Microalgal-Based Carbon Sequestration by Converting LNG-Fired Waste CO2 into Red Gold Astaxanthin: The Potential Applicability
Abstract
:1. Introduction
2. Materials and Methods
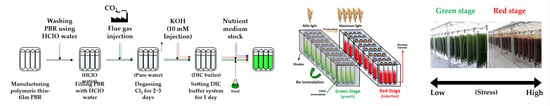
2.1. Establishment of Outdoor Microalgal Culture System Using LNG-Fired Flue Gas
2.2. Pure Culture System of H. pluvialis Without Microbial Contamination
2.3. Algal Strains and Culture Conditions
2.4. Analytical Methods
2.4.1. Analysis of Cell Growth
2.4.2. Analysis of Astaxanthin
2.4.3. Analysis of Lipid
2.4.4. Analysis of Carbohydrate
2.4.5. Analysis of Protein
2.4.6. Ball Milling Method to Rupture H. pluvialis Red Cysts and KFDA Approval of the Extracts
2.4.7. Physiological Studies of H. pluvialis Biomass on Poultry Feeding
3. Results
3.1. Outdoor Biomass and Astaxanthin Production Using LNG-fired Flue Gas During Spring and Summer Seasons
3.2. Outdoor Lipid and Carbohydrate Production Using LNG-Fired Flue Gas During Spring and Summer Seasons
3.3. KFDA Approval and Physiological Studies of H. pluvialis Extracts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Metz, B.; Davidson, O.; Coninck, H.C.D.; Loos, M.; Meyer, L.A. Special Report on Carbon Dioxide Capture and Storage; IPCC, Ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Davis, W.J. The relationship between atmospheric carbon dioxide concentration and global temperature for the last 425 million years. Climate 2017, 5, 76. [Google Scholar] [CrossRef]
- Murai, S.; Fujioka, Y. Challenges to the carbon dioxide capture and storage (CCS) technology. IEEJ Trans. Electr. Electron. Eng. 2008, 3, 37–42. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Chiu, S.-Y.; Huang, T.-T.; Dai, L.; Hsu, L.-K.; Lin, C.-S. Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl. Energy 2012, 93, 176–183. [Google Scholar] [CrossRef]
- Wilson, M.H.; Groppo, J.; Placido, A.; Graham, S.; Morton, S.A.; Santillan-Jimenez, E.; Shea, A.; Crocker, M.; Crofcheck, C.; Andrews, R. CO2 recycling using microalgae for the production of fuels. Appl. Petrochem. Res. 2014, 4, 41–53. [Google Scholar] [CrossRef]
- Lara-Gil, J.A.; Senés-Guerrero, C.; Pacheco, A. Cement flue gas as a potential source of nutrients during CO2 mitigation by microalgae. Algal Res. 2016, 17, 285–292. [Google Scholar] [CrossRef]
- Hong, M.-E.; Choi, Y.Y.; Sim, S.J. Effect of red cyst cell inoculation and iron(II) supplementation on autotrophic astaxanthin production by Haematococcus pluvialis under outdoor summer conditions. J. Biotechnol. 2016, 218, 25–33. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Joun, J.M.; Lee, J.; Hong, M.E.; Pham, H.M.; Chang, W.S.; Sim, S.J. Development of large-scale and economic pH control system for outdoor cultivation of microalgae Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2017, 244, 1235–1244. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Hong, M.E.; Jin, E.S.; Woo, H.M.; Sim, S.J. Improvement in modular scalability of polymeric thin-film photobioreactor for autotrophic culturing of Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2017, 249, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.D.; Sim, S.J. Direct extraction of astaxanthin from Haematococcus culture using vegetable oils. Biotechnol. Lett. 2008, 30, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.D.; Han, S.J.; Kim, J.P.; Kim, M.S.; Oh, Y.K.; Sim, S.J. Hydrogen production by the hyperthermophilic eubacterium, Thermotoga neapolitana, using cellulose pretreated by ionic liquid. Int. J. Hydrog. Energy 2008, 33, 5161–5168. [Google Scholar] [CrossRef]
- Pham, H.-M.; Kwak, H.S.; Hong, M.-E.; Lee, J.W.; Chang, W.S.; Sim, S.J. Development of an X-Shape airlift photobioreactor for increasing algal biomass and bio-diesel production. Bioresour. Technol. 2017, 239, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Lee, J.S.; Choi, J.W.; Shin, Y.S.; Sung, Y.J.; Hong, M.E.; Kwak, H.S.; Kim, C.Y.; Sim, S.J. Performance and potential appraisal of various microalgae as direct combustion fuel. Bioresour. Technol. 2019, 273, 341–349. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.E.; Choi, S.P.; Park, Y.I.; Kim, Y.K.; Chang, W.S.; Kim, B.W.; Sim, S.J. Astaxanthin production by a highly photosensitive Haematococcus mutant. Process Biochem. 2012, 47, 1972–1979. [Google Scholar] [CrossRef]
- Hong, M.E.; Choi, H.I.; Kwak, H.S.; Hwang, S.-W.; Sung, Y.J.; Chang, W.S.; Sim, S.J. Rapid selection of astaxanthin-hyperproducing Haematococcus mutant via azide-based colorimetric assay combined with oil-based astaxanthin extraction. Bioresour. Technol. 2018, 267, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Panis, G.; Rosales Carreon, J. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 28, 175–190. [Google Scholar] [CrossRef]
- Bubrick, P. Production of astaxanthin from Haematococcus. Bioresour. Technol. 1991, 38, 237–239. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Kadam, K.L. Power plant flue gas as a source of CO2 for microalgae cultivation: Economic impact of different process options. Energy Convers. Manag. 1997, 38, S505–S510. [Google Scholar] [CrossRef]
- Doucha, J.; Straka, F.; Lívanský, K. Utilization of flue gas for cultivation of microalgae (Chlorella sp.) in an outdoor open thin-layer photobioreactor. J. Appl. Phycol. 2005, 17, 402–412. [Google Scholar] [CrossRef]
- Borkenstein, C.G.; Knoblechner, J.; Frühwirth, H.; Schagerl, M. Cultivation of Chlorella emersonii with flue gas derived from a cement plant. J. Appl. Phycol. 2011, 23, 131–135. [Google Scholar] [CrossRef]
- Li, F.-F.; Yang, Z.-H.; Zeng, R.; Yang, G.; Chang, X.; Yan, J.-B.; Hou, Y.-L. Microalgae capture of CO2 from actual flue gas discharged from a combustion chamber. Ind. Eng. Chem. Res. 2011, 50, 6496–6502. [Google Scholar] [CrossRef]
- Douskova, I.; Doucha, J.; Livansky, K.; Machat, J.; Novak, P.; Umysova, D.; Zachleder, V.; Vitova, M. Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl. Microbiol. Biotechnol. 2009, 82, 179–185. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Chen, W.; Qiao, Y.; He, C.; Wang, Q. Evaluation of an oil-producing green alga Chlorella sp. C2 for biological deNOx of industrial flue gases. Environ. Sci. Technol. 2014, 48, 10497–10504. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Kim, Y.; Wu, X.; Berzin, I.; Merchuk, J.C. Air-lift bioreactors for algal growth on flue gas: Mathematical modeling and pilot-plant studies. Ind. Eng. Chem. Res. 2005, 44, 6154–6163. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Chen, T.-Y.; Chang, Y.-B.; Chiu, T.-W.; Lin, H.-Y.; Chen, C.-D.; Chang, J.-S.; Lin, C.-S. Utilization of carbon dioxide in industrial flue gases for the cultivation of microalgae Chlorella sp. Bioresour. Technol. 2014, 166, 485–493. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef]
- Hong, M.E.; Hwang, S.K.; Chang, W.S.; Kim, B.W.; Lee, J.W.; Sim, S.J. Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber-Weiss reaction. Appl. Microbiol. Biotechnol. 2015, 99, 5203–5215. [Google Scholar] [CrossRef]
- Kang, C.D.; Sim, S.J. Selective extraction of free astaxanthin from Haematococcus culture using a tandem organic solvent system. Biotechnol. Prog. 2007, 23, 866–871. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A lipid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Gutman, J.; Zarka, A.; Boussiba, S. The host-range of Paraphysoderma sedebokerensis, a chytrid that infects Haematococcus pluvialis. Eur. J. Phycol. 2009, 44, 509–514. [Google Scholar] [CrossRef]
- Gutman, J.; Zarka, A.; Boussiba, S. Evidence for the involvement of surface carbohydrates in the recognition of Haematococcus pluvialis by the parasitic blastoclad Paraphysoderma sedebokerensis. Fungal Biol. 2011, 115, 803–811. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Effect of pH on growth and lipid accumulation kinetics of the microalga Chlorella vulgaris grown heterotrophically under sulfur limitation. Bioresour. Technol. 2016, 219, 694–701. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Aishvarya, V.; Pradhan, N.; Nayak, R.R.; Sukla, L.B.; Mishra, B.K. Enhanced inorganic carbon uptake by Chlorella sp. IMMTCC-2 under autotrophic conditions for lipid production and CO2 sequestration. J. Appl. Phycol. 2012, 24, 1455–1463. [Google Scholar] [CrossRef]
- Nayak, M.; Rath, S.S.; Thirunavoukkarasu, M.; Panda, P.K.; Mishra, B.K.; Mohan-ty, R.C. Maximizing biomass productivity and CO2 biofixation of microalga, Scenedesmus sp. by using sodium hydroxide. J. Microbiol. Biotechnol. 2013, 23, 1260–1268. [Google Scholar] [CrossRef]
- Zawar, P.; Javalkote, V.; Burnap, R.; Mahulikar, P.; Puranik, P. CO2 capture using limestone for cultivation of the freshwater microalga Chlorella sorokiniana PAZ and the cyanobacterium Arthrospira sp. VSJ. Bioresour. Technol. 2016, 221, 498–509. [Google Scholar] [CrossRef]
- Fan, J.; Cui, Y.; Wan, M.; Wang, W.; Li, Y. Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol. Biofuels 2014, 7, 17. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Hong, M.E.; Chang, W.S.; Sim, S.J. Enhanced biodiesel production in Neochloris oleoabundans by a semi-continuous process in two stage photobioreactors. Bioprocess Biosyst. Eng. 2015, 38, 1415–1421. [Google Scholar] [CrossRef]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Dominguez-Bocanegra, A.R.; Legarreta, I.G.; Jeronimo, F.M.; Campocosio, A.T. Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2004, 92, 209–214. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae). Eur. J. Phycol. 2008, 43, 365–376. [Google Scholar] [CrossRef]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2014, 119, 345–350. [Google Scholar] [CrossRef]
- Wan, M.; Zhang, J.; Hou, D.; Fan, J.; Li, Y.; Huang, J.; Wang, J. The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation. Bioresour. Technol. 2014, 167, 276–283. [Google Scholar] [CrossRef]
- García-Malea López, M.C.; Del Río Sánchez, E.; Casas López, J.L.; Acién Fernán-dez Sevilla, J.M.; Rivas, J.; Guerrero, M.G.; Molina Grima, E. Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. J. Biotechnol. 2006, 123, 329–342. [Google Scholar] [CrossRef]
- Chen, H.-W.; Yang, T.-S.; Chen, M.-J.; Chang, Y.-C.; Lin, C.-Y.; Wang, E.-C.; Ho, C.-L.; Huang, K.-M.; Yu, C.-C.; Yang, F.-L.; et al. Application of power plant flue gas in a photobioreactor to grow Spirulina algae, and a bioactivity analysis of the algal water-soluble polysaccharides. Bioresour. Technol. 2012, 120, 256–263. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Fraeye, I.; Bruneel, C.; Lemahieu, C.; Buyse, J.; Muylaert, K.; Foubert, I. Dietary enrichment of eggs with omega-3 fatty acids: A review. Food Res. Int. 2012, 48, 961–969. [Google Scholar] [CrossRef]
- Ao, T.; Macalintal, L.M.; Paul, M.A.; Pescatore, A.J.; Cantor, A.H.; Ford, M.J.; Timmons, B.; Dawson, K.A. Effects of supplementing microalgae in laying hen diets on productive performance, fatty-acid profile, and oxidative stability of eggs. J. Appl. Poultry Res. 2015, 24, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Świątkiewicz, S.; Arczewska-włosk, A.; Józefiak, D. Application of microalgae biomass in poultry nutrition. World Poult. Sci. J. 2016, 71, 663–672. [Google Scholar] [CrossRef]
- Elwinger, K.; Lignell, A.; Wilhelmson, M. Astaxanthin rich algal meal (Haematococcus pluvialis) as carotenoid source in feed for laying hens. In Proceedings of the VII European Symposium on the Quality of Eggs and Egg Products, Poznan, Poland, 21–26 September 1997; pp. 52–59. [Google Scholar]
- Yang, Y.X.; Kim, Y.J.; Jin, Z.; Lohakare, J.D.; Kim, C.H.; Ohh, S.H.; Lee, S.H.; Choi, J.Y.; Chae, B.J. Effects of dietary supplementation of astaxanthin on production performance, egg quality in layers and meat quality in finishing pigs. Asian-Australas. J. Anim. Sci. 2006, 19, 1019–1025. [Google Scholar] [CrossRef]
- Hende, S.V.D.; Vervaeren, H.; Boon, N. Flue gas compounds and microalgae: (Bio-)chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 2012, 30, 1405–1424. [Google Scholar] [CrossRef]






| Spring | Summer | |||
|---|---|---|---|---|
| Wild Type (72 Days) | Mutant (57 Days) | Wild Type (45 Days) | Mutant (36 Days) | |
| Biomass yield (g L−1) | 3.989 | 4.31 | 4.161 | 4.564 |
| Biomass productivity (g L−1 day−1) | 0.055 | 0.075 | 0.092 | 0.127 |
| Astaxanthin content (mg g−1) | 33.60 | 36.78 | 40.02 | 44.02 |
| Astaxanthin accumulation rate (mg g−1 day−1) | 0.466 | 0.645 | 0.889 | 1.223 |
| Astaxanthin yield (mg L−1) | 134.04 | 158.52 | 166.43 | 196.88 |
| Astaxanthin productivity (mg L−1 day−1) | 1.861 | 2.781 | 3.698 | 5.469 |
| CO2 removal rate (g L−1 day−1) 1 | 0.125 | 0.171 | 0.209 | 0.287 |
| Items | Standard Amount | Result | Decision |
|---|---|---|---|
| Characteristic | It should have an inherent color and glossy, lacking unpleasant flavor and odor | It is a dark reddish-brown viscous fluid lacking unpleasant flavor and odor | Accepted |
| Astaxanthin content | 50 mg g−1 | 54.39 mg g−1 | Accepted |
| Heavy metal | Total Pb (<1 mg kg) | 0.0181 mg kg−1 | Accepted |
| Total Cd (<1 mg kg) | 0.0083 mg kg−1 | Accepted | |
| Total Hg (<1 mg kg) | 0.0070 mg kg−1 | Accepted | |
| Total As (<1 mg kg) | 0.1175 mg kg−1 | Accepted | |
| Contaminant | Bacteria negative | Negative | Accepted |
| Solvent remnant | Acetone (<30 mg kg−1) | Negative | Accepted |
| Parameters 1 | Unit | Regular Range | 0% Biomass | 0.1% Biomass | 0.2% Biomass | Remarks 2 | Contents |
|---|---|---|---|---|---|---|---|
| RBC | M μL−1 | 2.0–6.0 | 3.61 | 3.72 | 3.99 | A | O2 carrier function |
| WBC | K μL−1 | 10.0–30.0 | 13.99 | 13.83 | 18.58 | A | Immune function |
| Lymphocytes | % | 50–100 | 73.35 | 68.68 | 68.47 | A | Immune function |
| Monocytes | % | 5–15 | 9.11 | 10.25 | 10.35 | A | Immune function |
| Eosinophils | % | 0–3 | 1.81 | 2.08 | 2.41 | A | Defense function (parasite) |
| Basophils | % | 0–1 | 0.48 | 0.50 | 0.60 | A | Defense function (parasite) |
| Neutrophils | % | 10–30 | 17.64 | 19.19 | 21.97 | A | Phagocytosis function |
| Hemoglobin | g dL−1 | 10–20 | 12.20 | 12.90 | 13.48 | A | O2 carrier function |
| Hematocrit | % | 30–50 | 35.22 | 36.18 | 39.54 | A | Red cell amount (RBC volume B volume−1) |
| MCV | fL | 50–150 | 97.86 | 99.14 | 99.28 | A | Average RBC size |
| MCH | pg | 20–40 | 33.98 | 34.72 | 33.80 | A | Hemoglobin amount per RBC |
| MCHC | g dL−1 | 25–50 | 34.74 | 37.70 | 34.48 | A | Hemoglobin amount relative to the size of the cell per red blood cell |
| Platelet | K μL−1 | 800–1200 | 993.0 | 992.0 | 1010.8 | A | Congelation of blood |
| ALP | U L−1 | 800–1600 | 1200.8 | 1112.6 | 1145.4 | B | Liver function index (bile excretion) |
| TBIL | mg dL−1 | 1.5–3.5 | 2.52 | 2.38 | 2.20 | B | Liver function index (Jaundice) |
| BUN | mg dL−1 | 3.0–6.0 | 4.64 | 4.30 | 4.22 | B | Kidney function index (Urea concentration in blood) |
| PBR Type | Culture Volume | Microalgae | Targeted Products | Biomass Productivity (g L−1 day−1) | CO2 Removal Rate 1 | Flue Gas Composition (Autotrophic Condition) | References |
|---|---|---|---|---|---|---|---|
| Open thin-layer PBR | 330 L | Chlorella vulgaris P12 | - | 22.8 g m−2 day−1 | 42.8 (Calculated) | 7% CO2, 9% O2, 27 ppm NOx, 2 ppm CO (LNG-fired heat and power plant) | [22] |
| Airlift PBR | 5.5 L | Chlorella emersonii | Biodiesel | 0.14 | 0.23 (Presented) | 15% CO2 (cement plant, coal-fired) | [23] |
| Air-lift PBR | 100 L | Scendenesmus obliquus (mutant WUST4) | Biodiesel | 0.11 | 0.21 (Calculated) | 18% CO2, 2% O2, 200 ppm Sox, 150 ppm NOx | [24] |
| Airlift PBR | 1000 L | Scenedesmus acutus UTEX B72 | Biodiesel | 23 g m−2 day−1 | 43.24 (Calculated) | 9% CO2, 53 ppm NOx, 28 ppm Sox (coal-fire power plant) | [5] |
| Column PBR | 0.18 L | Chlorella vulgaris P12 | Biodiesel | 2.5 | 4.4 (Presented) | 13% CO2, 10% O2 (waste-fired power plant) | [25] |
| Column PBR | 0.8 L | Chlorella sp. (mutant MB-9) | Biodiesel | 0.21 | 0.39 (Calculated) | 20% CO2, 70% CH4, 50 ppm or below H2S (desulfied biogas) | [4] |
| Column PBR | 3 L | Chlorella sp. C2 | Biodiesel | 0.093 | 0.17 (Calculated) | 15% CO2, 300 ppm NO (caprolactam production plant) | [26] |
| Column PBR | 30 L | Dunaliella tertiolecta (UTEX LB999) | - | 2.4 × 109 cells L−1 day−1 | - | 8% CO2, 20 ppm NOx (cogeneration power plant) | [27] |
| Column PBR | 50 L | Chlorella sp. MTF-15 | Biodiesel | 0.515 | 0.968 (Calculated) | 23% CO2, 4% O2, 78 ppm NOx, 87 ppm Sox (coke oven in steel corporatioin) | [28] |
| Customized PBR | 1 L | Desmodesmus abundans UTEX2976 | Biodiesel | 0.227 | 0.416 (Presented) | 25% CO2, 800 ppm NOx, 200 ppm SOx (cement plant) | [6] |
| PET PBR | 15 L | Spirulina sp. J774A.1 | Polysaccharide, C-phycocyanin | 0.118 | 0.22 (Calculated) | 12% CO2 (coal-fired power plant) | [51] |
| Thin-film column PBR | 5 L | Haematococcus pluvialis NIES-144 | Astaxanthin | 0.065 | 0.147 (Calculated) | 3.5% CO2, 10% O2, 20 ppm NOx, 3 ppm CO (LNG-fired heat and power plant) | [8] |
| Thin-film column PBR | 100 L | Haematococcus pluvialis (mutant M160) | Astaxanthin | 0.127 | 0.187 (Calculated | 3.5% CO2, 10% O2, 20 ppm NOx, 3 ppm CO (LNG-fired heat and power plant) | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.E.; Chang, W.S.; Patel, A.K.; Oh, M.S.; Lee, J.J.; Sim, S.J. Microalgal-Based Carbon Sequestration by Converting LNG-Fired Waste CO2 into Red Gold Astaxanthin: The Potential Applicability. Energies 2019, 12, 1718. https://doi.org/10.3390/en12091718
Hong ME, Chang WS, Patel AK, Oh MS, Lee JJ, Sim SJ. Microalgal-Based Carbon Sequestration by Converting LNG-Fired Waste CO2 into Red Gold Astaxanthin: The Potential Applicability. Energies. 2019; 12(9):1718. https://doi.org/10.3390/en12091718
Chicago/Turabian StyleHong, Min Eui, Won Seok Chang, Anil Kumar Patel, Mun Sei Oh, Jong Jun Lee, and Sang Jun Sim. 2019. "Microalgal-Based Carbon Sequestration by Converting LNG-Fired Waste CO2 into Red Gold Astaxanthin: The Potential Applicability" Energies 12, no. 9: 1718. https://doi.org/10.3390/en12091718