Numerical Study of the Structure and NO Emission Characteristics of N2- and CO2-Diluted Tubular Diffusion Flames
Abstract
:1. Introduction
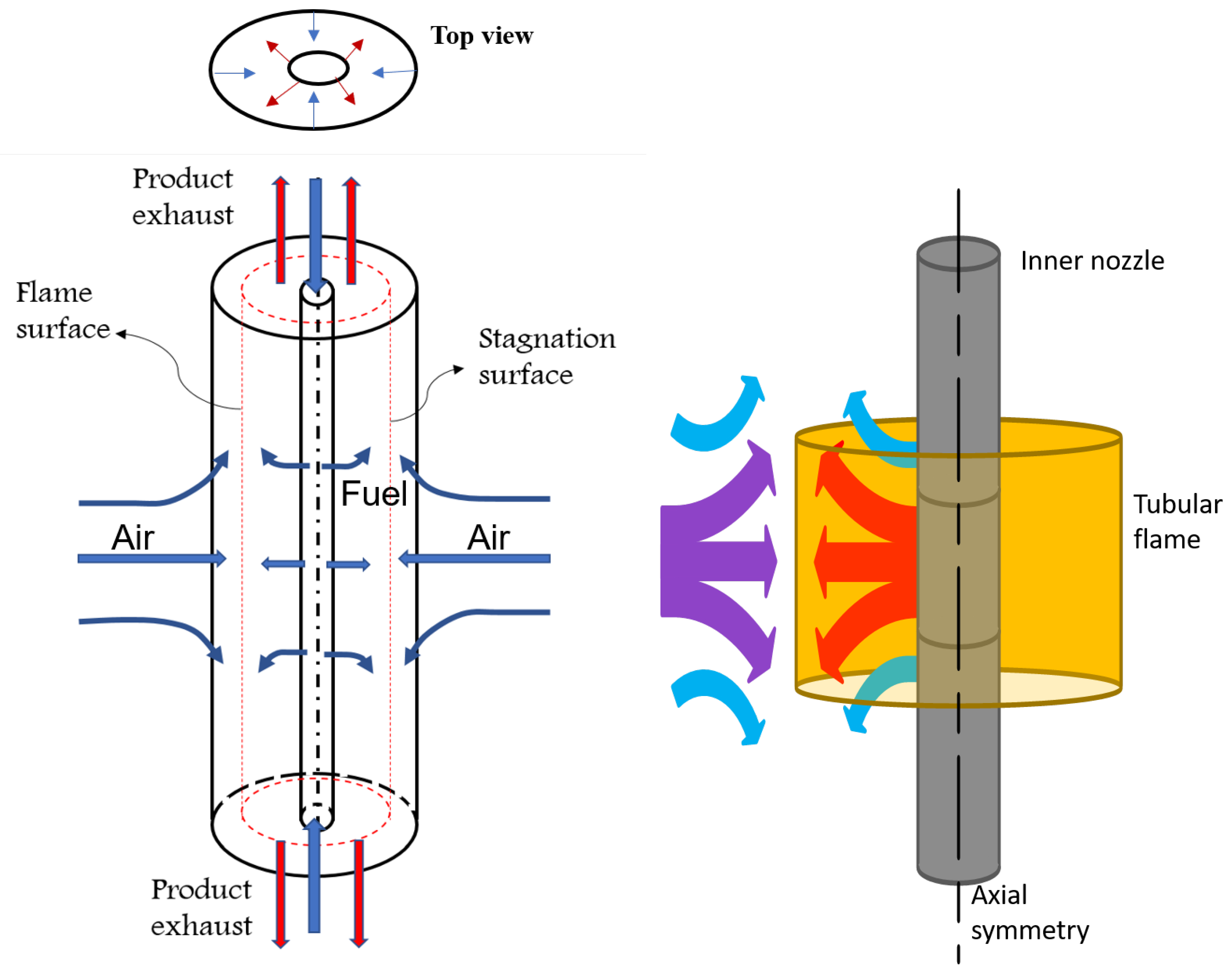
2. Burner Geometry and Numerical Model
Quantitative Reaction Pathway Diagrams
3. Results
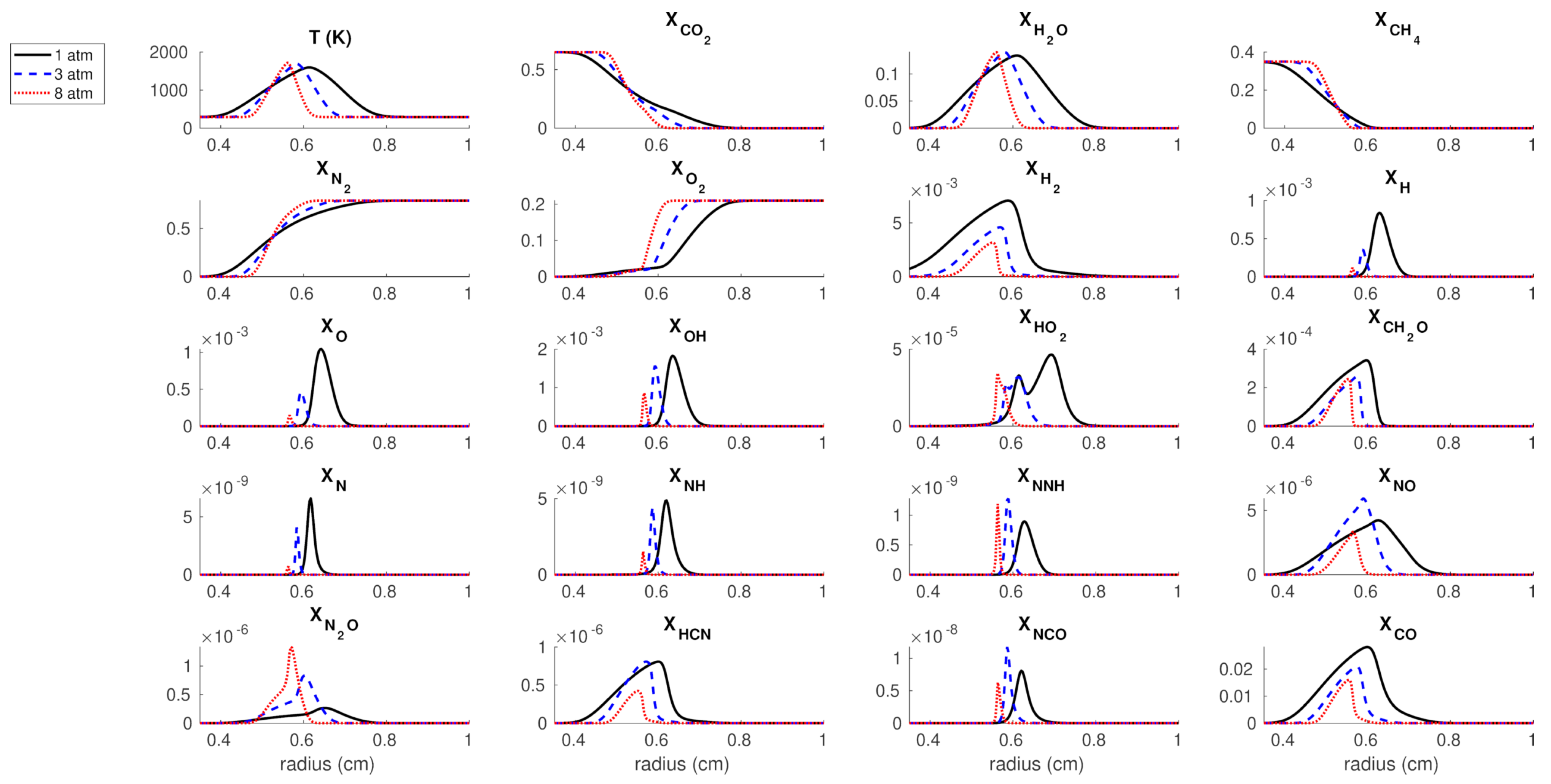
3.1. Flame Structure
3.2. Quantitative Reaction Pathway Diagrams (QRPDs)
3.3. Analysis of Reaction Rates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pitz, R.W.; Hu, S.T.; Wang, P.Y. Tubular premixed and diffusion flames: Effect of stretch and curvature. Prog. Energy Combust. Sci. 2014, 42, 1–34. [Google Scholar] [CrossRef]
- Hall, C.A.; Kulatilaka, W.D.; Gord, J.R.; Pitz, R.W. Quantitative atomic hydrogen measurements in premixed hydrogen tubular flames. Combust. Flame 2014, 161, 2924–2932. [Google Scholar] [CrossRef]
- Hall, C.A.; Pitz, R.W. Numerical simulation of premixed H2–air cellular tubular flames. Combust. Theory Model. 2016, 20, 328–348. [Google Scholar] [CrossRef]
- Mosbacher, D.M.; Wehrmeyer, J.A.; Pitz, R.W.; Sung, C.J.; Byrd, J.L. Experimental and numerical investigation of premixed tubular flames. Proc. Combust. Inst. 2002, 29, 1479–1486. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, S.T.; Pitz, R.W. Extinction and cellular instability of premixed tubular flames. Proc. Combust. Inst. 2009, 32, 1141–1147. [Google Scholar] [CrossRef]
- Shopoff, S.W.; Wang, P.Y.; Pitz, R.W. The effect of stretch on cellular formation in non-premixed opposed-flow tubular flames. Combust. Flame 2011, 158, 876–884. [Google Scholar] [CrossRef]
- Shopoff, S.W.; Wang, P.Y.; Pitz, R.W. Experimental study of cellular instability and extinction of non-premixed opposed-flow tubular flames. Combust. Flame 2011, 158, 2165–2177. [Google Scholar] [CrossRef]
- Hall, C.A.; Pitz, R.W. Major species investigation of non-premixed cellular tubular flame. In Proceedings of the 54th AIAA Aerospace Sciences Meeting, San Diego, CA, USA, 4–8 January 2016. Number 1203. [Google Scholar]
- Hall, C.A.; Pitz, R.W. Experimental and numerical study of H2-air non-premixed cellular tubular flames. Proc. Combust. Inst. 2017, 36, 1595–1602. [Google Scholar] [CrossRef]
- Wang, P.Y.; Hu, S.T.; Pitz, R.W. Numerical investigation of the curvature effects on diffusion flames. Proc. Combust. Inst. 2007, 31, 989–996. [Google Scholar] [CrossRef]
- Hu, S.T.; Pitz, R.W.; Wang, Y. Extinction and near-extinction instability of non-premixed tubular flames. Combust. Flame 2009, 156, 90–98. [Google Scholar] [CrossRef]
- Tinker, D.C.; Hall, C.A.; Pitz, R.W. Measurement and simulation of partially-premixed cellular tubular flames. Proc. Combust. Inst. 2019, 37, 2021–2028. [Google Scholar] [CrossRef]
- Hu, S.T.; Pitz, R.W. Structural study of non-premixed tubular hydrocarbon flames. Combust. Flame 2009, 156, 51–61. [Google Scholar] [CrossRef]
- Li, Q.; Fernandez, L.; Zhang, P.Y.; Wang, P.Y. Stretch and curvature effects on NO emission of H2/air diffusion flames. Combust. Sci. Technol. 2015, 187, 1520–1541. [Google Scholar] [CrossRef]
- Suenaga, Y.; Kitano, M.; Yanaoka, H. Extinction of cylindrical diffusion flame. J. Thermal Sci. Technol. 2011, 6, 323–332. [Google Scholar] [CrossRef]
- Suenaga, Y.; Yanaoka, H.; Momotori, D. Influences of stretch and curvature on the temperature of stretched cylindrical diffusion flames. J. Thermal Sci. Technol. 2016, 11, JTST0028. [Google Scholar] [CrossRef]
- Nishioka, M.; Inagaki, K.; Ishizuka, S.; Takeno, T. Effects of pressure on structure and extinction of tubular flame. Combust. Flame 1991, 86, 90–100. [Google Scholar] [CrossRef]
- Glassman, I.; Yetter, R.A.; Glumac, N.G. Combustion, 5th ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Correa, S.M. A review of NOx formation under gas-turbine combustion conditions. Combust. Sci. Technol. 1993, 87, 329–362. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.T.; Park, J.S.; Kim, J.S.; Kim, S.; Kim, T.K. A study on H2-air counterflow flames in highly preheated air diluted with CO2. Energy Fuels 2005, 19, 2254–2260. [Google Scholar] [CrossRef]
- Lim, J.; Gore, J.; Viskanta, R. A study of the effects of air preheat on the structure of methane/air counterflow diffusion flames. Combust. Flame 2000, 121, 262–274. [Google Scholar] [CrossRef]
- Rørtveit, G.J.; Hustad, J.E.; Li, S.C.; Williams, F.A. Effects of diluents on NOx formation in hydrogen counterflow flames. Combust. Flame 2002, 130, 48–61. [Google Scholar] [CrossRef]
- Yang, K.H.; Shih, H.Y. NO formation of opposed-jet syngas diffusion flames: Strain rate and dilution effects. Int. Hydrog. Energy 2017, 42, 24517–24531. [Google Scholar] [CrossRef]
- Shih, H.Y.; Hsu, J.R. Computed NOx emission characteristics of opposed-jet syngas diffusion flames. Combust. Flame 2012, 159, 1851–1863. [Google Scholar] [CrossRef]
- Hu, S.T.; Wang, P.Y.; Pitz, R.W.; Smooke, M.D. Experimental and numerical investigation of non-premixed tubular flames. Proc. Combust. Inst. 2007, 31, 1093–1099. [Google Scholar] [CrossRef]
- Schlichting, H. Boundary-Layer Theory, 7th ed.; McGraw-Hill: New York, NY, USA, 1979. [Google Scholar]
- Wang, P.Y.; Wehrmeyer, J.A.; Pitz, R.W. Stretch rate of tubular premixed flames. Combust. Flame 2006, 145, 401–414. [Google Scholar] [CrossRef]
- Matalon, M. On flame stretch. Combust. Sci. Technol. 1983, 31, 169–181. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. GRI-Mech 3.0. 2000. Available online: http://www.me.berkeley.edu/gri_mech (accessed on 10 October 2018).
- Grcar, J.F.; Day, M.S.; Bell, J.B. Conditional and Opposed Reaction Path Diagrams for the Analysis of Fluid-Chemistry Interactions; LBNL Report No. 52164; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2003.
- Warnatz, J.; Maas, U.; Dibble, R.W. Combustion, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Brown, T.M.; Tanoff, M.A.; Osborne, R.J.; Pitz, R.W.; Smooke, M.D. Experimental and numerical investigation of laminar hydrogen-air counterflow diffusion flames. Combust. Sci. Technol. 1997, 129, 71–88. [Google Scholar] [CrossRef]






| (cm/s) | (cm/s) | (1/s) | k (1/s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N2-diluted case | 0.35 | 0.65 | 0 | 15 | 0.21 | 0.79 | 25 | 15 | 88 |
| CO2-diluted case | 0.35 | 0 | 0.65 | 15.09 | 0.21 | 0.79 | 30.24 | 20 | 88 |
| Diluent | Pressure (atm) | (K) | (K) | |
|---|---|---|---|---|
| N2 | 1 | 2040 | 1770 | −13.2 |
| N2 | 3 | 2050 | 1851 | −9.7 |
| N2 | 8 | 2057 | 1898 | −7.7 |
| CO2 | 1 | 1923 | 1598 | −16.9 |
| CO2 | 3 | 1930 | 1692 | −13.3 |
| CO2 | 8 | 1936 | 1719 | −11.2 |
| Route | Reaction |
|---|---|
| Thermal | N + NO ↔ N2 + O |
| N + O2 ↔ NO + O | |
| N + OH ↔ NO + H | |
| Fenimore prompt | HCN + O ↔ NCO + H |
| NCO + H ↔ NH + CO | |
| NH + H ↔ N + H2 | |
| NH + OH ↔ N + H2O | |
| N + O2 ↔ NO + O | |
| N + OH ↔ NO + H | |
| N2O | N2 + O (+ M ) ↔ N2O (+ M ) |
| N2O + O ↔ 2NO | |
| NNH | N2 + H + M ↔ NNH + M |
| NNH + O ↔ NO + NH |
| 1 atm | 3 atm | 8 atm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N2 Case | CO2 Case | Reduce by | N2 Case | CO2 Case | Reduce by | N2 Case | CO2 Case | Reduce by | |
| N | 33 | 20 | 20 | ||||||
| NH | 20 | 17 | 15 | ||||||
| NNH | 2.5 | 2.3 | 3.3 | ||||||
| NO | 10 | 8.3 | 10 | ||||||
| N2O | 1 | 1.7 | 1.5 | ||||||
| HCN | 21 | 17 | 33 | ||||||
| NCO | 16 | 11 | 13 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devathi, H.; Hall, C.A.; Pitz, R.W. Numerical Study of the Structure and NO Emission Characteristics of N2- and CO2-Diluted Tubular Diffusion Flames. Energies 2019, 12, 1490. https://doi.org/10.3390/en12081490
Devathi H, Hall CA, Pitz RW. Numerical Study of the Structure and NO Emission Characteristics of N2- and CO2-Diluted Tubular Diffusion Flames. Energies. 2019; 12(8):1490. https://doi.org/10.3390/en12081490
Chicago/Turabian StyleDevathi, Harshini, Carl A. Hall, and Robert W. Pitz. 2019. "Numerical Study of the Structure and NO Emission Characteristics of N2- and CO2-Diluted Tubular Diffusion Flames" Energies 12, no. 8: 1490. https://doi.org/10.3390/en12081490






