The Membrane-Less Microbial Fuel Cell (ML-MFC) with Ni-Co and Cu-B Cathode Powered by the Process Wastewater from Yeast Production
Abstract
:1. Introduction
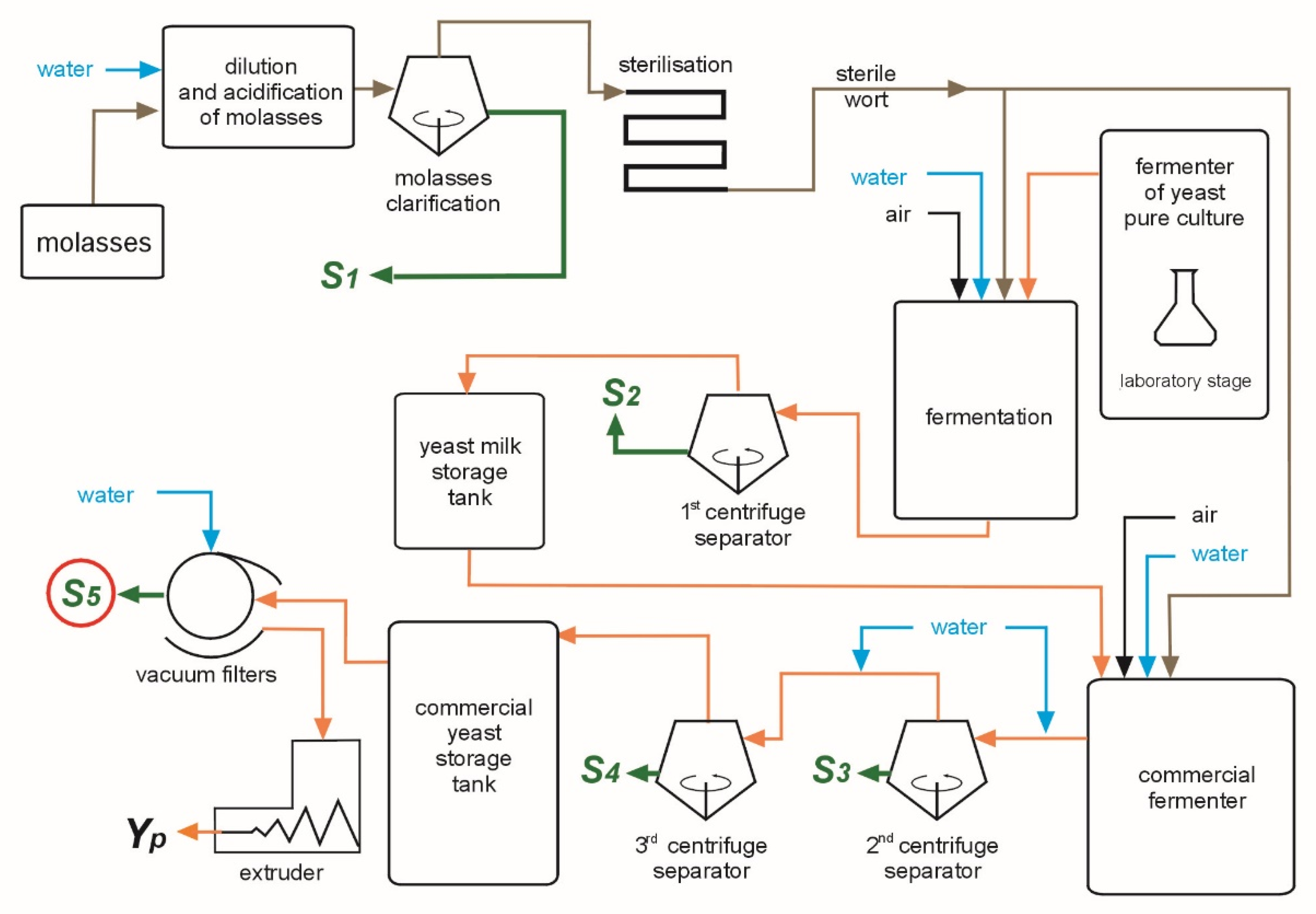
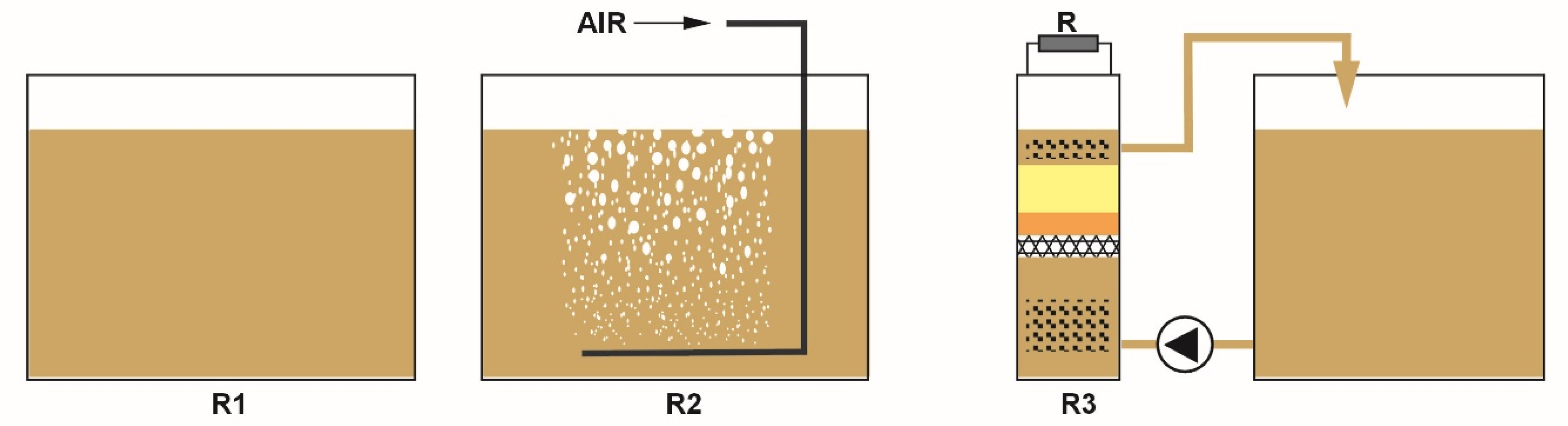
2. Materials and Methods
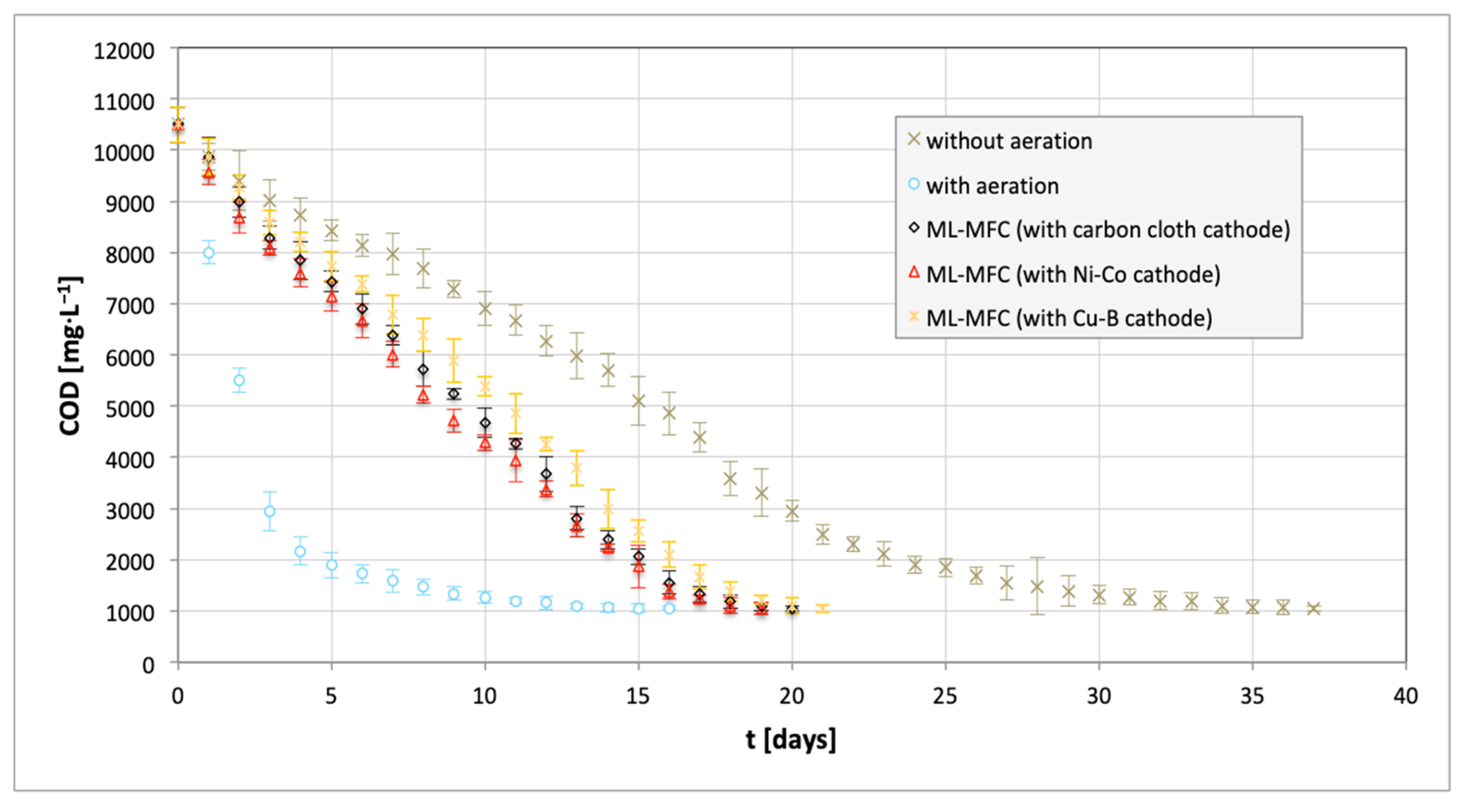
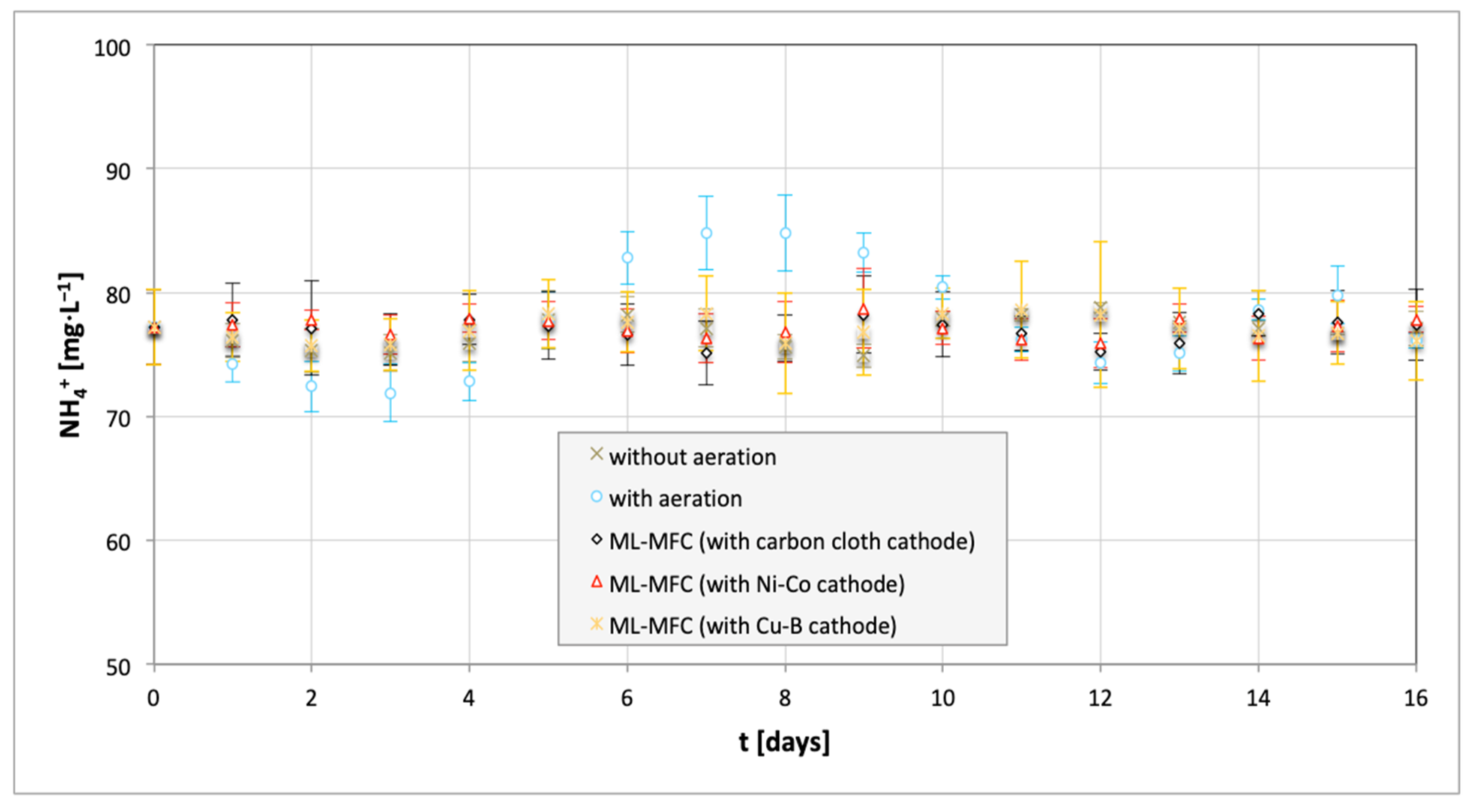
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Logan, B. Microbial Fuel Cells; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Cusick, R.D.; Kim, Y.; Logan, B.E. Energy capture from thermolytic solutions in microbial reverse-electrodialysis cells. Science 2012, 335, 1474–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, A.E.; Nevin, K.P. Microbial fuel cells, a current review. Energies 2010, 3, 899. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: Design, major elements, and scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef] [Green Version]
- Berk, R.S.; Canfield, J.H. Bioelectrochemical energy conversion. Appl. Microbiol. 1964, 12, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Rabaey, K.; Alterman, P.; Clauwaert, P.; de Schamphelaire, L.; Boon, N.; Verstraete, W. Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng. Life Sci. 2006, 6, 285–292. [Google Scholar]
- Chiao, M.; Lam, K.B.; Lin, L. Micromachined microbial and photosynthetic fuel cells. J. Micromech. Microeng. 2006, 16, 2547–2553. [Google Scholar] [CrossRef]
- Lee, J.; Phung, N.T.; Chang, I.S.; Kim, B.H.; Sung, H.C. Use of acetate for enrichment of electrochemically active microorga-nisms and their 16S rDNA analyses. FEMS Microbiol. Lett. 2003, 223, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Mitra, P.; Hill, G.A. Continuous microbial fuel cell using a photoautotrophic cathode and a fermentative anode. Can. J. Chem. Eng. 2012, 90, 1006–1010. [Google Scholar] [CrossRef]
- Prasad, D.; Arun, S.; Murugesan, M.; Padmanaban, S.; Satyana- rayanan, R.S.; Berchmans, S.; Yegnaraman, V. Direct electron transfer with yeast cells and construction of a mediatorless microbial fuel cells. Biosens. Bioelectron. 2007, 22, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A mediator-less microbial fuel cell using a metal reducing bacterium Shewanella Putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, B.H.; Kim, H.S.; Kim, H.J.; Kim, G.T.; Kim, M.; Chang, I.S.; Park, Y.K.; Chang, H.I. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 2001, 7, 297–306. [Google Scholar] [CrossRef]
- Pham, C.A.; Jung, S.J.; Phung, N.T.; Lee, J.; Chang, I.S.; Kim, B.H.; Yi, H.; Chun, J. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila isolated from a microbial fuel cell. FEMS Microbiol. Lett. 2003, 223, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Schaetzle, O.; Barrière, F.; Baronian, K. Bacteria and yeasts as catalysts in microbial fuel cells: Electron transfer from microorganisms to electrodes for green electricity. Energy Environ. Sci. 2008, 1, 607–620. [Google Scholar] [CrossRef]
- Sivasankar, V.; Mylsamy, P.; Omine, K. Microbial Fuel Cell Technology for Bioelectricity, 1st ed.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Permana, D. Performance of Single Chamber Microbial Fuel Cell (SCMFC) for biological treatment of tofu wastewater. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Saint Petersburg, Russia, 17–18 April 2019; Volume 277, p. 012008. [Google Scholar] [CrossRef]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical effects accompanying the decomposition organic compounds. Proc. R. Soc. Lond. Ser. B 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Davis, J.B.; Yarbrough, H.F., Jr. Preliminary experiments on a microbial fuel cell. Science 1962, 137, 615–616. [Google Scholar] [CrossRef]
- Lewis, K. Symposium on bioelectrochemistry of microorganisms: IV. Biochemical fuel cells. Bacteriol. Rev. 1966, 30, 101–113. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schroder, U.; Keller, J.; Verstraete, W.; Rabaey, K. microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Min, B.; Cheng, S.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005, 39, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, P.P.; Włodarczyk, B. Preparation and analysis of Ni–Co catalyst use for electricity production and COD reduction in microbial fuel cells. Catalysts 2019, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, P.P.; Włodarczyk, B. Wastewater treatment and electricity production in a microbial fuel cell with Cu–B alloy as the cathode catalyst. Catalysts 2019, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Zhao, F.; Schröder, U.; Scholz, F. Interfacing electrocatalysis and biocatalysis with tungsten carbide: A high-performance, noble-metal-free microbial fuel cell. Angew. Chem. Int. Ed. 2006, 45, 6658–6661. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wu, Y.; Cao, D.; Zhao, L.; Sun, Q. Electricity generation and modeling of microbial fuel cell from continuous beer brewery wastewater. Bioresour. Technol. 2009, 100, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Juang, D.F.; Lee, C.H.; Hsueh, S.C.; Chou, H.Y. Power generation capabilities of microbial fuel cells with different oxygen supplies in the cathodic chamber. Appl. Biochem. Biotechnol. 2012, 167, 714–731. [Google Scholar] [CrossRef]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.J.; Lee, H. Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci. Technol. 2008, 57, 1117–1121. [Google Scholar] [CrossRef]
- Włodarczyk, B.; Włodarczyk, P.P. Analysis of the potential of an increase in yeast output resulting from the application of additional process wastewater in the evaporator station. Appl. Sci. 2019, 9, 2282. [Google Scholar] [CrossRef] [Green Version]
- Kalyuzhnyi, S.; Gladchenko, M.; Starostina, E.; Shcherbakov, S.; Versprille, A. Combined biological and physico-chemical treatment of baker’s yeast wastewater. Water Sci. Technol. 2005, 52, 175–181. [Google Scholar] [CrossRef]
- Catalkaya, E.C.; Sengul, F. Application of box-wilson experimental design method for the photodegradation of bakery’s yeast industry with UV/H2O2 and UV/H2O2/Fe(II) process. J. Hazard. Mater. 2006, 128, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zub, S.; Kurisso, T.; Menert, A.; Blonskaja, V. Combined biological treatment of high-sulphate wastewater from yeast production. Water Environ. J. 2008, 22, 274–286. [Google Scholar] [CrossRef]
- Gengec, E.; Kobya, M.; Demirbas, E.; Akyol, A.; Oktor, K. Optimization of baker’s yeast wastewater using response surface methodology by electrocoagulation. Desalination 2012, 286, 200–209. [Google Scholar] [CrossRef]
- Wu, W.; Niu, H.; Yang, D.; Wang, S.-B.; Wang, J.; Lin, J.; Hu, C. Controlled layer-by-layer deposition of carbon nanotubes on electrodes for microbial fuel cells. Energies 2019, 12, 363. [Google Scholar] [CrossRef] [Green Version]
- Sudirjo, E.; Buisman, C.J.; Strik, D.P. Activated carbon mixed with marine sediment is suitable as bioanode material for spartina anglica sediment/plant microbial fuel cell: Plant growth, electricity generation, and spatial microbial community diversity. Water 2019, 11, 1810. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.; Xu, Y.; Lu, C.; Lai, W.; Xie, G.; Zheng, L.; Talawar, M.P.; Du, Q.; Li, G. Enhancing stability of microalgae biocathode by a partially submerged carbon cloth electrode for bioenergy production from wastewater. Energies 2019, 12, 3229. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Harnisch, F.; Fricke, K.; Schroeder, U.; Climent, V.; Feliu, J.M. The study of electrochemically active microbial biofilms on different carbon-based anode materials in microbial fuel cells. Biosens. Bioelectron. 2010, 25, 2167–2171. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.; Zhuang, L. Polypyrrole/carbon black composite as a novel oxygen reduction catalyst for microbial fuel cells. J. Power Sources 2010, 195, 3490–3493. [Google Scholar] [CrossRef]
- Zuo, K.; Liang, S.; Liang, P.; Zhou, X.; Sun, D.; Zhang, X.; Huang, X. Carbon filtration cathode in microbial fuel cell to enhance wastewater treatment. Bioresour. Technol. 2015, 185, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Erbay, C.; Yang, G.; de Figueiredo, P.; Sadr, R.; Yu, C.; Han, A. Three-dimensional porous carbon nanotube sponges for high-performance anodes of microbial fuel cells. J. Power Sources 2015, 298, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Asensio, Y.; Montes, I.B.; Fernández-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Selection of cheap electrodes for two-compartment microbial fuel cells. J. Electroanal. Chem. 2017, 785, 235–240. [Google Scholar] [CrossRef]
- Penteado, E.D.; Fernández-Marchante, C.M.; Zaiat, M.; Cañizares, P.; Gonzalez, E.R.; Rodrigo, M.A. Influence of carbon electrode material on energy recovery from winery wastewater using a dual-chamber microbial fuel cell. Environ. Technol. 2017, 38, 1333–1341. [Google Scholar] [CrossRef]
- Sanchez, D.V.P.; Huynh, P.; Kozlov, M.E.; Baughman, R.H.; Vidic, R.D.; Yun, M. Carbon nanotube/platinum (Pt) sheet as an improved cathode for microbial fuel cells. Energy Fuels 2010, 24, 5897–5902. [Google Scholar] [CrossRef]
- Martin, E.; Tartakovsky, B.; Savadogo, O. Cathode materials evaluation in microbial fuel cells: A comparison of carbon, Mn2O3, Fe2O3 and platinum materials. Electrochim. Acta 2011, 58, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.M.; Jin, S.; Wang, J.; Zhu, C.; Urynowiczcz, M.A. Lead dioxide as an alternative catalyst to platinum in microbial fuel cells. Electrochem. Commun. 2007, 9, 1730–1734. [Google Scholar] [CrossRef]
- Liew, K.B.; Daud, W.R.W.; Ghasemia, M.; Leong, J.X.; Lim, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrog. Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Zhuang, L.; Li, W.; Zhou, S.; Zhang, J. Manganese dioxide as an alternative cathodic catalyst to platinum in microbial fuel cells. Biosens. Bioelectron. 2009, 24, 2825–2829. [Google Scholar] [CrossRef]
- Santoro, C.; Lei, Y.; Li, B.; Cristianid, P. Power generation from wastewater using single chamber microbial fuel cells (MFCs) with platinum-free cathodes and pre-colonized anodes. Biochem. Eng. J. 2012, 62, 8–16. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, H.; Liu, H.; Zhou, X. Self-sustained electrochemical promotion catalysts for partial oxidation reforming of heavy hydrocarbons. Int. J. Hydrog. Energy 2012, 37, 17928–17935. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Zhou, X.; Liu, H.; Wei, Y.; Pramuanjaroenkij, A.; Bordas, A.; Page, M.; Cai, S.; Zhang, X. Development and study of self-sustained electrochemical promotion catalysts for hydrocarbon reforming. In ECS Transactions, 2nd ed.; Electrochemical Society Inc.: Pennington, NJ, USA, 2013; Volume 58, pp. 243–254. [Google Scholar] [CrossRef]
- Osorio de la Rosa, E.; Vázquez Castillo, J.; Carmona Campos, M.; Barbosa Pool, G.R.; Becerra Nuñez, G.; Castillo Atoche, A.; Ortegón Aguilar, J. Plant microbial fuel cells–based energy harvester system for self-powered IoT applications. Sensors 2019, 19, 1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, C.; Mollica, A.; Féron, D.; Basséguy, R.; Etcheverry, L.; Bergel, A. Marine microbial fuel cell: Use of stainless steel electrodes as anode and cathode materials. Electrochim. Acta 2006, 53, 468–473. [Google Scholar] [CrossRef] [Green Version]
- Chae, K.J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 2008, 22, 169–176. [Google Scholar] [CrossRef]
- Oh, S.E.; Logan, B.E. Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl. Microbiol. Biotechnol. 2006, 70, 162–169. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Wu, C.C.; Lee, C.Y.; Shih, E.P. Microbial fuel cell performance of multiwall carbon nanotubes on carbon cloth as electrodes. J. Power Sources 2009, 194, 199–205. [Google Scholar] [CrossRef]
- Tardast, A.; Najafpour, G.D.; Rahimnejad, M.; Amiri, A. Bioelectrical power generation in a membrane less microbial fuel cell. World App. Sci. J. 2012, 16, 179–182. [Google Scholar]
- Ghangrekar, M.M.; Shinde, V.B. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol. 2007, 98, 2879–2885. [Google Scholar] [CrossRef]
- Jang, J.K.; Pham, T.H.; Chang, I.S.; Kang, K.H.; Moon, H.; Cho, K.S.; Kim, B.H. Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process. Biochem. 2004, 39, 1007–1012. [Google Scholar] [CrossRef]
- Huggins, T.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Energy and performance comparison of microbial fuel cell and conventional aeration treating of wastewater. J. Microb. Biochem. Technol. 2013, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, P.P.; Włodarczyk, B. Ni-Co alloy as catalyst for fuel electrode of hydrazine fuel cell. China-USA Bus. Rev. 2015, 14, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, B.; Włodarczyk, P.P. Comparsion of Cu-B alloy and stainless steel as electrode material for microbial fuel cell. In Renewable Energy Sources: Engineering, Technology, Innovation ICORES 2018; Wróbel, M., Jewiarz, M., Szlęk, A., Eds.; Springer Nature Switzerland AG: Basel, Switzerland, 2020; pp. 1057–1063. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Microbial fuel cell with Ni-Co cathode powered with yeast wastewater. Energies 2018, 11, 3194. [Google Scholar] [CrossRef] [Green Version]
- Fogelman, S.; Zhao, H.; Blumenstein, M. A rapid analytical method for predicting the oxygen demand of wastewater. Anal. Bioanal. Chem. 2006, 386, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Fizyczno-Chemiczne Badanie Wody I Ścieków; Arkady: Warszawa, Poland, 1999. [Google Scholar]
- Gadzała-Kopciuch, R.; Buszewski, B. Fizykochemiczne Metody Analizy W Chemii Środowiska; Wydawnictwo Uniwersytetu Mikołaja Kopernika: Toruń, Poland, 2003. [Google Scholar]
- Bielański, A. Podstawy Chemii Nieorganicznej; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2013. [Google Scholar]
- Schweda, E. Chemia Nieorganiczna; Wydawnictwo Medpharm: Wrocław, Poland, 2014. [Google Scholar]
- Łomotowski, J.; Szpindor, A. Nowoczesne Systemy Oczyszczania Ścieków; Arkady: Warsaw, Poland, 2002. [Google Scholar]
- Ren, Z.; Yan, H.; Wang, W.; Mench, M.M.; Regan, J.M. Characterization of microbial fuel cells at microbially and electrochemically meaningful time scales. Environ. Sci. Technol. 2011, 45, 2435–2441. [Google Scholar] [CrossRef]

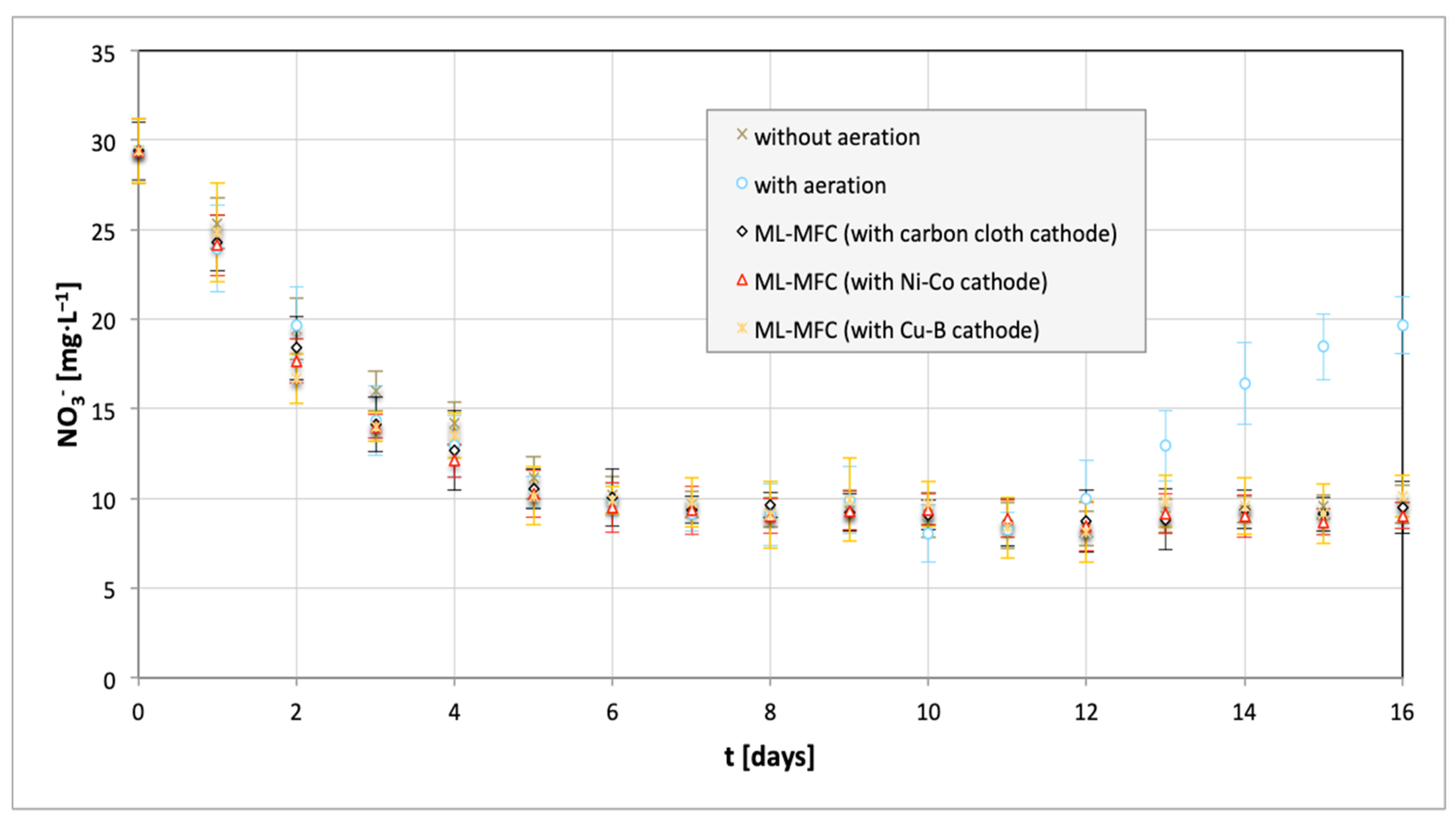
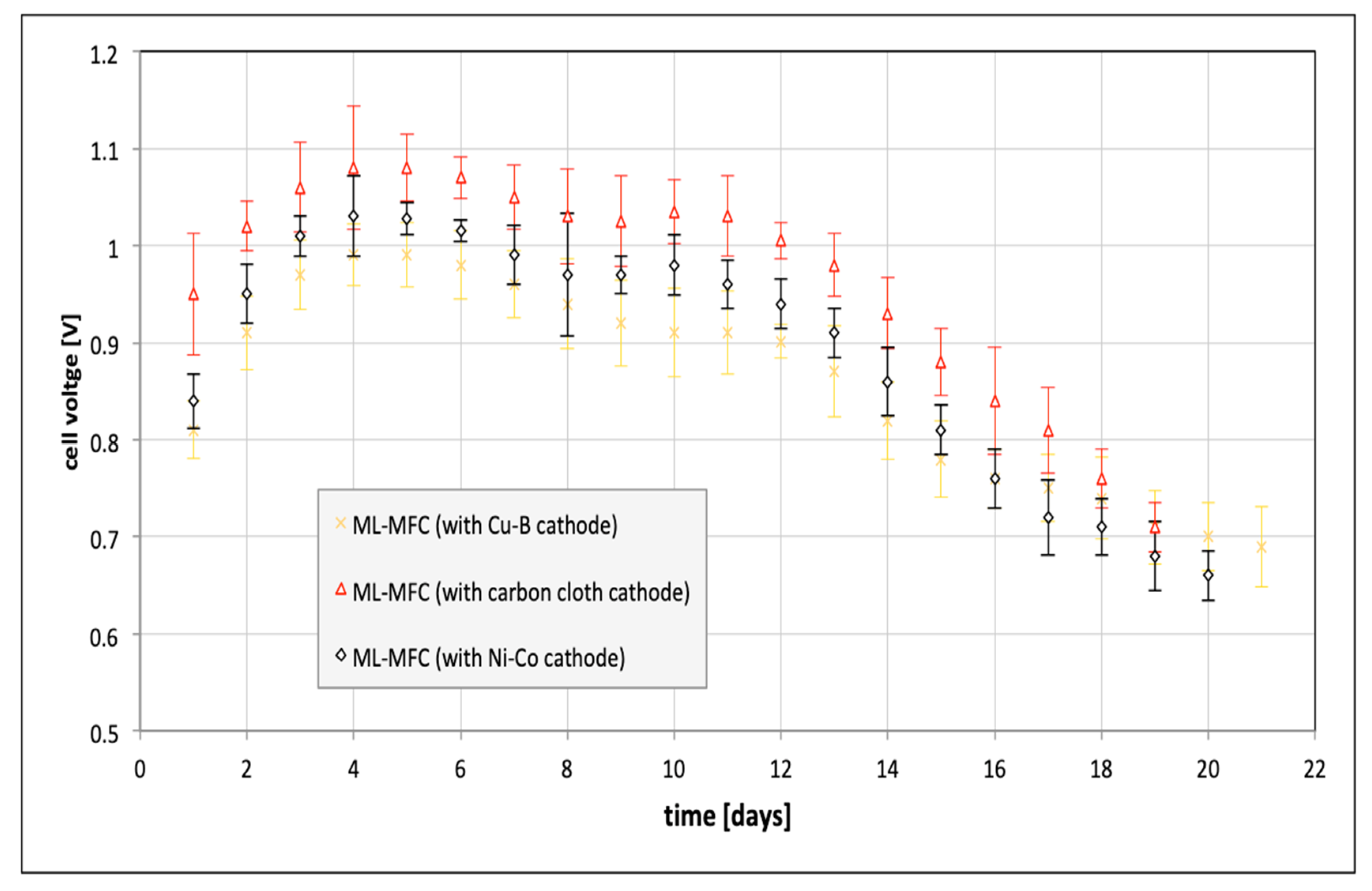
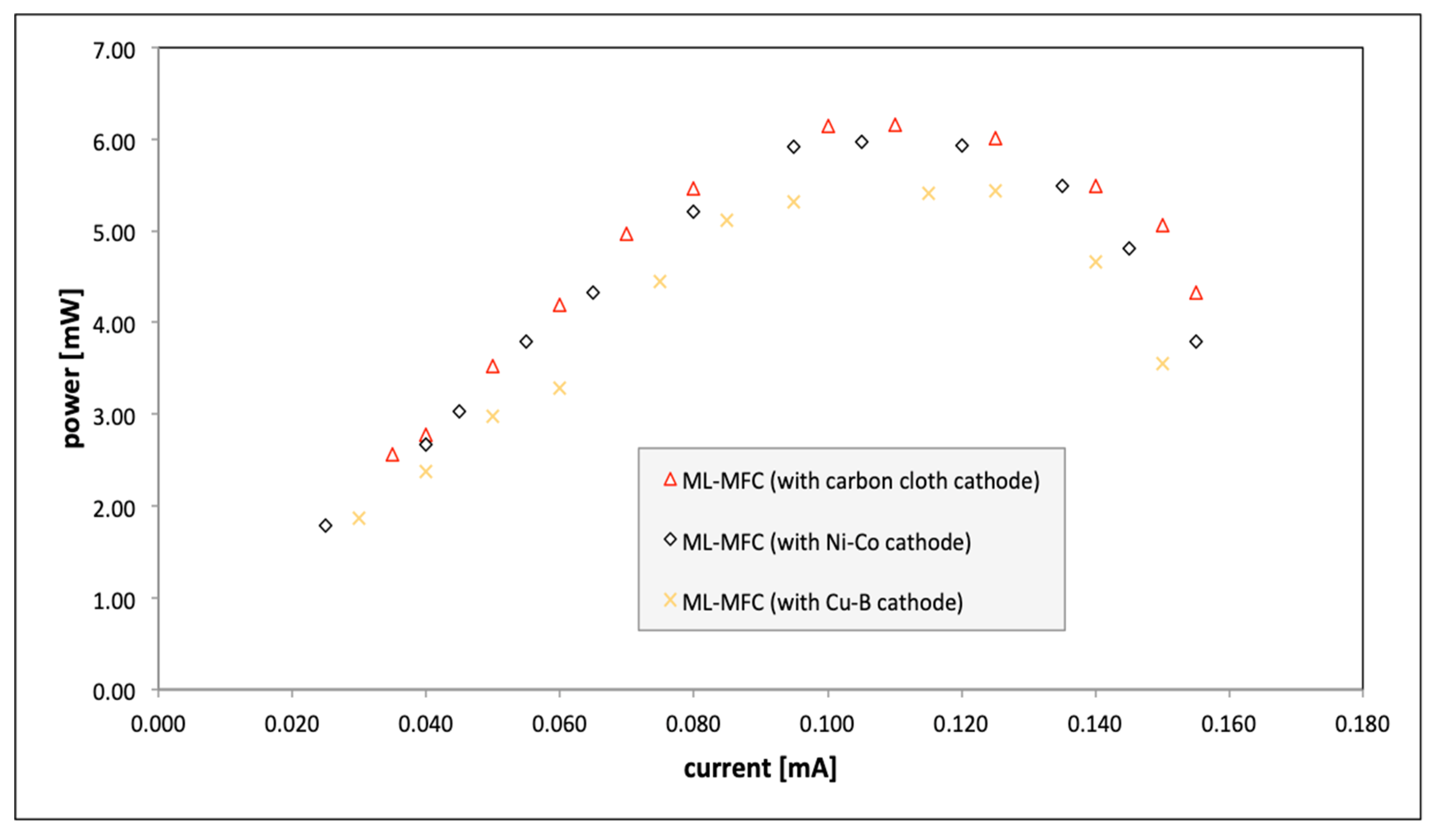
| Parameter | Value |
|---|---|
| COD [mg⋅L−1] | 10,500 ± 347 |
| NH4+ [mg⋅L−1] | 77.2 ± 3.3 |
| NO3− [mg⋅L−1] | 29.4 ± 1.8 |
| pH | 6.0–7.0 |
| Electrode | Current Density [mA·cm−2] | Power [mW] |
|---|---|---|
| carbon cloth | 0.19 | 5.99 |
| Ni–Co | 0.38 | 6.19 |
| Cu–B | 0.32 | 5.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, B.; Włodarczyk, P.P. The Membrane-Less Microbial Fuel Cell (ML-MFC) with Ni-Co and Cu-B Cathode Powered by the Process Wastewater from Yeast Production. Energies 2020, 13, 3976. https://doi.org/10.3390/en13153976
Włodarczyk B, Włodarczyk PP. The Membrane-Less Microbial Fuel Cell (ML-MFC) with Ni-Co and Cu-B Cathode Powered by the Process Wastewater from Yeast Production. Energies. 2020; 13(15):3976. https://doi.org/10.3390/en13153976
Chicago/Turabian StyleWłodarczyk, Barbara, and Paweł P. Włodarczyk. 2020. "The Membrane-Less Microbial Fuel Cell (ML-MFC) with Ni-Co and Cu-B Cathode Powered by the Process Wastewater from Yeast Production" Energies 13, no. 15: 3976. https://doi.org/10.3390/en13153976





