Column Leaching Tests to Valorize a Solid Waste from the Decommissioning of Coal-Fired Power Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Sampling
2.2. Pre-Washing of Sample
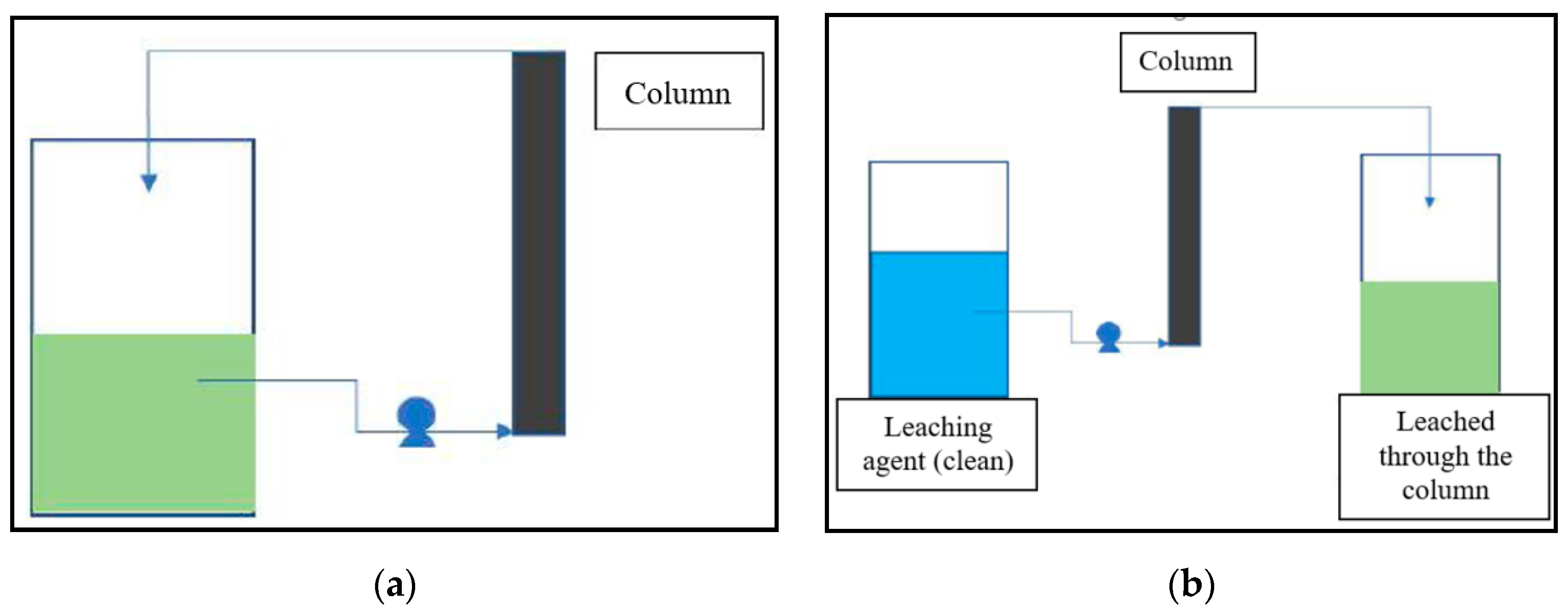
2.3. Acid Leaching Tests in Columns
2.4. Analysis of Elution Stability after Column Leaching Tests
2.5. Treatment for Obtaining an Inert Solid
- 1st stage: An acid solution of 0.3 M sulfuric acid at pH value of 0.5 was pumped through the column. 10 L of solution was distributed through the column at constant flow rate over 120 min.
- stage: 10 L of water was distributed through the column at constant flow rate over 120 min.
- stage: A basic solution of 0.1 M NaOH at pH value of 13 was pumped through the column. 10 L of solution was distributed through the column at constant flow rate over 120 min.
- stage: 10 L of water was distributed through the column at constant flow rate over 120 min.
3. Results
3.1. Characterization of Solid Waste
3.2. Column Leaching Tests
3.2.1. Effect of Pre-Washing
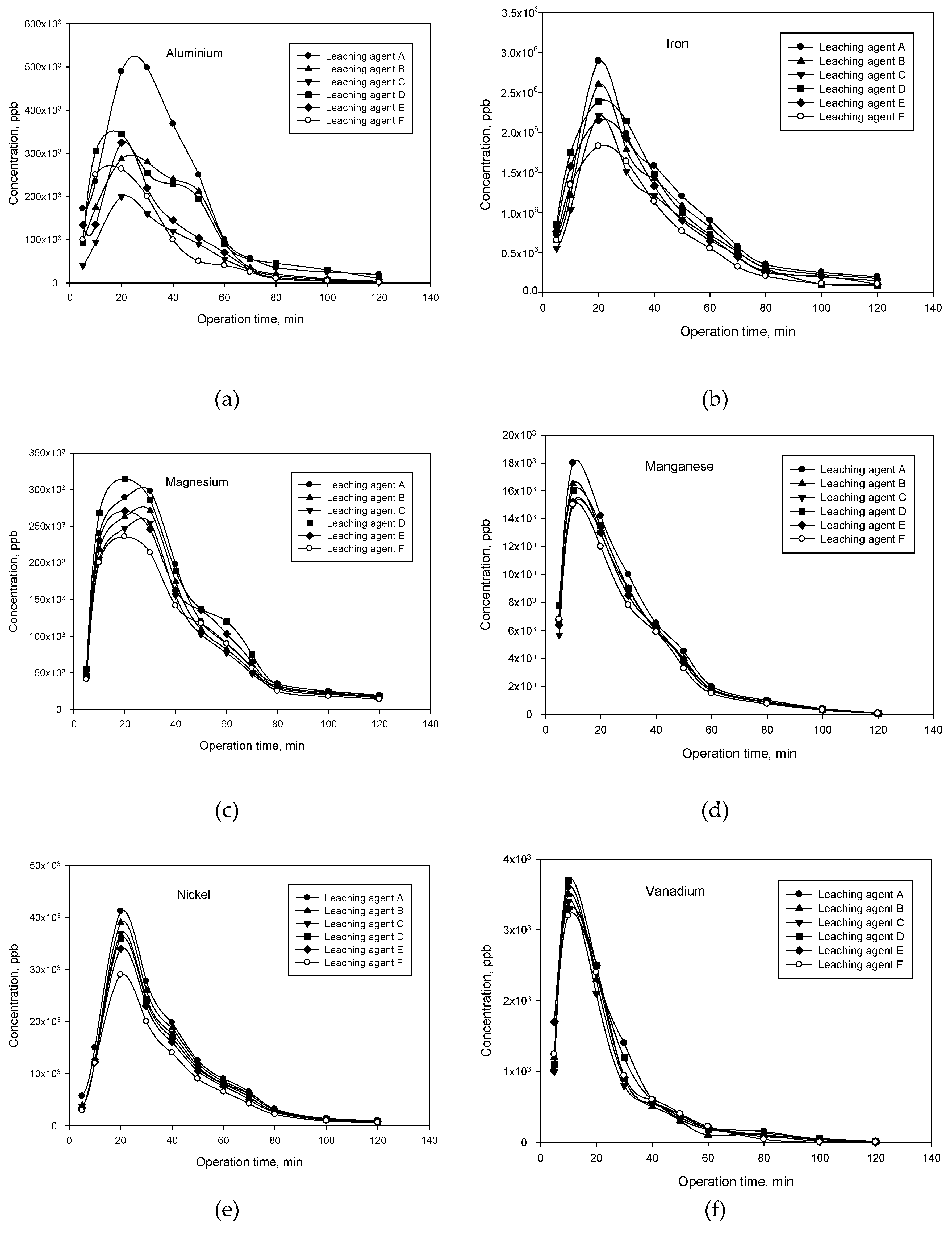
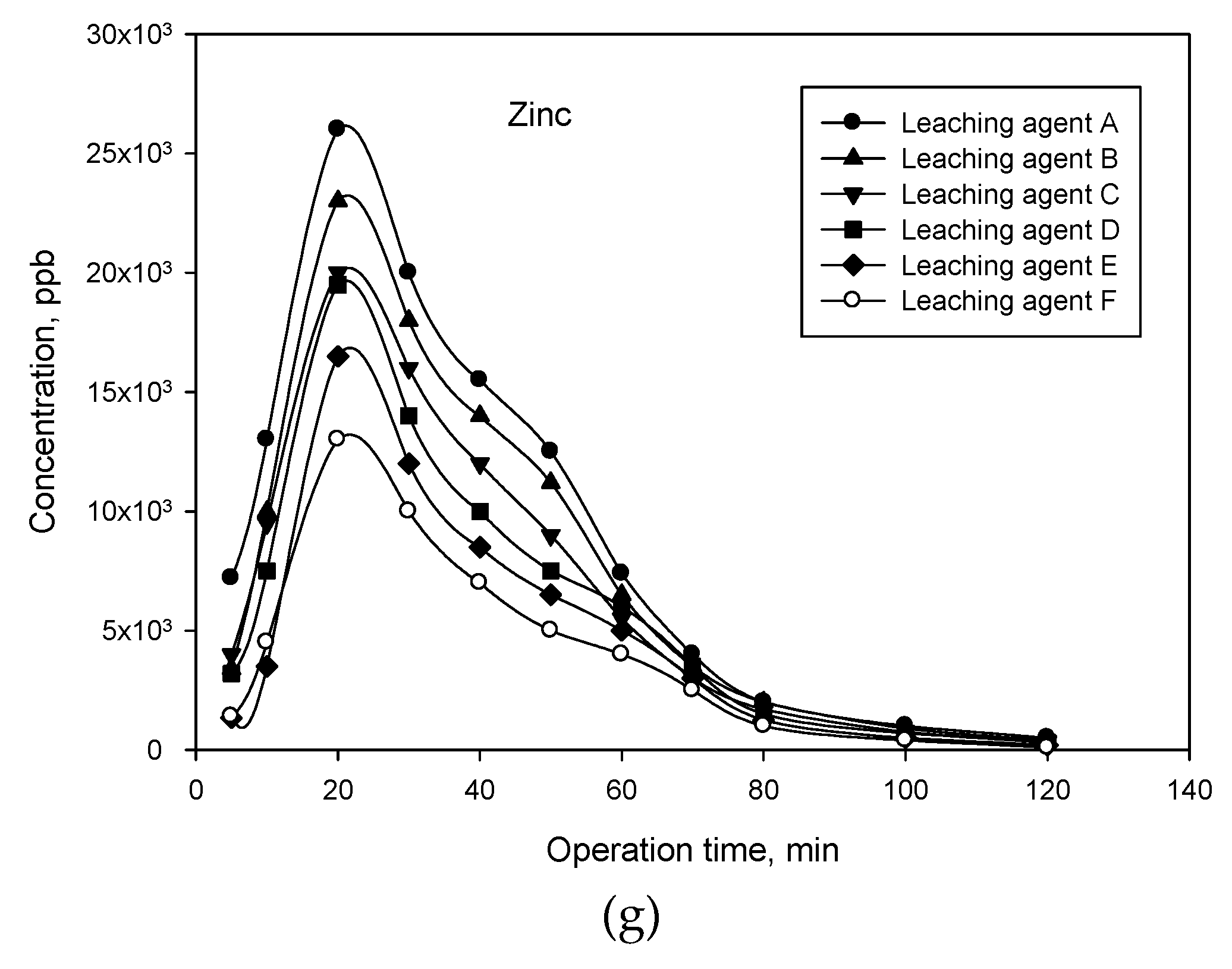
3.2.2. Study of Metal Leaching as a Function of Acid Leaching Agent and Leaching Conditions
3.2.3. Study of Metal Leaching in Different Column Operating Modes
3.2.4. Effect of an Assistant Agent (Forced Aeration) on Extraction Efficiencies
3.3. Elution Stability after Leaching Tests
3.4. Comparison Among Treatment and Disposal Technologies (In Terms of Cost)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Gusano, D.; Iribarren, D.; Dufour, J. Is coal extension a sensible option for energy planning? A combined energy systems modeling and life cycle assessment approach. Energy Policy 2018, 114, 413–421. [Google Scholar] [CrossRef]
- World Coal Association. Coal and Electricity. 2018. Available online: http://www.worldcoal.org/coal/uses-of-coal/coal-electricity/ (accessed on 22 April 2019).
- Heinrichs, H.U.; Markewitz, P. A coal phase-out in Germany—Clean, efficient and affordable? Energy Procedia 2015, 75, 2541–2547. [Google Scholar] [CrossRef]
- Kefford, B.M.; Ballinger, B.; Schmeda-Lopez, D.R.; Greig, C.; Smart, S. The early retirement challenge for fossil fuel power plants in deep decarbonisation scenarios. Energy Policy 2018, 119, 294–306. [Google Scholar] [CrossRef]
- Markewitz, P.; Robinius, M.; Stolten, D. The future of fossil fired power plants in Germany—A lifetime analysis. Energies 2018, 11, 1616. [Google Scholar] [CrossRef]
- Amft, M.; Leisvik, M.; Carroll, S. Applying and adapting the Swedish regulatory system for decommissioning to nuclear power reactors—The regulator’s perspective. J. Environ. Radioact. 2019, 196, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, T.; Hooper, A.; O’Connor, B.; Miller, E.; Jarratt, A. Foamed concrete facilitates decommissioning of nuclear plant. Proc. Inst. Civ. Eng. Energy 2013, 166, 58–66. [Google Scholar] [CrossRef]
- Goudeau, V.; Daniel, B.; Dubot, D. Mobile laboratories: An innovative and efficient solution for radiological characterization of sites under or after decommissioning. J. Environ. Radioact. 2019, 196, 194–198. [Google Scholar] [PubMed]
- Juodis, L.; Maceika, E.; Plukis, A.; Dacquait, F.; Genin, J.-B.; Benier, G. Assessment of radioactive contamination in primary circuit of WWER-440 type reactors by computer code OSCAR for the decommissioning case. Prog. Nucl. Energy 2019, 110, 191–198. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.Y.; Song, J.S. A study on characteristics and internal exposure evaluation of radioactive aerosols during pipe cutting in decommissioning of nuclear power plant. Nucl. Eng. Technol. 2018, 50, 1088–1098. [Google Scholar] [CrossRef]
- Mossini, E.; Parma, G.; Rossi, F.M.; Giola, M.; Cammi, A.; Macerata, E.; Padovani, E.; Mariani, M. Monte Carlo integrated approach to radiological characterization for nuclear facilities decommissioning. Radiat. Eff. Defects Solids 2018, 173, 772–783. [Google Scholar] [CrossRef]
- Mossini, E.; Codispoti, L.; Giola, M.; Castelli, L.; Macerata, E.; Porta, A.; Campi, F.; Mariani, M. Topsoil radiological characterisation of L-54M reactor surroundings preliminary to decommissioning operations. J. Environ. Radioact. 2019, 196, 187–193. [Google Scholar] [CrossRef]
- Volk, R.; Hübner, F.; Hünlich, T.; Schultmann, F. The future of nuclear decommissioning—A worldwide market potential study. Energy Policy 2019, 124, 226–261. [Google Scholar] [CrossRef]
- Podgorodetskii, G.S.; Gorbunov, V.B.; Agapov, E.A.; Erokhov, T.V.; Kozlova, O.N. Challenges and opportunities of utilization of ash and slag waste of tpp (thermal power plant). Part 2. Izvestiya Visshikh Uchebnykh Zavedenii. Chernaya Metallurgiya = Izvestiya. Ferr. Metall. 2018, 61, 557–563. (In Russian) [Google Scholar]
- Kinoshita, T.; Akita, S.; Kobayashi, N.; Nii, S.; Kawaizumi, F.; Takahashi, K. Metal recovery from non-mounted printed wiring boards via hydrometallurgical processing. Hydrometallurgy 2003, 69, 73–79. [Google Scholar] [CrossRef]
- Chauhan, G.; Jadhao, P.R.; Pant, K.K.; Nigam, K.D.P. Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment: Challenges & opportunities—A review. J. Environ. Chem. Eng. 2018, 6, 1288–1304. [Google Scholar]
- Jadhav, U.U.; Hocheng, H. A review of recovery of metals from industrial waste. J. Achiev. Mater. Manuf. Eng. 2012, 54, 159–167. [Google Scholar]
- Brunori, C.; Balzamo, S.; Morabito, R. Comparison between different leaching/extraction tests for the evaluation of metal release from fly ash. Int. J. Environ. Anal. Chem. 1999, 75, 19–31. [Google Scholar] [CrossRef]
- Komonweeraket, K.; Cetin, B.; Aydilek, A.H.; Benson, C.H.; Edil, T.B. Effects of pH on the leaching mechanisms of elements from fly ash mixed soils. Fuel 2015, 140, 788–802. [Google Scholar] [CrossRef]
- Yang, S.; Wei, K.; Ma, W.; Xie, K.; Wu, J.; Lei, Y. Kinetic mechanism of aluminum removal from diamond wire sawpowder in HCl solution. J. Hazard. Mater. 2019, 368, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, G.; Mohapatra, S.K. Leaching characteristics of heavy metal in bottom ash from indian thermal power plant. J. Sci. Ind. Res. 2017, 76, 255–258. [Google Scholar]
- Gomes, H.I.; Funari, V.; Mayes, W.M.; Rogerson, M.; Prior, T.J. Recovery of Al, Cr and V from steel slag by bioleaching: Batch and column experiments. J. Environ. Manag. 2018, 222, 30–36. [Google Scholar] [CrossRef]
- Ishigaki, T.; Nakanishi, A.; Tateda, M.; Ike, M.; Fujita, M. Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulphur-oxidizing and iron-oxidizing bacteria. Chemosphere 2005, 60, 1087–1094. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Wang, Q.; Wu, T. Heavy metals extraction from municipal solid waste incineration fly ash using adapted metal tolerant Aspergillus niger. Biosour. Technol. 2008, 100, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Komonweeraket, K.; Cetin, B.; Benson, C.H.; Aydilek, A.H.; Edil, T.B. Leaching characteristics of toxic constituents from coal fly ash mixed soils under the influence of pH. Waste Manag. 2015, 38, 174–184. [Google Scholar] [CrossRef]
- Rivas, E.; Calero, M.; Amor, C.; Blázquez, G.; Martín-Lara, M.A.; Pérez, A. Mixed solid waste from the decommissioning of coal-fired power plants as a resource of high value metals. Process Saf. Environ. Prot. 2019. paper accepted for publication. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, T.; Miao, Y.; Lan, T.; Liu, D. Characteristic of leaching toxicity of lead in flying ash with pretreatment effect. Chin. J. Environ. Eng. 2017, 11, 4808–4814. [Google Scholar]
- Jayaranjan, M.L.D.; Hullebusch, E.D.; Annachhatre, A.P. Reuse options for coal fired power plant bottom ash and fly ash. Rev. Environ. Sci. Biotechnol. 2014, 13, 467–486. [Google Scholar] [CrossRef]
- Li, S.; Qin, S.; Kang, L.; Liu, J.; Wang, J.; Li, Y. An efficient approach for lithium and aluminum recovery from coal fly ash by pre-desilication and intensified acid leaching processes. Metals 2017, 7, 272. [Google Scholar] [CrossRef]
- Chichester, D.L.; Landsberger, S. Determination of the leaching dynamics of metals from municipal solid waste incinerator fly ash using a column test. J. Air Waste Manag. Assoc. 1996, 46, 643–649. [Google Scholar] [CrossRef]
- Zhang, F.S.; Itoh, H. Extraction of metals from municipal solid waste incinerator fly ash by hydrothermal process. J. Hazard. Mater. 2006, 136, 663–670. [Google Scholar] [CrossRef]
- Nagib, S.; Inoue, K. Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching. Hydrometallurgy 2000, 56, 269–292. [Google Scholar] [CrossRef]
- Funari, V.; Mäkinen, J.; Salmine, J.; Braga, R.; Dinelli, E.; Revitzer, H. Metal removal from municipal solid waste incineration fly ash: A comparison between chemical leaching and bioleaching. Waste Manag. 2017, 60, 397–406. [Google Scholar] [CrossRef]
- Wang, H.; Fan, X.; Wang, Y.; Li, W.; Zhan, Y.M.; Wu, G. Comparative leaching of six toxic metals from raw and chemically stabilized MSWI fly ash using citric acid. J. Environ. Manag. 2018, 208, 15–23. [Google Scholar] [CrossRef]
- Ferraro, A.; Fabbricino, M.; van Hullebusch, E.D.; Esposito, G.; Pirozzi, F. Effect of soil/contamination characteristics and process operational conditions on aminopolycarboxylates enhanced soil washing for heavy metals removal: A review. Rev. Environ. Sci. Biol. 2015, 15, 111–145. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, Y.; Tang, J.; Chen, D.; Li, X.; Lin, Q.; Yin, G.; Zhang, M.; Hu, H. Potassium lignosulfonate as a washing agent for remediating lead and copper co-contaminated soils. Sci. Total Environ. 2019, 658, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Zhang, L.; Dong, Z.; Namioka, T.; Yamada, N.; Ninomiya, Y. Study on the species of heavy metals in MSW incineration fly ash and their leaching behavior. Fuel Process. Technol. 2016, 152, 108–115. [Google Scholar] [CrossRef]
- Quina, M.J.; Bordado, J.C.M.; Quinta-Ferreira, R.M. The influence of pH on the leaching behaviour of inorganic components from municipal solid waste APC residues. Waste Manag. 2009, 29, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Kawahata, H.; Takeuchi, M.; Ohta, H.; Ono, Y. Temperature and pH Dependence of Some Metals Leaching from Fly Ash of Municipal Solid Waste. Resour. Geol. 2008, 55, 357–372. [Google Scholar] [CrossRef]



| Parameter | Average | Standard Deviation, SD | |
|---|---|---|---|
| Elemental analysis, % | C | 4.32 | 0.28 |
| H | 0.12 | 0.02 | |
| N | 0.00 | 0.01 | |
| S | 2.03 | 0.16 | |
| Ash content, % | 92.30 | 2.31 | |
| Major elements and toxic metals content, mg/kg | Al | 108600 | 8500 |
| Ca | 45000 | 4120 | |
| Fe | 34640 | 3910 | |
| K | 15500 | 1844 | |
| Mg | 5558 | 584 | |
| Na | 4222 | 470 | |
| Ti | 3656 | 390 | |
| V | 1954 | 260 | |
| Cr | 1951 | 134 | |
| Mn | 416 | 48.1 | |
| Ni | 195 | 12.4 | |
| Zn | 78 | 7.61 | |
| Column Configuration | Extraction Efficiency, % | |||||||
|---|---|---|---|---|---|---|---|---|
| Al | Fe | Mg | Mn | Ni | V | Zn | ||
| One single stage—Without recirculation | 30 min | 0.16 | 2.64 | 2.04 | 1.49 | 6.26 | 0.06 | 10.96 |
| 60 min | 0.31 | 4.83 | 3.70 | 2.22 | 10.94 | 0.07 | 20.58 | |
| 90 min | 0.34 | 5.76 | 4.28 | 2.41 | 12.53 | 0.08 | 23.27 | |
| 120 min | 0.35 | 5.99 | 4.43 | 2.43 | 12.75 | 0.08 | 23.62 | |
| One single stage—With total recirculation (at stationary state) | 0.36 | 6.04 | 4.66 | 2.71 | 12.08 | 0.14 | 24.41 | |
| Two consecutive stages | 0.48 | 6.40 | 5.11 | 2.83 | 12.64 | 0.34 | 25.28 | |
| Column Configuration | Extraction Efficiency, % | ||||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Mg | Mn | Ni | V | Zn | |
| One single stage—With total recirculation(at stationary state) | 0.46 | 8.05 | 5.38 | 2.75 | 11.66 | 2.49 | 54.24 |
| Two consecutive stages | 0.57 | 9.29 | 6.91 | 3.08 | 12.13 | 2.83 | 60.03 |
| Element | Before Acid Leaching (Original Contaminated Waste) | After Acid Leaching | Complete Leaching Treatment | Limit Values for Inert Landfill |
|---|---|---|---|---|
| As | 0.02 | 0.02 | 0.01 | 0.5 |
| Ba | 0.26 | 0.05 | 0.04 | 20 |
| Cd | 0.06 | 0.00 | 0.00 | 0.04 |
| Cr | 0.00 | 0.01 | 0.01 | 0.5 |
| Cu | 0.12 | 0.01 | 0.01 | 2 |
| Hg | 0.07 | 0.07 | 0.01 | 0.01 |
| Mo | 0.01 | 0.02 | 0.01 | 0.5 |
| Ni | 26.53 | 0.02 | 0.02 | 0.4 |
| Pb | 0.03 | 0.02 | 0.01 | 0.5 |
| Sb | 0.13 | 0.12 | 0.05 | 0.06 |
| Se | 0.02 | 0.04 | 0.00 | 0.1 |
| Zn | 19.29 | 0.09 | 0.08 | 4 |
| Type of Waste | Solidification/Treatment | Disposal |
|---|---|---|
| Hazardous waste | Encapsulation 90–100 €/t | Landfilling 150–300 €/t |
| Chemical Fixation 180–200 €/t | Incineration 500–600 €/t | |
| Inert waste | Leaching 30–60 €/t | Landfilling 50–150 €/t |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, E.; Martín-Lara, M.Á.; Blázquez, G.; Pérez, A.; Calero, M. Column Leaching Tests to Valorize a Solid Waste from the Decommissioning of Coal-Fired Power Plants. Energies 2019, 12, 1684. https://doi.org/10.3390/en12091684
Rivas E, Martín-Lara MÁ, Blázquez G, Pérez A, Calero M. Column Leaching Tests to Valorize a Solid Waste from the Decommissioning of Coal-Fired Power Plants. Energies. 2019; 12(9):1684. https://doi.org/10.3390/en12091684
Chicago/Turabian StyleRivas, Ernesto, María Ángeles Martín-Lara, Gabriel Blázquez, Antonio Pérez, and Mónica Calero. 2019. "Column Leaching Tests to Valorize a Solid Waste from the Decommissioning of Coal-Fired Power Plants" Energies 12, no. 9: 1684. https://doi.org/10.3390/en12091684





