charging procedure.
2.4. Set-Up of the Experimental Conditions
3. Results of the Experiments
3.1. Investigating Compressive Strength of Composite Honeycombs
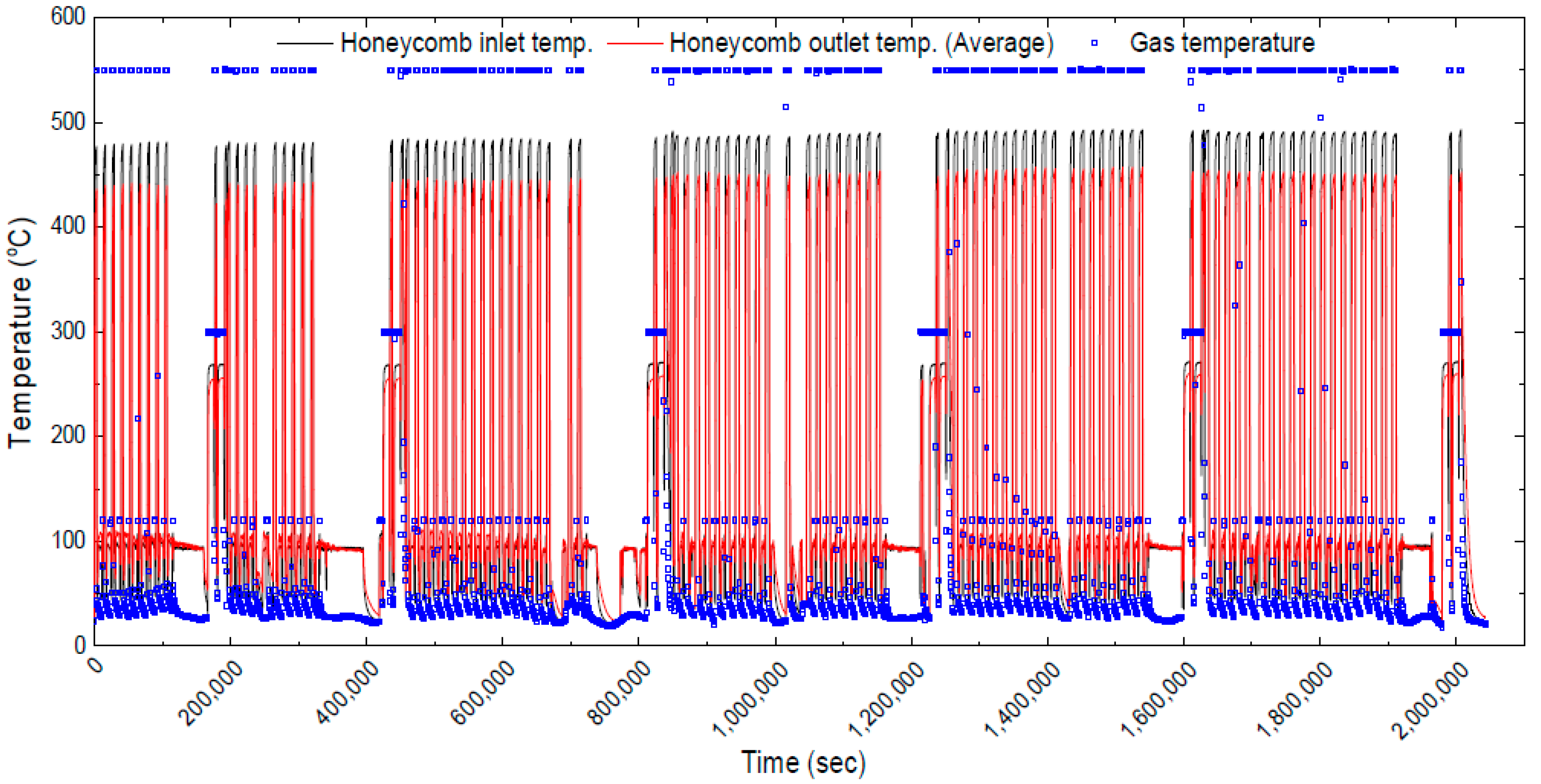
3.2. Investigating the Thermochemical Heat Charging Behaviors
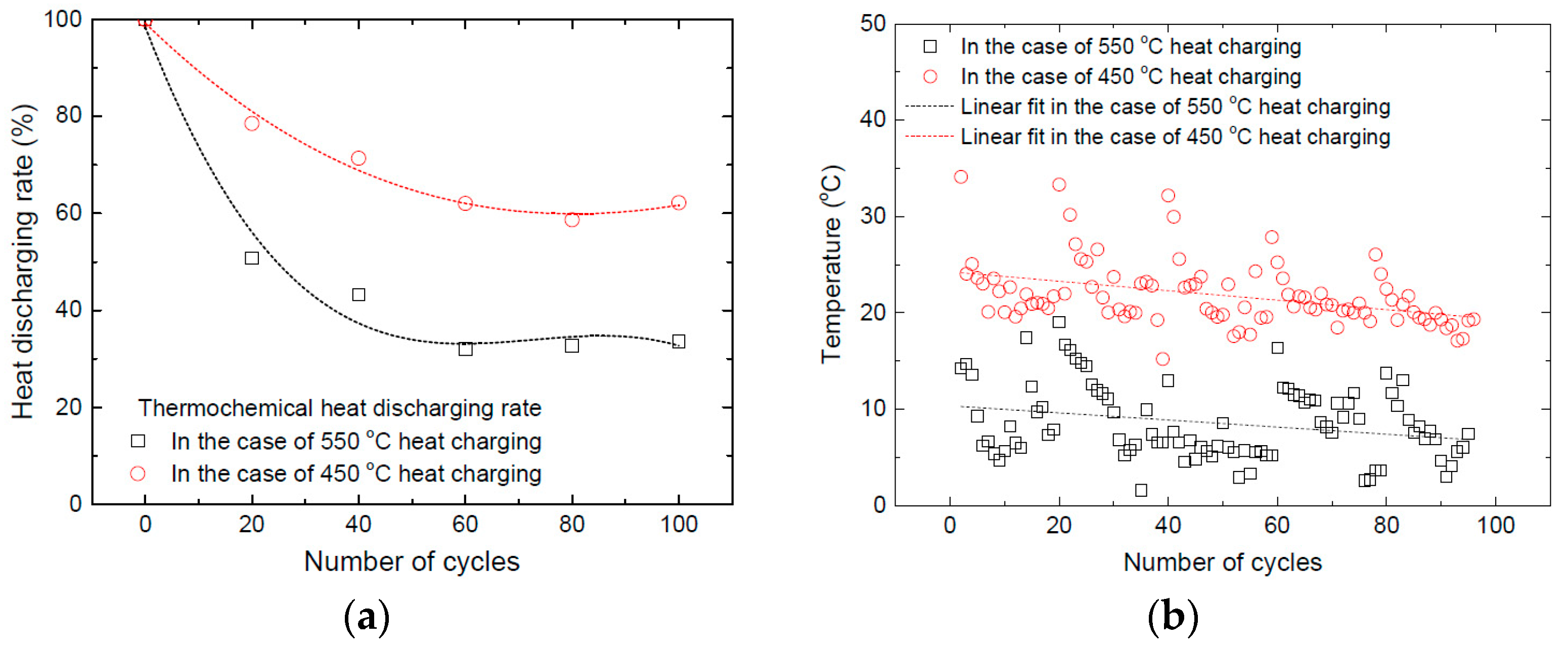
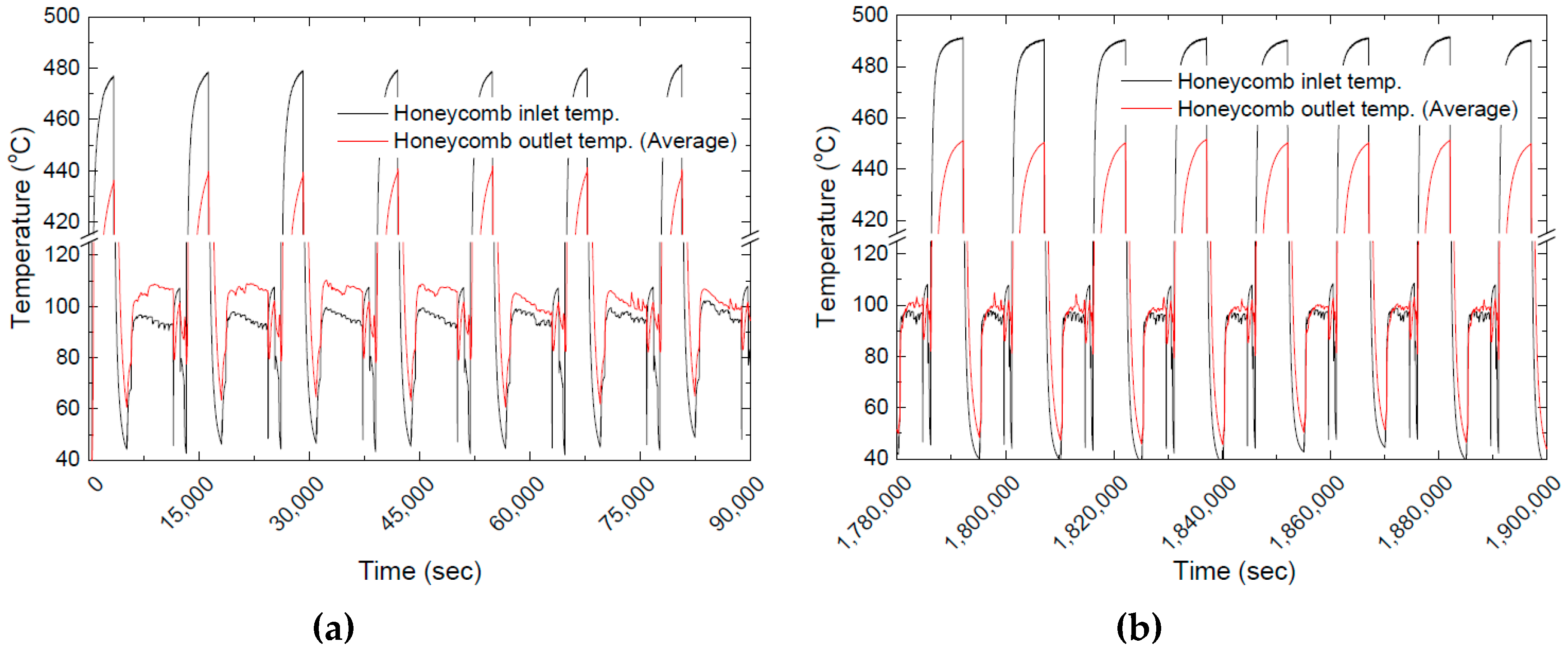
3.3. Effect of Long-Term Cyclic Operation: Heat Charging Capability
3.4. Effect of Long-Term Cyclic Operation: Heat Discharging Capability
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Papapetrou, M.; Kosmadakisb, G.; Cipollinaa, A.; Commarea, U.; Micale, G. Industrial waste heat: Estimation of the technically available resource in the EU per industrial sector, temperature level and country. Appl. Therm. Eng. 2018, 138, 207–216. [Google Scholar] [CrossRef]
- Fleiter, T.; Steinbah, J.; Ragwitz, M. Mapping and Analyses of the Current and Future (2020–2030) Heating/Cooling Fuel Deployment (Fossil/Renewables). Available online: https://ec.europa.eu/energy/sites/ener/files/documents/Report%20WP1.pdf (accessed on 10 February 2019).
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: An EU Strategy on Heating and Cooling 2016. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/1_EN_ACT_part1_v14.pdf (accessed on 10 February 2019).
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 1: Testing of cobalt oxide-based powders. Sol. Energy 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Singh, A.; Tescari, S.; Lantin, G.; Agrafiotis, C.; Roeb, M.; Sattler, C. Solar thermochemical heat storage via the Co3O4/CoO looping cycle: Storage reactor modelling and experimental validation. Sol. Energy 2017, 144, 453–465. [Google Scholar] [CrossRef]
- Randhir, K.; King, K.; Rhodes, N.; Li, L.; Hahn, D.; Mei, R.; AuYeung, N.; Klausnera, J. Magnesium-manganese oxides for high temperature thermochemical energy storage. J. Energy Storage 2019, 21, 559–610. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.; Sanchez-Jimenez, P.; Sanchez-Jimenez, P.; Perejon, A.; Perez-Maqueda, L. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in concentrated solar power plants. Sol. Energy 2017, 153, 188–199. [Google Scholar] [CrossRef]
- Tescari, S.; Singh, A.; Oliveira, L.; Breuer, S.; Agrafiotis, C.; Roeb, M.; Sattler, C.; Marcher, J.; Pagkoura, C.; Karagiannakis, G.; et al. Experimental proof of concept of a pilot-scale thermochemical storage unit. AIP Conf. Proc. 2017, 1850, 090006. [Google Scholar]
- Yan, J.; Zhao, C. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage. Appl. Energy 2016, 175, 277–284. [Google Scholar] [CrossRef]
- Dai, L.; Long, X.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Appl. Therm. Eng. 2018, 133, 261–268. [Google Scholar] [CrossRef]
- Kuwata, K.; Esaki, T.; Iwase, D.; Ito, H.; Li, S.; Yang, X.; Huang, H.; Kobayashi, N. Long-Term Durability and Reactivation of Thermochemical Heat Storage Driven by the CaO/Ca(OH)2 Reversible Reaction. J. Mater. Sci. Chem. Eng. 2017, 5, 23–32. [Google Scholar]
- Schmidt, M.; Linder, M. Power generation based on the Ca(OH)2/ CaO thermochemical storage system—Experimental investigation of discharge operation modes in lab scale and corresponding conceptual process design. Appl. Energy 2017, 203, 594–607. [Google Scholar] [CrossRef]
- Schmidt, M.; Szczukowski, C.; Roßkopf, C.; Linder, M.; Wörner, A. Experimental results of a 10 kW high temperature thermochemical storage reactor based on calcium hydroxide. Appl. Therm. Eng. 2014, 62, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Nakahata, J.; Yoshizawa, Y. Durability characteristics of the hydration of magnesium oxide under repetitive reaction. J. Mater. Sci. 1999, 34, 475–480. [Google Scholar] [CrossRef]
- Thomas, J.J.; Musso, S.; Prestini, I. Kinetics and Activation Energy of Magnesium Oxide Hydration. J. Am. Ceram. Soc. 2014, 97, 275–282. [Google Scholar] [CrossRef]
- Shand, M. Calcination of Magnesia Hydroxide and Carbonate. In The Chemistry and Technologies of Magnesia; Shand, M.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 83–96. [Google Scholar]
- Gravogl, G.; Knoll, C.; Welch, J.; Artner, W.; Freiberger, N.; Nilica, R.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Werner, A.; et al. Cycle Stability and Hydration Behavior of Magnesium Oxide and Its Dependence on the Precursor-Related Particle Morphology. Nanomaterials 2018, 8, 795. [Google Scholar] [CrossRef]
- Van Essen, V.M.; Zondag, H.A.; Cot Gores, J.; Bleijendaal, L.P.J.; Bakker, M.; Schuitema, R.; van Helden, W.; He, Z.; Rindt, C. Characterization of MgSO4 Hydrate for Thermochemical Seasonal Heat Storage. J. Sol. Energy Eng. 2009, 131. [Google Scholar] [CrossRef]
- Krönauera, A.; Lävemanna, E.; Brücknera, S.; Hauera, A. Mobile Sorption Heat Storage in Industrial Waste Heat Recovery. Energy Procedia 2015, 73, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Yim, T.; Kim, H.; Lee, J. Cyclic Assessment of Magnesium Oxide with Additives as a Thermochemical Material to Improve the Mechanical Strength and Chemical Reaction. Energies 2018, 11, 2366. [Google Scholar] [CrossRef]
- Criado, Y.; Alonso, M.; Abanades, J. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016, 135, 800–809. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Lanza, M.; Milone, C. Thermochemical performance of carbon nanotubes based hybrid materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 181, 232–243. [Google Scholar] [CrossRef]
- Mastronardo, E.; Kato, Y.; Bonaccorsi, L.; Piperopoulos, E.; Milone, C. Thermochemical Storage of Middle Temperature Wasted Heat by Functionalized C/Mg(OH)2 Hybrid Materials. Energies 2017, 10, 70. [Google Scholar] [CrossRef]
- Shkatulov, A.; Ryu, J.; Kato, Y.; Aristov, Y. Composite material Mg(OH)2/vermiculite: A promising new candidate for storage of middle temperature heat. Energy 2012, 44, 1028–1034. [Google Scholar] [CrossRef]
- Karagiannakis, G.; Pagkoura, C.; Halevasa, E.; Baltzopoulou, P.; Konstandopoulos, A. Cobalt/cobaltous oxide based honeycombs for thermochemical heat storage in future concentrated solar power installations: Multi-cyclic assessment and semi-quantitative heat effects estimations. Sol. Energy 2016, 133, 394–407. [Google Scholar] [CrossRef]
- Li, Y.; Perera, S.; Crittenden, B.; Bridgwater, J. The effect of the binder on the manufacture of a 5A zeolite monolith. Powder Technol. 2001, 116, 85–96. [Google Scholar] [CrossRef]







| Step | Process | Gas Supply | Reactant Supply | Gas Heater | Steam Boiler | Time (min) |
|---|---|---|---|---|---|---|
| 1 | Stabilizing the gas flow for preparing heat charging | On | Off | Off | Off | 5 |
| 2 | Heat charging—dehydration | On | Off | On | Off | 90 |
| 3 | TCM cooling down | On | Off | Off | Off | 50 |
| 4 | Optional process—measuring the amount of heat charging by thermochemical reaction | On | Off | On | Off | 90 |
| 5 | Heat discharging—hydration | Off | On | Off | On | 70 |
| 6 | Post drying after heat discharging | On | Off | On | Off | 15 |
| Heat Charging Only by Thermochemical Reaction | Number of Honeycomb | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Maximum value of instantaneous reaction (W) | 117.4 | 105.7 | 115.7 | 121.9 | 110.7 | 122.2 | 116.8 |
| Total amount of accumulated reaction (Wh) | 50.8 | 38.7 | 46.9 | 47.1 | 43.1 | 49.3 | 52.7 |
| Heat Charging Density (GJ/m3) | Number of Honeycomb | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| At the beginning of cycle | 2.06 | 1.96 | 1.90 | 2.00 | 2.15 | 1.99 | 2.11 |
| At the end of cycle | 1.14 | 1.01 | 1.20 | 1.00 | 1.30 | 1.11 | 1.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Yim, T.; Kim, H.J.; Hong, S.; Seo, D.W.; Kim, H.S. Investigation on Long Term Operation of Thermochemical Heat Storage with MgO-Based Composite Honeycombs. Energies 2019, 12, 1262. https://doi.org/10.3390/en12071262
Lee JY, Yim T, Kim HJ, Hong S, Seo DW, Kim HS. Investigation on Long Term Operation of Thermochemical Heat Storage with MgO-Based Composite Honeycombs. Energies. 2019; 12(7):1262. https://doi.org/10.3390/en12071262
Chicago/Turabian StyleLee, Jae Yong, Taesu Yim, Hyouck Ju Kim, Sungkook Hong, Doo Won Seo, and Hong Soo Kim. 2019. "Investigation on Long Term Operation of Thermochemical Heat Storage with MgO-Based Composite Honeycombs" Energies 12, no. 7: 1262. https://doi.org/10.3390/en12071262




