Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoparticles
2.2. Grafting of Dopamine on Fe3O4 Nanoparticles
2.3. Characterization of Nanoparticles and PDA-Nanoparticles Complex
- (a)
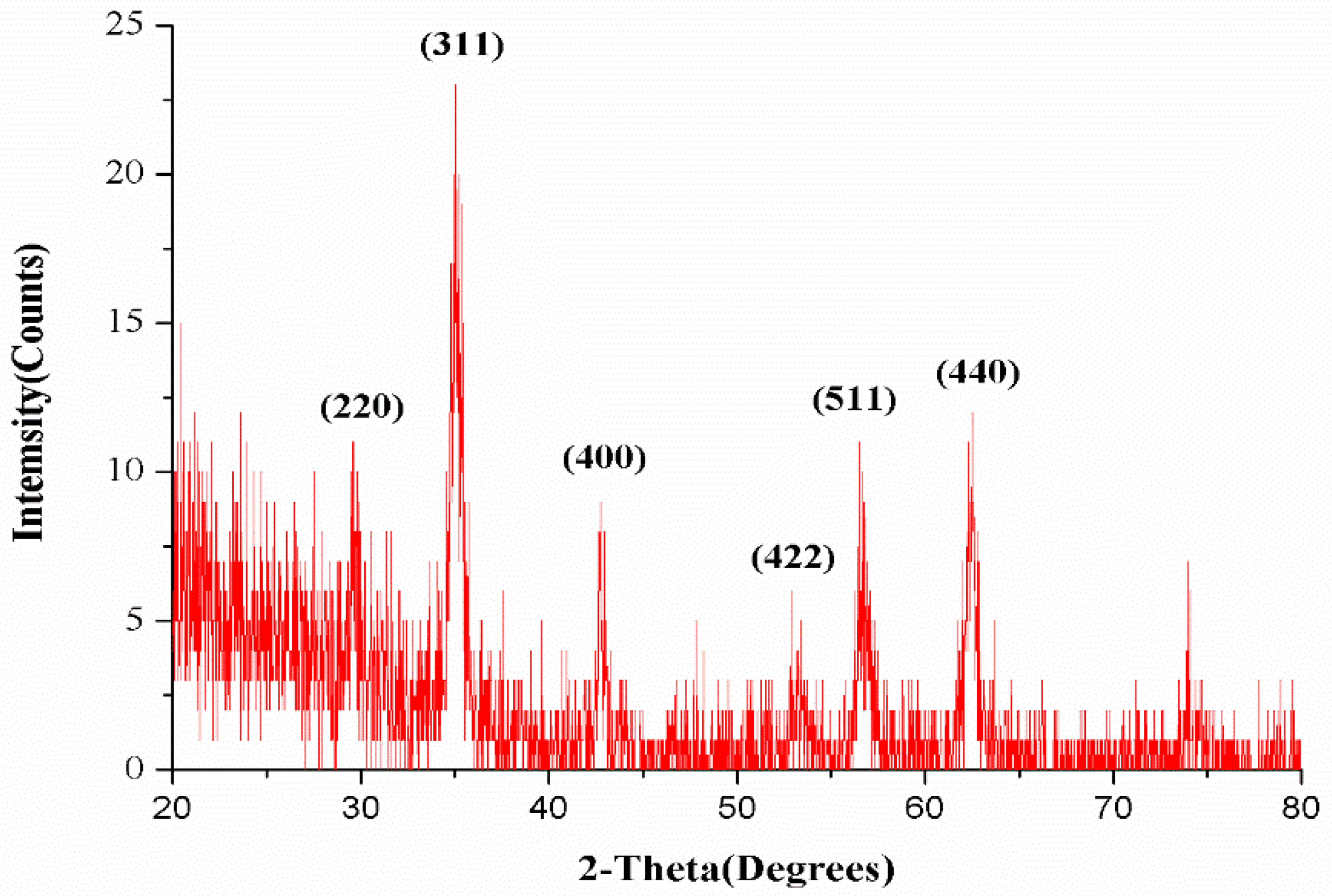
- Nanoparticles were characterized by X-ray diffraction, which helps to identify the crystal phase, dimensions and size of the crystalline material. X’pertpro (PANalyatical) XRD model was used for this purpose. The XRD pattern of Fe3O4 was attained by use of Cu K alpha radiation of wave length 1.54 Å. The patron was taken between the range 20–80 Ɵ with 0.02 scan step size.
- (b)
- Morphological characterization was carried out by TEM (Tecnai G2 F20 U-TWIN) with an accelerating voltage of 200 kV.
- (c)
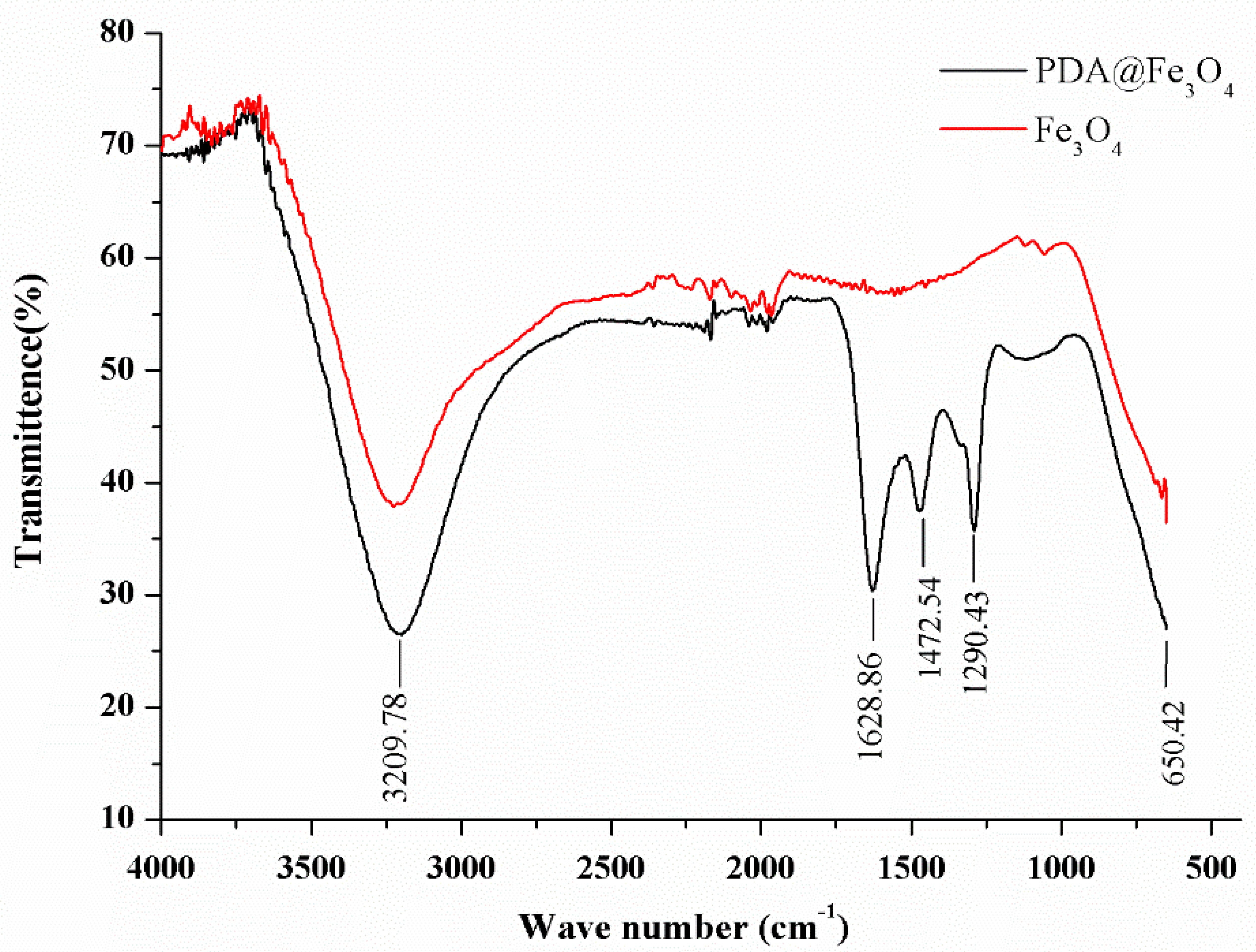
- Bare nanoparticles and the nanoparticles after grafting of polydopamine were analysed by FTIR in the scanning range 400–4000 cm−1, using the Cary 630 Agilent FTIR spectrometer to check the formation/purity of nanoparticles and to confirm the attachment of dopamine polymers on nanoparticle surface.
2.4. Lipase Immobilization on Modified Nanoparticles
2.5. Lipase Activity Assay (Free and Immobilized Form)
2.6. Collection and Characterization of Feedstock
2.7. Experimental Design
2.8. Recovery and Recycling of Nano-Biocatalyst
2.9. Characterization of Biodiesel
2.10. Selection of Suitable Models for Optimization
3. Results and Discussion
3.1. Synthesis and Evaluation of Nano-Biocatalyst Effectiveness
3.1.1. XRD Analysis
3.1.2. TEM Analysis
3.1.3. FTIR Analysis of Nanoparticles
3.1.4. Lipase Activity Assay
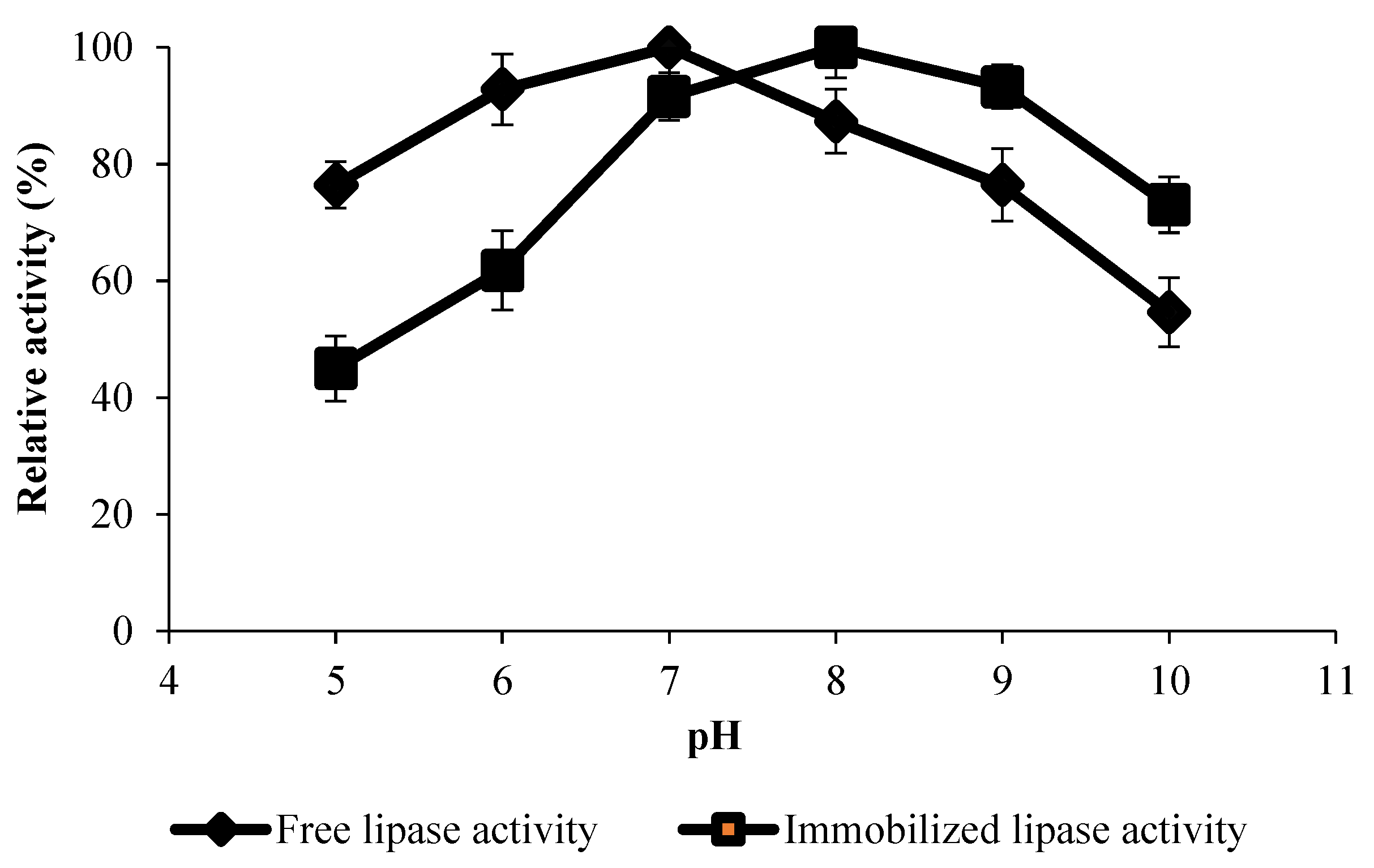
3.1.5. Effect of pH on Nano-Biocatalyst Activity
3.1.6. Effect of Temperature on Activity of Nano-Biocatalyst
3.2. Process Optimization/Characterization
3.2.1. Characteristics of Feedstock
3.2.2. Composition Analysis of Produced Biodiesel by GC-MS
3.2.3. FTIR Monitoring of Biodiesel Production
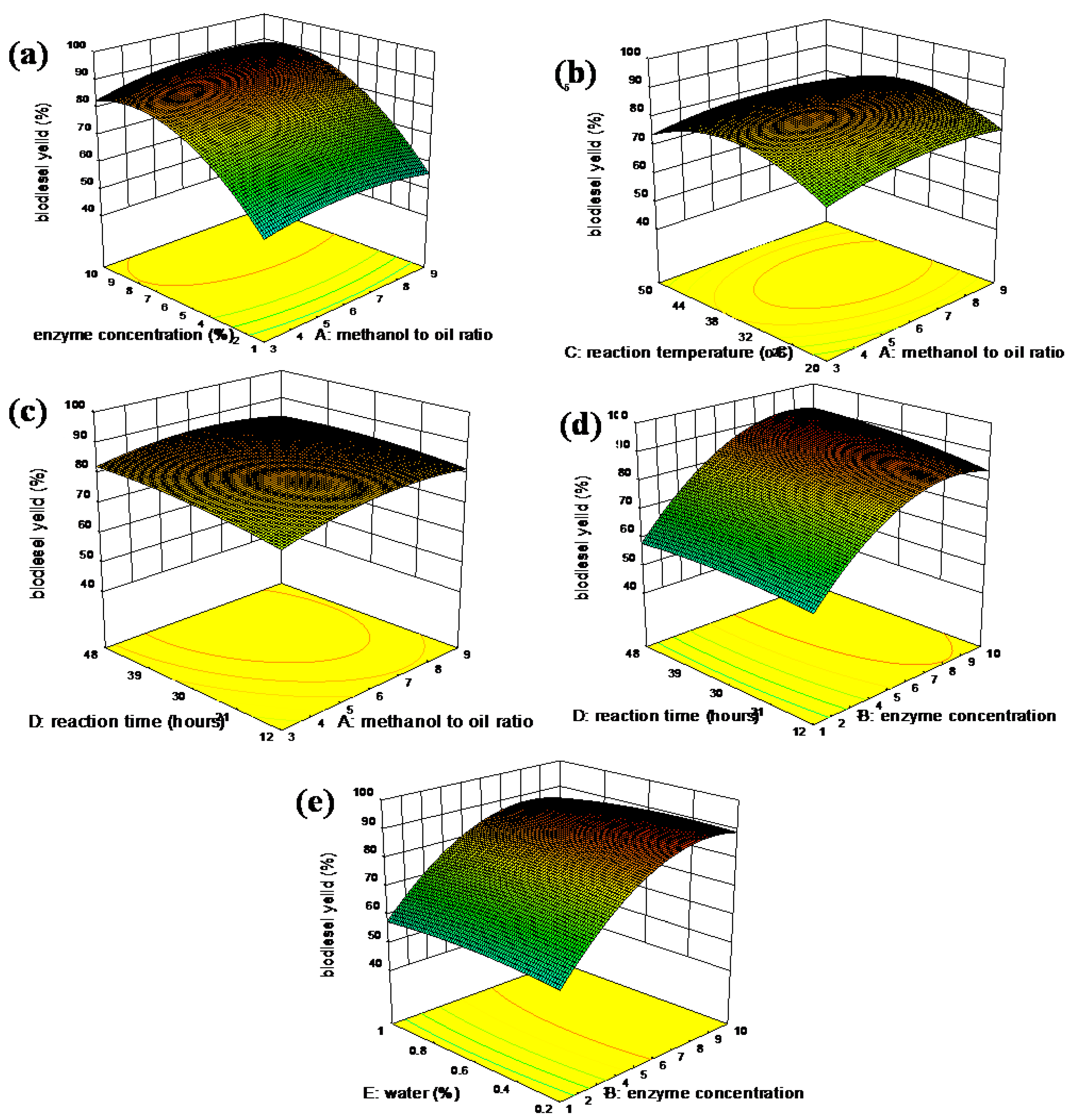
3.2.4. Optimized Reaction Parameters
3.2.5. Model Validation
3.2.6. ANOVA for Response Surface Quadratic Models for Biodiesel Production
AC − 1.13 AD + 0.76 AE + 0.51 BC + 1.47 BD − 0.97 BE − 0.57 CD + 0.22 CE + 0.053
DE − 3.37 A2 − 11.87 B2 − 8.90 C2 − 1.57 D2 − 1.72 E2
3.2.7. Response Surface (RS) Plots of Interacting Terms
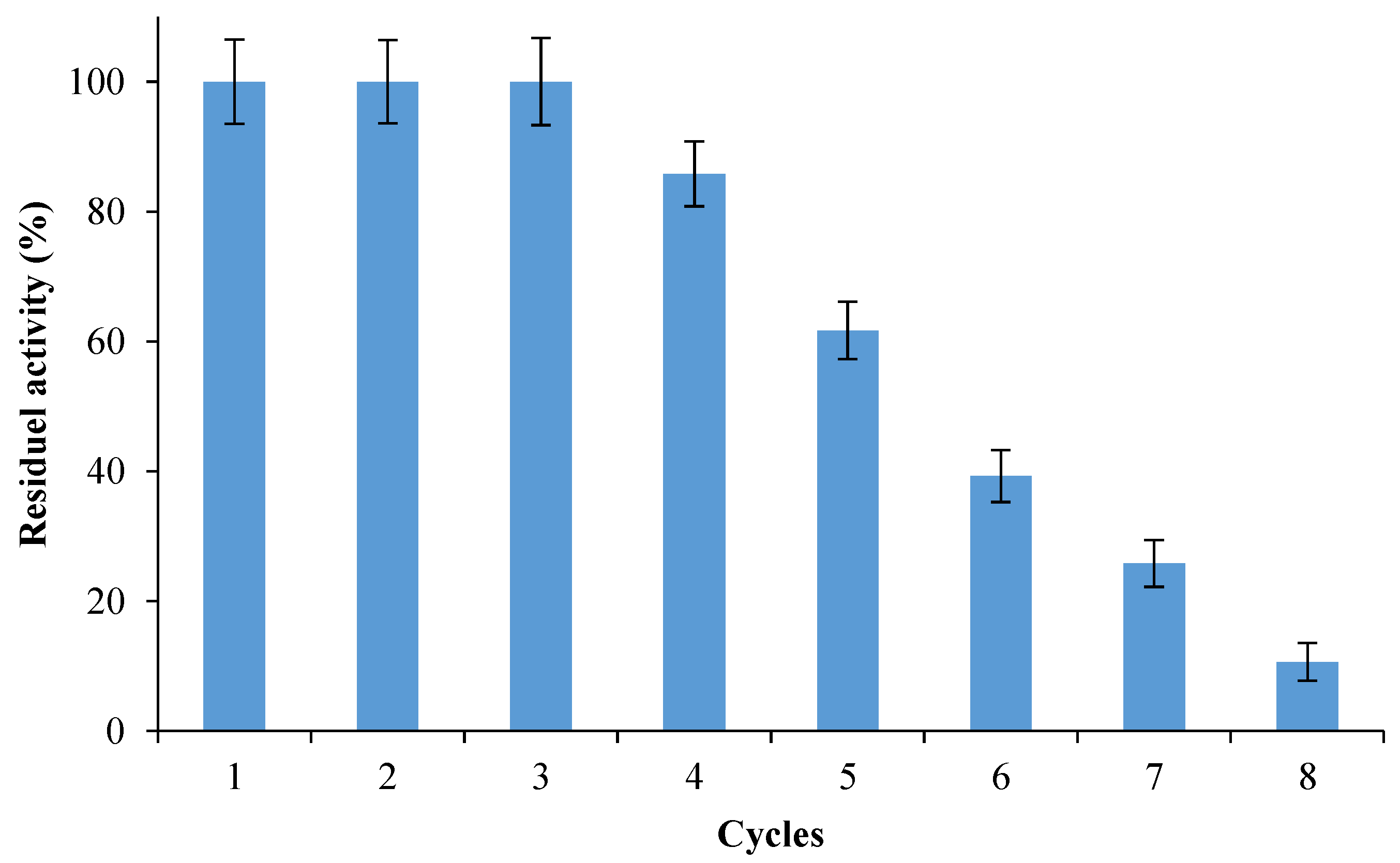
3.2.8. Recovery and Reusability of Nano-Biocatalyst
3.2.9. Physical Properties of Biodiesel
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shirneshan, A. HC, CO, CO2 and NOx emission evaluation of a diesel engine fueled with waste frying oil methyl ester. Procedia-Soc. Behav. Sci. 2013, 75, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Prasad, R. Triglycerides-based diesel fuels. Renew. Sustain. Energy Rev. 2000, 4, 111–133. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Aladedunye, F.A.; Przybylski, R. Degradation and Nutritional Quality Changes of Oil During Frying. J. Am. Oil Chem. Soc. 2008, 86, 149–156. [Google Scholar] [CrossRef]
- Paul, S.; Mittal, G.S. Regulating the use of degraded oil/fat in deep-fat/oil food frying. Crit. Rev. Food Sci. Nutr. 1997, 37, 635–662. [Google Scholar] [CrossRef]
- Raqeeb, M.A.; Bhargavi, R. Biodiesel production from waste cooking oil. J. Chem. Pharm. Res. 2015, 7, 670–681. [Google Scholar]
- Zhang, H.; Ding, J.; Zhao, Z. Microwave assisted esterification of acidified oil from waste cooking oil by CERP/PES catalytic membrane for biodiesel production. Bioresour. Technol. 2012, 123, 72–77. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals’ feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Köse, Ö.; Tüter, M.; Aksoy, H.A. Immobilized Candida antarctica lipase-catalyzed alcoholysis of cotton seed oil in a solvent-free medium. Bioresour. Technol. 2002, 83, 125–129. [Google Scholar] [CrossRef]
- Bisen, P.S.; Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G. Biodiesel production with special emphasis on lipase-catalyzed transesterification. Biotechnol. Lett. 2010, 32, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Zuhair, S.; Dowaidar, A.; Kamal, H. Dynamic modeling of biodiesel production from simulated waste cooking oil using immobilized lipase. Biochem. Eng. J. 2009, 44, 256–262. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Gonçalves, R.V. Magnetic nanocatalysts: Supported metal nanoparticles for catalytic applications. Nanotechnol. Rev. 2013, 2, 5. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kamiya, S. Use of magnetic particles isolated from magnetotactic bacteria for enzyme immobilization. Appl. Microbiol. Biotechnol. 1987, 26, 328–332. [Google Scholar] [CrossRef]
- Wang, C.; Han, H.; Jiang, W.; Ding, X.; Li, Q.; Wang, Y. Immobilization of thermostable lipase QLM on core-shell structured polydopamine-coated Fe3O4 nanoparticles. Catalysts 2017, 7, 49. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Rivera, J.G.; He, L.; Kulkarni, H.; Lee, D.K.; Messersmith, P.B. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol. 2011, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Li, D.; Zhang, L.; Li, J.; Wang, E. Monodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug delivery. Biomaterials 2009, 30, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Dumri, K.; Hung Anh, D. Immobilization of Lipase on Silver Nanoparticles via Adhesive Polydopamine for Biodiesel Production. Enzyme Res. 2014, 2014, 389739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabbir, A.; Mukhtar, H. Optimization process for enhanced extracellular lipases production from a new isolate of Aspergillus terreus ah-F2. Pak. J. Bot. 2018, 50, 1571–1578. [Google Scholar]
- Andrade, M.F.C.; Parussulo, A.L.A.; Netto, C.G.C.M.; Andrade, L.H.; Toma, H.E. Lipase immobilized on polydopamine-coated magnetite nanoparticles for biodiesel production from soybean oil. Biofuel Res. J. 2016, 3, 403–409. [Google Scholar] [CrossRef]
- Li, Y.X. Optimization of biodiesel production from rice bran oil via immobilized lipase catalysis. Afr. J. Biotechnol. 2011, 10, 16314–16324. [Google Scholar]
- Li, Y.X.; Dong, B.X. Optimization of Lipase-Catalyzed Transesterification of Cotton Seed Oil for Biodiesel Production Using Response Surface Methodology. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H.; Al-Resayes, S.I. Post-functionalization of polymeric mesoporous C@Zn core-shell spheres used for methyl ester production. Renew. Energy 2016, 99, 1235–1243. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, B.; Zhao, F.; Feng, L. Synthesis and properties of MPEG-coated superparamagnetic magnetite nanoparticles. J. Nanomater. 2012, 2012, 607296. [Google Scholar] [CrossRef]
- Chen, D.; Tang, Q.; Li, X.; Zhou, X.; Zang, J.; Xue, W.-Q.; Xiang, J.-Y.; Guo, C.-Q. Biocompatibility of magnetic Fe3O4 nanoparticles and their cytotoxic effect on MCF-7 cells. Int. J. Nanomed. 2012, 7, 4973. [Google Scholar] [CrossRef] [Green Version]
- Baharfar, R.; Mohajer, S. Synthesis and Characterization of Immobilized Lipase on Fe3O4 Nanoparticles as Nano biocatalyst for the Synthesis of Benzothiazepine and Spirobenzothiazine Chroman Derivatives. Catal. Lett. 2016, 146, 1729–1742. [Google Scholar] [CrossRef]
- Noshadi, I.; Amin, N.A.S.; Parnas, R.S. Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid: Optimization using response surface methodology (RSM). Fuel 2012, 94, 156–164. [Google Scholar] [CrossRef]
- Uzun, B.B.; Kılıç, M.; Özbay, N.; Pütün, A.E.; Pütün, E. Biodiesel production from waste frying oils: Optimization of reaction parameters and determination of fuel properties. Energy 2012, 44, 347–351. [Google Scholar] [CrossRef]
- Kannan, G.; Anand, R. Effect of injection pressure and injection timing on DI diesel engine fuelled with biodiesel from waste cooking oil. Biomass Bioenergy 2012, 46, 343–352. [Google Scholar] [CrossRef]
- Omar, W.N.N.W.; Amin, N.A.S. Optimization of heterogeneous biodiesel production from waste cooking palm oil via response surface methodology. Biomass Bioenergy 2011, 35, 1329–1338. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Reddy, H.K.; Muppaneni, T.; Deng, S. Biodiesel production from waste cooking oil using sulfuric acid and microwave irradiation processes. J. Environ. Protect. 2012, 3, 107. [Google Scholar] [CrossRef] [Green Version]
- Dubé, M.A.; Zheng, S.; McLean, D.D.; Kates, M. A comparison of attenuated total reflectance-FTIR spectroscopy and GPC for monitoring biodiesel production. J. Am. Oil Chem. Soc. 2004, 81, 599–603. [Google Scholar] [CrossRef]
- Siatis, N.; Kimbaris, A.; Pappas, C.; Tarantilis, P.; Polissiou, M. Improvement of biodiesel production based on the application of ultrasound: Monitoring of the procedure by FTIR spectroscopy. J. Am. Oil Chem. Soc. 2006, 83, 53–57. [Google Scholar] [CrossRef]
- Soares, I.P.; Rezende, T.F.; Silva, R.C.; Castro, E.V.R.; Fortes, I.C. Multivariate calibration by variable selection for blends of raw soybean oil/biodiesel from different sources using Fourier transform infrared spectroscopy (FTIR) spectra data. Energy Fuel 2008, 22, 2079–2083. [Google Scholar] [CrossRef]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Production of biodiesel by enzymatic transesterification of waste sardine oil and evaluation of its engine performance. Heliyon 2017, 3, e00486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Kulschewski, T.; Sasso, F.; Secundo, F.; Lotti, M.; Pleiss, J. Molecular mechanism of deactivation of C. antarctica lipase B by methanol. J. Biotechnol. 2013, 168, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Lipase NS81006 immobilized on Fe3O4 magnetic nanoparticles for biodiesel production. Ovidius Univ. Ann. Chem. 2016, 27, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Iso, M.; Chen, B.; Eguchi, M.; Kudo, T.; Shrestha, S. Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J. Mol. Catal. B Enzymat. 2001, 16, 53–58. [Google Scholar] [CrossRef]
- Mumtaz, M.W.; Adnan, A.; Mahmood, Z.; Mukhtar, H.; Malik, M.F.; Qureshi, F.A.; Raza, A. Biodiesel from Waste Cooking Oil: Optimization of Production and Monitoring of Exhaust Emission Levels From its Combustion in a Diesel Engine. Int. J. Green Energy 2012, 9, 685–701. [Google Scholar] [CrossRef]
- Andrade, I.C.; Vargas, F.E.S.; Fajardo, C.A.G. Biodiesel production from waste cooking oil by enzymatic catalysis process. J. Chem. Eng. 2013, 7, 993–1000. [Google Scholar]
- Li, Q.; Yan, Y. Production of biodiesel catalyzed by immobilized Pseudomonas cepacia lipase from Sapiumsebiferum oil in micro-aqueous phase. Appl. Energy 2010, 87, 3148–3154. [Google Scholar] [CrossRef]
- Wu, W.; Foglia, T.; Marmer, W.; Phillips, J. Optimizing production of ethyl esters of grease using 95% ethanol by response surface methodology. J. Am. Oil Chem. Soc. 1999, 76, 517–521. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Yan, Y. Optimization of lipase-catalyzed transesterification of lard for biodiesel production using response surface methodology. Appl. Biochem. Biotechnol. 2010, 160, 504–515. [Google Scholar] [CrossRef] [PubMed]





| Feedstock | Catalyst | Catalyst Conc. (%) | Methanol to Oil Ratio (%) | Reaction Time (h) | Reaction Temp. (°C) | Water Conc. (%) | Biodiesel Yield (%) |
|---|---|---|---|---|---|---|---|
| WCO | Fe3O4_PDA_Lipase | 10 | 6:1 | 30 | 37 | 0.6 | 92 |
| Source | Df | Sum of Squares | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 20 | 15,145.61 | 757.28 | 151.53 | <0.0001 |
| A—methanol to oil ratio | 1 | 132.56 | 132.56 | 26.53 | <0.0001 |
| B—enzyme concentration | 1 | 7374.92 | 7374.92 | 1475.73 | <0.0001 |
| C—reaction temperature | 1 | 32.22 | 32.22 | 6.45 | 0.0167 |
| D—reaction time | 1 | 170.11 | 170.11 | 34.04 | <0.0001 |
| E—water | 1 | 15.64 | 15.64 | 3.13 | 0.0874 |
| AB | 1 | 67.57 | 67.57 | 13.52 | 0.0010 |
| AC | 1 | 25.34 | 25.34 | 5.07 | 0.0321 |
| AD | 1 | 41.18 | 41.18 | 8.24 | 0.0076 |
| AE | 1 | 18.45 | 18.45 | 3.69 | 0.0645 |
| BC | 1 | 8.44 | 8.44 | 1.69 | 0.2041 |
| BD | 1 | 69.33 | 69.33 | 13.87 | 0.0008 |
| BE | 1 | 30.23 | 30.23 | 6.05 | 0.0201 |
| CD | 1 | 10.59 | 10.59 | 2.12 | 0.1563 |
| CE | 1 | 1.61 | 1.61 | 0.32 | 0.5744 |
| DE | 1 | 0.090 | 0.090 | 0.018 | 0.8940 |
| A2 | 1 | 28.12 | 28.12 | 5.63 | 0.0245 |
| B2 | 1 | 348.60 | 348.60 | 69.76 | <0.0001 |
| C2 | 1 | 188.78 | 188.78 | 37.78 | <0.0001 |
| D2 | 1 | 6.11 | 6.11 | 1.22 | 0.2779 |
| E2 | 1 | 7.33 | 7.33 | 1.47 | 0.2355 |
| Residual | 29 | 144.93 | 5.00 | ||
| Lack of Fit | 22 | 132.35 | 6.02 | 3.35 | 0.0531 |
| Pure Error | 7 | 12.58 | 1.80 | ||
| Cor Total | 49 | 15,290.53 |
| Property | Unit | Value | ASTM D | Method |
|---|---|---|---|---|
| Acid value | (mg KOH/g) | 0.21 ± 0.06 | <0.50 | ASTM D 664 |
| Kinematic viscosity (40 °C) | (mm2/s) | 4.10 ± 0.04 | 1.9–6.0 | ASTM D 445 |
| Flash point | (°C) | 170.41 ± 0.47 | >130 | ASTM D 93 |
| Pour point | (°C) | −3.0 ± 0.5 | - | ASTM D 97 |
| Density | (g mL−1) | 0.871 ± 0.320 | 0.86–0.90 | ASTM D 5002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies 2020, 13, 177. https://doi.org/10.3390/en13010177
Touqeer T, Mumtaz MW, Mukhtar H, Irfan A, Akram S, Shabbir A, Rashid U, Nehdi IA, Choong TSY. Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies. 2020; 13(1):177. https://doi.org/10.3390/en13010177
Chicago/Turabian StyleTouqeer, Tooba, Muhammad Waseem Mumtaz, Hamid Mukhtar, Ahmad Irfan, Sadia Akram, Aroosh Shabbir, Umer Rashid, Imededdine Arbi Nehdi, and Thomas Shean Yaw Choong. 2020. "Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization" Energies 13, no. 1: 177. https://doi.org/10.3390/en13010177