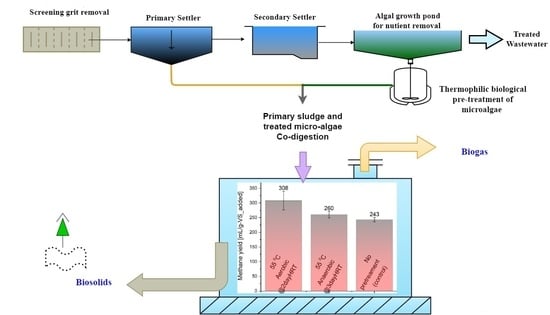
Synergistic Co-Digestion of Microalgae and Primary Sludge to Enhance Methane Yield from Temperature-Phased Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Substrate and Inoculum
2.1.1. Algal Biomass Preparation
2.1.2. Primary Sludge, Inoculum and the Sampling Plant
2.2. Phase One (Thermophilic Pre-Treatment of Microalgae)
2.3. Phase Two (Mesophilic Digestion) BMP Tests
2.4. Analytical Procedures and Equipment
2.5. Modeling Kinetics of BMP Assay
3. Results and Discussion
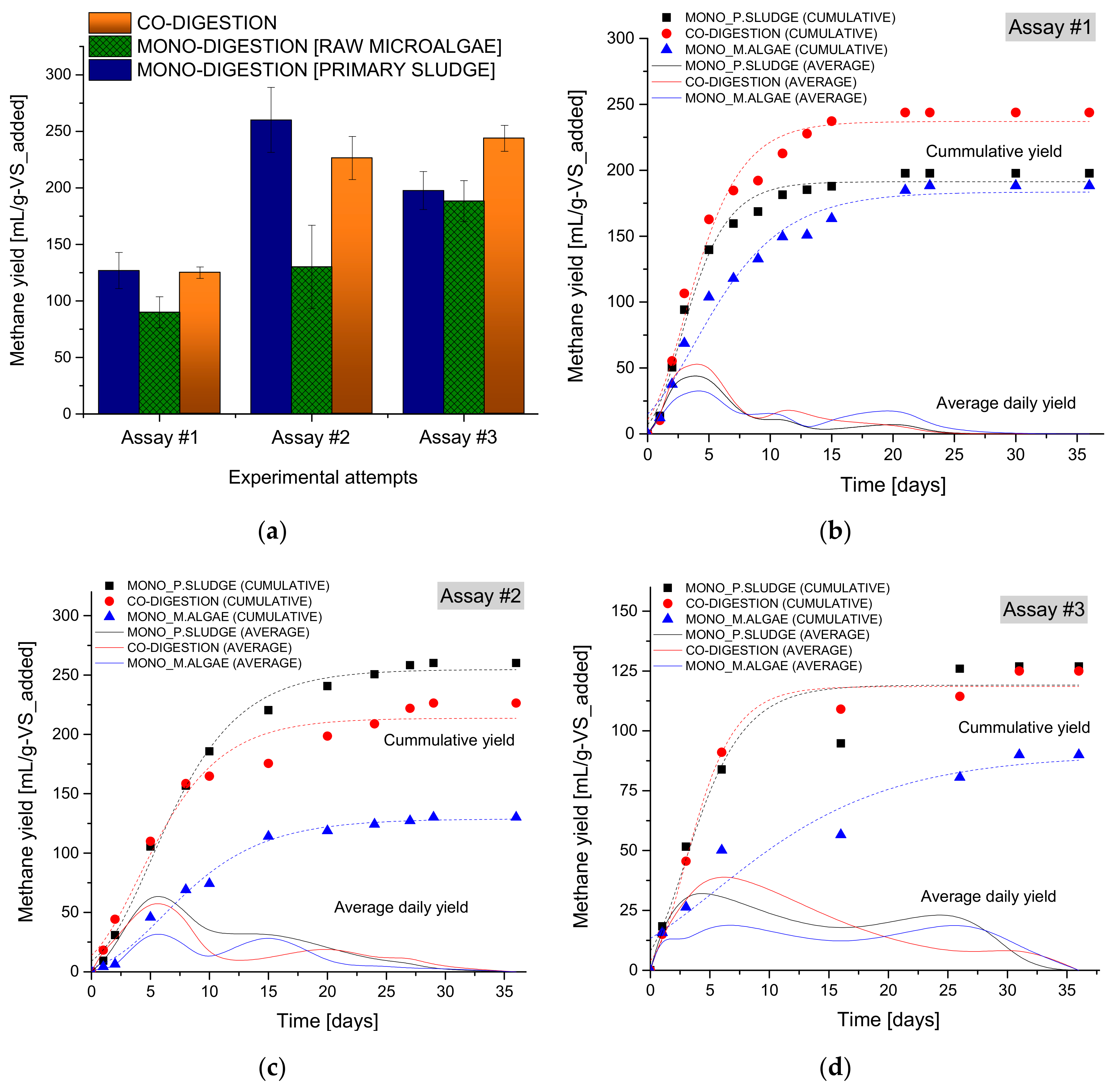
3.1. Effects of Co-Digestion on Methane Yield
3.2. The Dual-Effect of Co-Digestion and Pretreatment
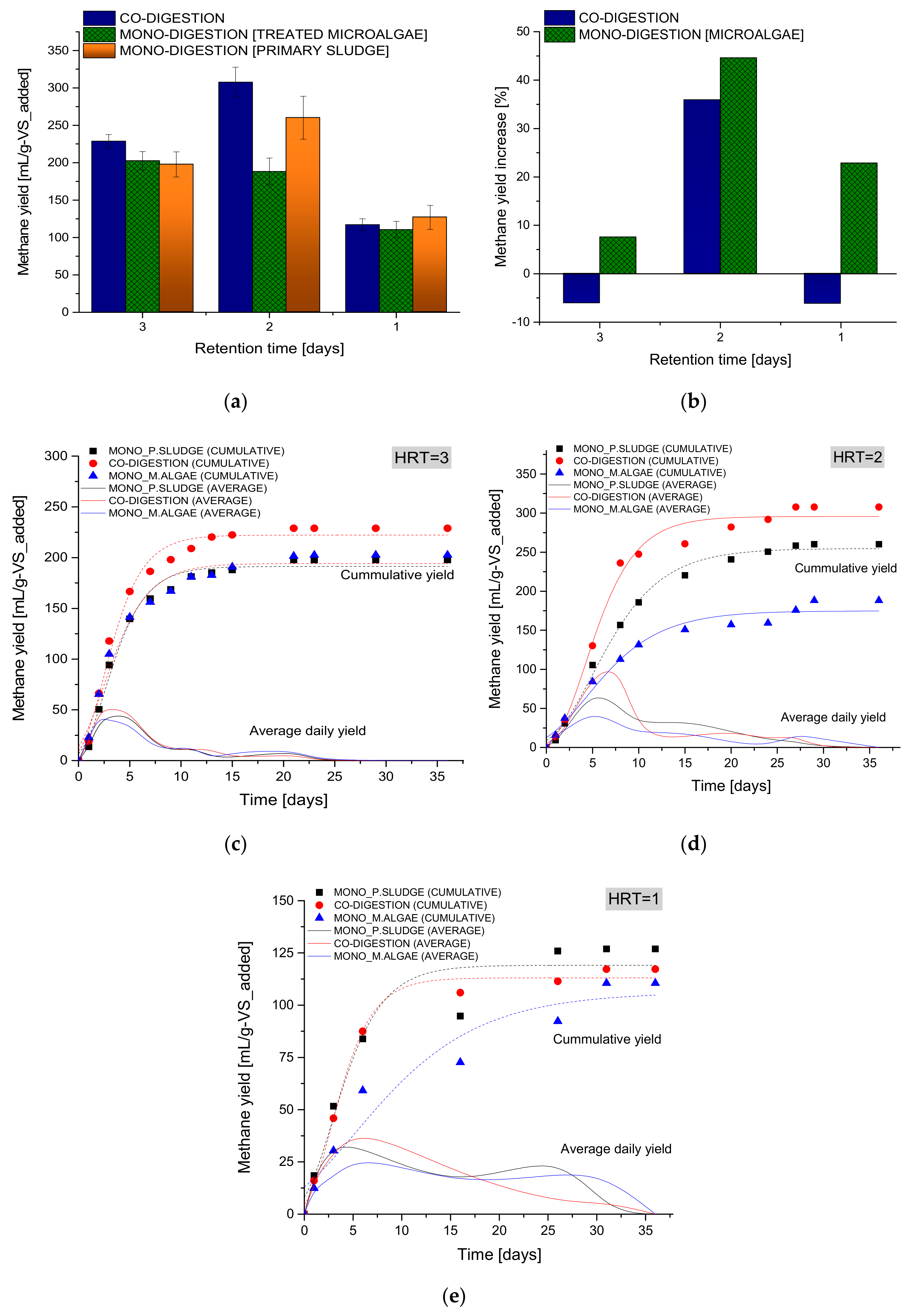
3.2.1. Co-Digestion after Aerobic Pretreatment
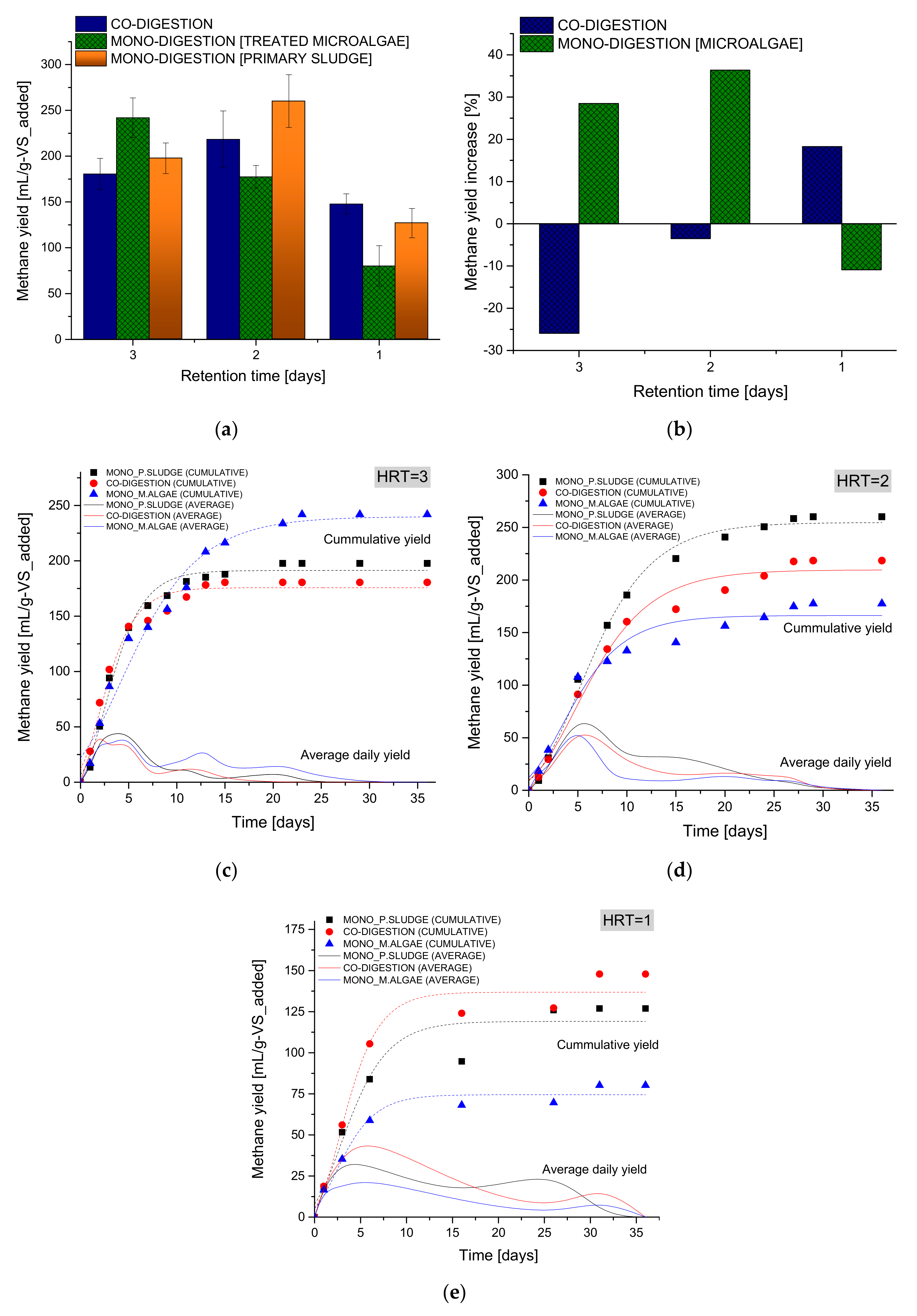
3.2.2. Co-Digestion after Anaerobic Pretreatment
3.2.3. Co-Digestion after 85 °C Anaerobic Pretreatment
3.3. Performance Comparison with Previous Works
3.4. Implications on the Circular Economy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghaffari, Y.; Gupta, N.K.; Bae, J.; Kim, K.S. One-step fabrication of Fe2O3/Mn2O3 nanocomposite for rapid photodegradation of organic dyes at neutral pH. J. Mol. Liq. 2020, 113691. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Yu, Z.M.; Liu, Y.; Jin, J.; Wu, K.; Deng, C.X.; Wei, W.; Wei, X.L.; Ni, B.-J. Green synthesis of Fe3O4@carbon filter media for simultaneous phosphate recovery and nitrogen removal from domestic wastewater in biological aerated filters. ACS Sustain. Chem. Eng. 2019, 7, 16698–16709. [Google Scholar] [CrossRef]
- Bao, T.; Yu, Z.M.; Damtie, M.M.; Wu, K.; Jin, J.; Zhang, Y.; Wei, X.L.; Frost, R.L. Use of autoclaved aerated concrete particles for simultaneous removal of nitrogen and phosphorus as filter media from domestic wastewater. Environ. Technol. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Kumar, N.; Kumar, V.; Negi, S.; Banerjee, C. Microalgae: A way forward approach towards wastewater treatment and bio-fuel production. In Applied Microbiology and Bioengineering; Shukla, P., Ed.; Academic Press: London, UK, 2019; pp. 229–243. ISBN 9780128154076. [Google Scholar]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Algaculture integration in conventional wastewater treatment plants: Anaerobic digestion comparison of primary and secondary sludge with microalgae biomass. Bioresour. Technol. 2015, 184, 236–244. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Garfí, M.; Ferrer, I. Pretreatment and co-digestion of microalgae, sludge and fat oil and grease (FOG) from microalgae-based wastewater treatment plants. Bioresour. Technol. 2020, 298, 122563. [Google Scholar] [CrossRef]
- Wang, Q.; Prasad, R.; Higgins, B.T. Aerobic bacterial pretreatment to overcome algal growth inhibition on high-strength anaerobic digestates. Water Res. 2019, 162, 420–426. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Phan, D.; Kopachevsky, A.M.; Chow, S.; Bouwer, E.J.; Betenbaugh, M.J. Synergistic co-digestion of wastewater grown algae-bacteria polyculture biomass and cellulose to optimize carbon-to-nitrogen ratio and application of kinetic models to predict anaerobic digestion energy balance. Bioresour. Technol. 2018, 269, 210–220. [Google Scholar] [CrossRef]
- Ge, H.; Jensen, P.D.; Batstone, D.J. Pre-treatment mechanisms during thermophilic–mesophilic temperature phased anaerobic digestion of primary sludge. Water Res. 2010, 44, 123–130. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Barragán-Trinidad, M.; Buitrón, G. Biological pretreatments of microalgal biomass for gaseous biofuel production and the potential use of rumen microorganisms: A review. Algal Res. 2016, 18, 341–351. [Google Scholar] [CrossRef]
- Lv, W.; Schanbacher, F.L.; Yu, Z. Putting microbes to work in sequence: Recent advances in temperature-phased anaerobic digestion processes. Bioresour. Technol. 2010, 101, 9409–9414. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Cho, H.U.; Park, S.K.; Ha, J.H.; Park, J.M. Influence of thermophilic aerobic digestion as a sludge pre-treatment and solids retention time of mesophilic anaerobic digestion on the methane production, sludge digestion and microbial communities in a sequential digestion process. Water Res. 2014, 48, 1–14. [Google Scholar] [CrossRef]
- Mshandete, A.; Björnsson, L.; Kivaisi, A.K.; Rubindamayugi, S.T.; Mattiasson, B. Enhancement of anaerobic batch digestion of sisal pulp waste by mesophilic aerobic pre-treatment. Water Res. 2005, 39, 1569–1575. [Google Scholar] [CrossRef]
- Wang, M.; Sahu, A.K.; Rusten, B.; Park, C. Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge. Bioresour. Technol. 2013, 142, 585–590. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Eskicioglu, C.; Garfí, M.; Carrère, H.; Ferrer, I. Anaerobic co-digestion of microalgal biomass and wheat straw with and without thermo-alkaline pretreatment. Bioresour. Technol. 2017, 237, 89–98. [Google Scholar] [CrossRef]
- Olsson, J.; Feng, X.M.; Ascue, J.; Gentili, F.G.; Shabiimam, M.A.; Nehrenheim, E.; Thorin, E. Co-digestion of cultivated microalgae and sewage sludge from municipal waste water treatment. Bioresour. Technol. 2014, 171, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jeon, J.H.; Shin, J.; Jang, H.M.; Kim, S.; Song, M.S.; Kim, Y.M. Quantitative and qualitative changes in antibiotic resistance genes after passing through treatment processes in municipal wastewater treatment plants. Sci. Total Environ. 2017, 605, 906–914. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF 2540 solids. In Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2018.
- Filer, J.; Ding, H.H.; Chang, S. Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of biochemical methane potential and kinetics on the anaerobic digestion of vegetable crop residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Lee, E.; Dilbeck, M.P.; Liebelt, M.; Zhang, Q.; Ergas, S.J. Thermal pretreatment of microalgae for biomethane production: Experimental studies, kinetics and energy analysis. J. Chem. Technol. Biotechnol. 2017, 92, 399–407. [Google Scholar] [CrossRef]
- Damtie, M.M.; Choi, J.-S.; Shon, H.K. Fluoride Removal and Nitrogen Recovery from Wastewater by Membrane Distillation Process. Ph.D. Thesis, University of Science and Technology (UST), Daejeon, Korea, 2020. [Google Scholar]
- Damtie, M.M.; Choi, J.-S. Modeling and application of direct contact membrane distillation for fluoride removal from aqueous solutions. Desalin. Water Treat. 2017, 97, 23–40. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Hosseinzadeh, A.; Frost, R.L.; Yu, Z.M.; Jin, J.; Wu, K. Catalytic degradation of P-chlorophenol by muscovite-supported nano zero valent iron composite: Synthesis, characterization, and mechanism studies. Appl. Clay Sci. 2020, 195, 105735. [Google Scholar] [CrossRef]
- Li, R.; Tan, W.; Zhao, X.; Dang, Q.; Song, Q.; Xi, B.; Zhang, X. Evaluation on the methane production potential of wood waste pretreated with NaOH and co-digested with pig manure. Catalysts 2019, 9, 539. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Bauddh, K.; Bux, F. (Eds.) Algae and environmental sustainability. In Developments in Applied Phycology; Springer: New Delhi, India, 2015; ISBN 9788132226390. [Google Scholar]
- Mendez, L.; Mahdy, A.; Timmers, R.A.; Ballesteros, M.; González-Fernández, C. Enhancing methane production of Chlorella vulgaris via thermochemical pretreatments. Bioresour. Technol. 2013, 149, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Carrère, H.; Garfí, M.; Ferrer, I. Enhancement of microalgae anaerobic digestion by thermo-alkaline pretreatment with lime (CaO). Algal Res. 2017, 24, 199–206. [Google Scholar] [CrossRef]
- Fuchs, A.; Lyautey, E.; Montuelle, B.; Casper, P. Effects of increasing temperatures on methane concentrations and methanogenesis during experimental incubation of sediments from oligotrophic and mesotrophic lakes. J. Geophys. Res. Biogeosci. 2016, 121, 1394–1406. [Google Scholar] [CrossRef] [Green Version]
- Schwede, S.; Rehman, Z.-U.; Gerber, M.; Theiss, C.; Span, R. Effects of thermal pretreatment on anaerobic digestion of Nannochloropsis salina biomass. Bioresour. Technol. 2013, 143, 505–511. [Google Scholar] [CrossRef]
- Bolzonella, D.; Cavinato, C.; Fatone, F.; Pavan, P.; Cecchi, F. High rate mesophilic, thermophilic, and temperature phased anaerobic digestion of waste activated sludge: A pilot scale study. Waste Manag. 2012, 32, 1196–1201. [Google Scholar] [CrossRef]
- Ge, H.; Jensen, P.D.; Batstone, D.J. Increased temperature in the thermophilic stage in temperature phased anaerobic digestion (TPAD) improves degradability of waste activated sludge. J. Hazard. Mater. 2011, 187, 355–361. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Salvadó, H.; Passos, F.; Garfí, M.; Ferrer, I. Strategies to optimize microalgae conversion to biogas: Co-digestion, pretreatment and hydraulic retention time. Molecules 2018, 23, 2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Kang, X.; Wang, Z.; Kong, X.; Li, L.; Sun, Y.; Zhu, S.; Feng, S.; Luo, X.; Lv, P. Enhancement of the energy yield from microalgae via enzymatic pretreatment and anaerobic co-digestion. Energy 2018, 164, 400–407. [Google Scholar] [CrossRef]
- Du, X.; Tao, Y.; Liu, Y.; Li, H. Stimulating methane production from microalgae by alkaline pretreatment and co-digestion with sludge. Environ. Technol. 2020, 41, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Anaerobic co-digestion on improving methane production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: Kinetic modeling and synergistic impact evaluation. Chem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Zamorano-López, N.; Borrás, L.; Seco, A.; Aguado, D. Unveiling microbial structures during raw microalgae digestion and co-digestion with primary sludge to produce biogas using semi-continuous AnMBR systems. Sci. Total Environ. 2020, 699, 134365. [Google Scholar] [CrossRef]
- Serna-García, R.; Zamorano-López, N.; Seco, A.; Bouzas, A. Co-digestion of harvested microalgae and primary sludge in a mesophilic anaerobic membrane bioreactor (AnMBR): Methane potential and microbial diversity. Bioresour. Technol. 2020, 298, 122521. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Garfí, M.; Matamoros, V.; Ferrer, I. Co-digestion of microalgae and primary sludge: Effect on biogas production and microcontaminants removal. Sci. Total Environ. 2019, 660, 974–981. [Google Scholar] [CrossRef]
- Cuadros-Blázquez, F.; González-González, A.; Sánchez-Sánchez, C.; Díaz-Rodríguez, V.; Cuadros-Salcedo, F. Waste valorization as an example of circular economy in extremadura (Spain). J. Clean. Prod. 2018, 181, 136–144. [Google Scholar] [CrossRef]
- Kisser, J.; Wirth, M.; de Gusseme, B.; van Eekert, M.; Zeeman, G.; Schoenborn, A.; Vinnerås, B.; Finger, D.C.; Kolbl-Repinc, S.; Bulc, T.G.; et al. A review of nature-based solutions for resource recovery in cities. Blue Green Syst. 2020, 2, 138–172. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Microalgae | Primary Sludge | Inoculum |
|---|---|---|---|
| pH | 7.7 | 6.1 | 7.61 |
| Total solids, TS (g/L) | 114.3 ± 0.9> | 49.3 ± 7.2 | 27.77 ± 0.8 |
| Volatile solids, VS (g/L) | 108.3 ± 0.2 | 33.6 ± 4.8 | 17.30 ± 2.1 |
| Total chemical oxygen demand, tCOD (g/L) | 103.5 ± 0.2 | 87.0 ± 4.4 | 14.31 ± 0.3 |
| Soluble chemical oxygen demand, sCOD (g/L) | 5.4 ± 0.0 | 11.2 ± 0.9 | 4.25 |
| Soluble carbohydrate (g/L) | 2.1 ± 0.1 | 0.8 ± 0.0 | - |
| Soluble protein (g/L) | 1.1 ± 0.1 | 2.7 ± 0.2 | 2.66 ± 0.4 |
| Model * Para Meters | ** Assay#1 | Assay#2 | Assay#3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mono-p.sludge | Mono-m.algae | Co-Digestion | Mono-p.sludge | Mono-m.algae | Co-Digestion | Mono-p.sludge | Mono-m.algae | Co-Digestion | |
| Go | 191.28 | 183.51 | 236.96 | 254.68 | 128.76 | 213.63 | 119.10 | 90.06 | 118.53 |
| λ | 0.44 | 0.34 | 0.33 | 0.85 | 1.32 | 0.04 | 0.03 | 2.95 | 0.52 |
| Rmax | 32.25 | 16.45 | 33.24 | 22.43 | 9.97 | 20.05 | 15.30 | 3.96 | 18.49 |
| R2 | 0.987 | 0.973 | 0.978 | 0.994 | 0.990 | 0.973 | 0.937 | 0.907 | 0.984 |
| * Model Para Meters | HRT = 3 | HRT = 2 | HRT = 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mono-p.sludge | Mono-m.algae | Co-Digestion | Mono-p.sludge | Mono-m.algae | Co-Digestion | Mono-p.sludge | Mono-m.algae | Co-Digestion | |
| Go | 191.27 | 194.24 | 222.2 | 254.68 | 174.86 | 295.68 | 119.10 | 106.17 | 113.06 |
| λ | 0.44 | 0.06 | 0.34 | 0.85 | 0.17 | 1.26 | 0.03 | 1.84 | 0.41 |
| Rmax | 32.25 | 29.17 | 38.8 | 22.43 | 14.33 | 35.86 | 15.30 | 5.49 | 17.67 |
| R2 | 0.987 | 0.975 | 0.985 | 0.99 | 0.973 | 0.99 | 0.937 | 0.917 | 0.99 |
| Model Parameters | HRT = 3 | HRT = 2 | HRT = 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mono-p.sludge | Mono-m.algae | Co-Digestion | Mono-p.sludge | Mono-m.algae | Co-Digestion | Mono-p.sludge | Mono-m.algae | Co-Digestion | |
| Go | 190.02 | 205.15 | 256.66 | 254.68 | 189.11 | 194.19 | 119.10 | 102.56 | 115.82 |
| λ | 0.47 | 0.27 | 0.63 | 0.85 | 0.18 | 0.74 | 0.03 | 0.15 | 0.44 |
| Rmax | 32.79 | 30.71 | 48.38 | 22.43 | 18.85 | 18.34 | 15.31 | 13.59 | 17.63 |
| R2 | 0.987 | 0.972 | 0.998 | 0.993 | 0.974 | 0.989 | 0.937 | 0.933 | 0.975 |
| Model Parameters | HRT = 3 | HRT = 2 | HRT = 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mono-p.sludge | Mono-m.algae | Co-Digestion | Mono-p.sludge | Mono-m.algae | Co-Digestion | Mono-p.sludge | Mono-m.algae | Co-Digestion | |
| Go | 191.28 | 239.93 | 175.72 | 254.69 | 166.21 | 209.61 | 119.10 | 74.48 | 136.85 |
| λ | 0.44 | 0.60 | 0.03 | 0.85 | 0.15 | 0.50 | 0.03 | 0.07 | 0.40 |
| Rmax | 32.24 | 18.94 | 31.46 | 22.43 | 17.35 | 18.13 | 15.31 | 11.40 | 21.28 |
| R2 | 0.987 | 0.973 | 0.977 | 0.994 | 0.96 | 0.983 | 0.9367 | 0.964 | 0.969 |
| SN | Type of Process | Type of Substrate | Experimental Conditions | Methane Yield | Reference |
|---|---|---|---|---|---|
| 1 | Thermal pretreatment and co-digestion | Microalgae, primary sludge and FOG | Thermal pretreatment (75 °C for 10 h), BMP Temp = 35 °C VS-based microalgae to sludge ratio = 50–50% | 237 ± 1, 298 ± 12, and 334 ± 1 mL CH4/gVS for FOG = 0%, FOG = 10%, and FOG = 20% resp. | [7] |
| 2 | Thermal pretreatment and co-digestion | Microalgae and primary sludge | Thermal pretreatment (120 °C for 40 min), BMP Temp = 35 °C, Microalgae and sludge ratio = varying | 261.7, 282.8 and 293.4 mL CH4/COD for ratio = 75%/25%, 50%/50% and 25%/75% resp. | [6] |
| 3 | Thermal pretreatment and co-digestion | Microalgae and Secondary sludge | Thermal pretreatment (120 °C for 40 min), BMP Temp = 35 °C, Microalgae and secondary sludge ratio = varying | ~150, 136 and 102 mL CH4/COD for ratio = 75%/25%, 50%/50% and 25%/75% resp. | |
| 4 | Thermo-alkaline pretreatment and co-digestion | Microalgal biomass and wheat straw | Thermo-alkaline pretreatment (72 °C for 24 h), HRT = 20 days, Total operation = 106 days, OLR = 1.5 g VS/L·day, BMP Temp = 37 ± 1 °C, microalgae to straw ratio (50–50%) | 0.24 L CH4/g vs. | [17] |
| 5 | Thermal pretreatment and co-digestion | Microalgae and primary sludge | Thermal pretreatment (75 °C for 10 h), HRT = 20/30 days, Microalgae/sludge ratio = 25%/75%, BMP Temp = 37 ± 1 °C, OLR = 1.17 (g VS/L·day) | 0.46 ± 0.27L CH4/g VS) | [35] |
| 6 | Enzymatic pretreatment and co-digestion | algal residues and Pennisetum hybrid energy grass | Mixed enzyme (Cellulase, Xynalase, Pectinase) algal to grass ratio = 1:3 (VS-based), BMP Temp = 35 °C | 207.35 ± 15.66 mLCH4/gVS | [36] |
| 7 | Alkaline pretreatment and co-digestion | Microalgae and mixed primary secondary sludge | Alkaline treatment (0.1 mol/L NaOH for 12 h), Microalgae to sludge ratio 2:1, BMP Temp = 35 ± 1°C, one month experiment | 298 mLCH4/gVS | [37] |
| 8 | Temperature phased Anaerobic co-digestion | Activated sludge | Pretreatment HRT=2 days pH=7, Temp = varying, BMP Temp = 37 °C | 160 mL/gVS and 300 mL/gVS for 50 °C and 65 °C resp. | [34] |
| 9 | Temperature phased co-digestion | Microalgae with primary sludge | Thermophilic Aerobic (HRT = 2 days) and anaerobic (HRT = 3 days) pretreatment at 55 °C, Sludge/algae ratio = 9 & ISR = 2, BMP Temp = 35 °C | 308 mL/g-VS and 260 mL/g-VS | This study |
| 10 | Only co-digestion | mixed microalgae and food waste | BMP Temp = 35 °C, microalgae to food waste ratio = 0.2:0.8, reaction day = 40 days | 639.8 ± 1.3 mL/g VS_added | [38] |
| 11 | Only co-digestion | Microalgae and primary sludge | AnMBR, SRT = 100 days, HRT = 30 days, OLR = 0.5 gVS/L d, Sludge to microalgae ratio = 62%:38%, BMP Temp = 35 °C | 228 ± 3 and 241 ± 18 mLCH4/gCOD for Vulgaris and Scenedesmus resp. | [39,40] |
| 12 | Only co-digestion | Microalgae and primary sludge | Microalgae to sludge ratio (25%/75% vs.-based) HRT = 20 days, BMP Temp = 37 ± 1 °C, OLR = 1.89 ± 0.26 kg VS/m3·day | 0.33 ± 0.05 m3 CH4/kg VS | [41] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damtie, M.M.; Shin, J.; Jang, H.M.; Kim, Y.M. Synergistic Co-Digestion of Microalgae and Primary Sludge to Enhance Methane Yield from Temperature-Phased Anaerobic Digestion. Energies 2020, 13, 4547. https://doi.org/10.3390/en13174547
Damtie MM, Shin J, Jang HM, Kim YM. Synergistic Co-Digestion of Microalgae and Primary Sludge to Enhance Methane Yield from Temperature-Phased Anaerobic Digestion. Energies. 2020; 13(17):4547. https://doi.org/10.3390/en13174547
Chicago/Turabian StyleDamtie, Mekdimu Mezemir, Jingyeong Shin, Hyun Min Jang, and Young Mo Kim. 2020. "Synergistic Co-Digestion of Microalgae and Primary Sludge to Enhance Methane Yield from Temperature-Phased Anaerobic Digestion" Energies 13, no. 17: 4547. https://doi.org/10.3390/en13174547