Investigating the Potential for Increased Energy Utilisation and Reduced CO2 Emissions at Mo Industrial Park
Abstract
:1. Introduction
- Stream data for temperature and enthalpy flows (, , ) and data on utility consumption was collected.
- Steam from by-product incineration was also considered as a source for process heat.
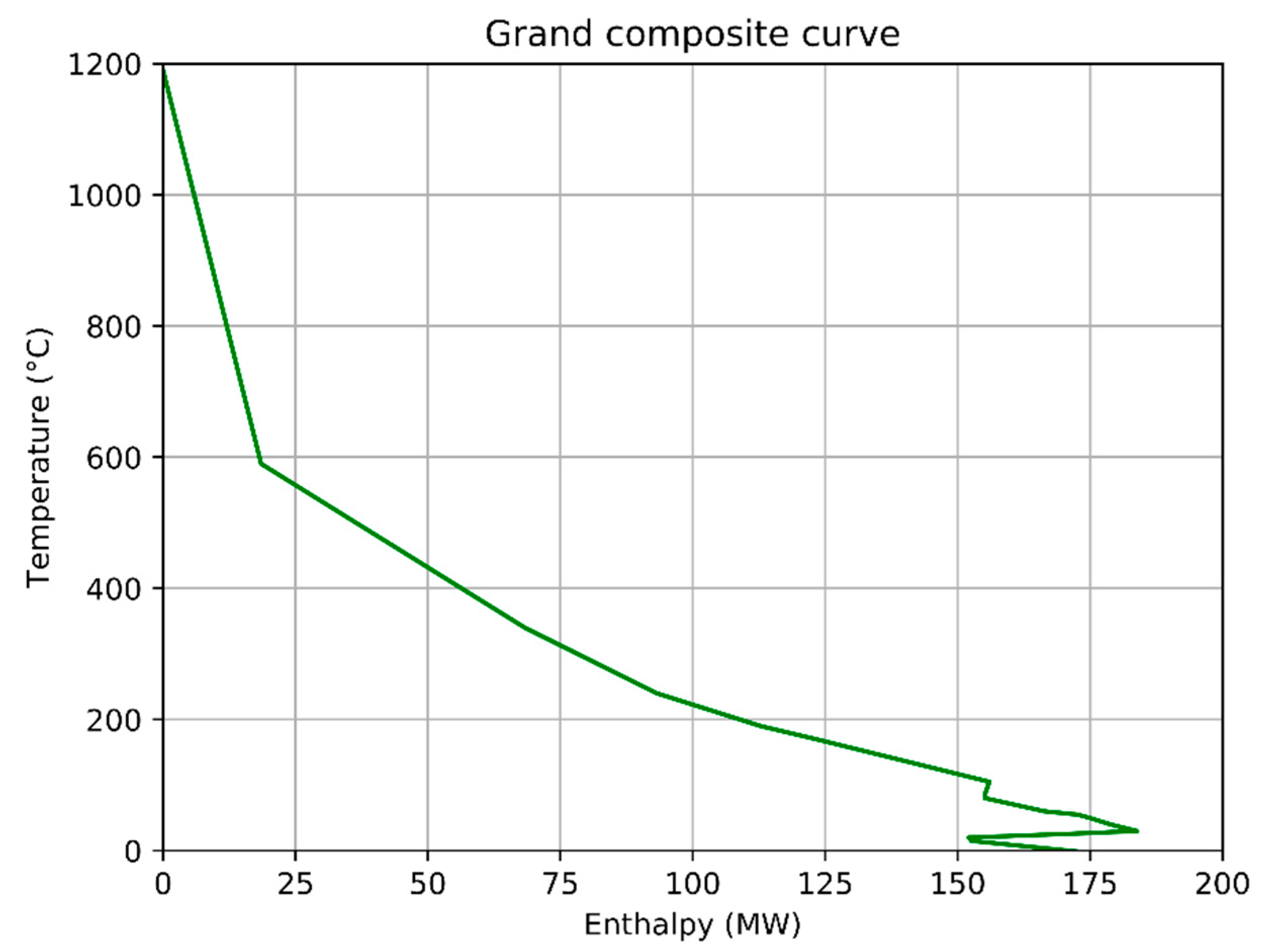
- The data was presented in so-called total site profiles (TSP) and total site composite curves (TSC). This analysis provided information on the site pinch temperature and was used to identify the most attractive measures to increase heat recovery.
- The sitewide potential for co-generation and measure for reducing the external cooling demand below ambient temperature was also analysed.
2. Methodology
2.1. Data Collection and Analysis
- “MIP CO nettverk” which redistributes carbon monoxide (CO)-rich flue gas (204 GWh) from Ferroglobe for heating purposes at Celsa, SMA Minerals, and Mo Fjernvarme
- “Mo Fjernvarme” which recovers latent heat from flue gas from Elkem (125 GWh) for use as district heating
- “Ranfjord fiskeprodukter” which utilises cooling water from Elkem (40 GWh).
2.2. Modelling
2.2.1. Silicon and Ferrosilicon Production
2.2.2. Waste Heat Recovery Unit
2.2.3. Carbon Capture
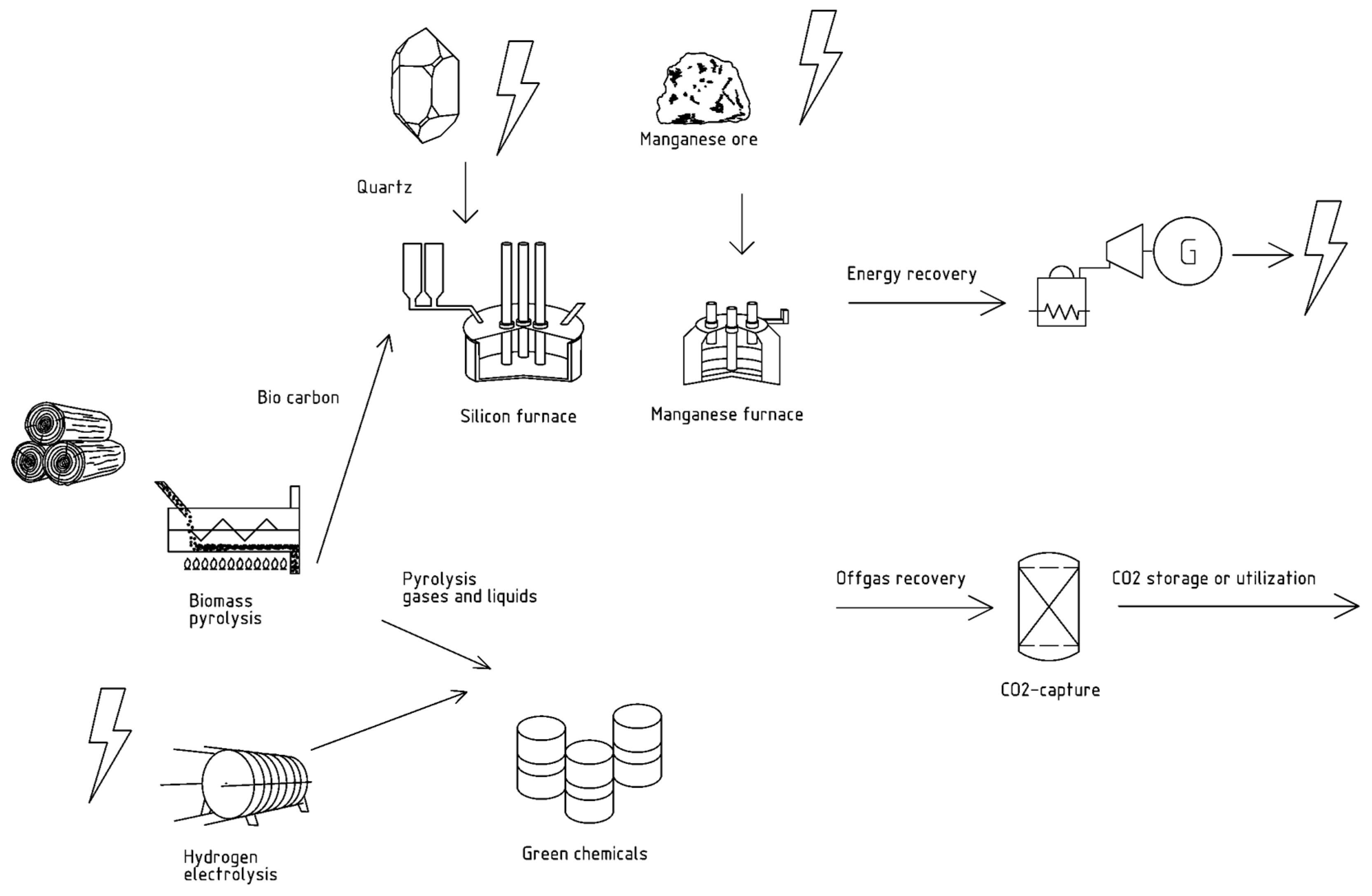
2.2.4. Biocarbon Production
2.3. Key Performance Indicators
- CO2 footprint as kg CO2 equivalents per kg metal produced (kgCO2/kg Me)
- Net electricity consumption as kWh(el) per kg metal produced (kWh/kg Me). Net electricity consumption is electricity input to SAF minus electricity produced in the waste heat recovery steam cycle
- Electricity and CO2 emission costs per kg metal produced (EUR/kg Me).
3. Potential New Energy Clients
3.1. Ferrosilicon Production
3.1.1. Semi-Closed Furnace with Recirculated Flue Gas
3.1.2. Biocarbon Production
3.1.3. CO2 Capture
3.1.4. Tradeoffs between Biocarbon Production and CO2 Capture
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CCS | Carbon capture and storage |
| FeMn | Ferromanganeses production |
| FeSi | Ferrosilicon production |
| KPI | Key performance indicator |
| MEA | Monoethanolamine |
| MIP | Mo industrial park |
| SAF | Submerged arch furnace |
| SRD | Specific reboiler duty (for regeneration of CO2 absorbent) |
Appendix A. Stream Data for MIP
| Process Stream | Tstart (°C) | Tend (°C) | kW/C | MW | GWh |
|---|---|---|---|---|---|
| Aga-Heat loss | 50 | 5 | 111 | 5 | 39 |
| BitFury-Heat loss to air | 70 | 5 | 683 | 44 | 350 |
| Celsa-Off gas | 250 | 25 | 63 | 14 | 112 |
| Celsa-Cooling water | 10 | 20 | 3439 | −34 | −271 |
| Celsa-Heat loss: Solids& Gas | 250 | 5 | 80 | 20 | 154 |
| Elkem-Mo Fjernvarme: Off gas to district heating | 350 | 180 | 81 | 14 | 108 |
| Elkem-Off gas pipe loss: Flue gas | 600 | 350 | 168 | 42 | 332 |
| Elkem-Off gas cooling: | 350 | 180 | 88 | 15 | 118 |
| Elkem-Off gas, unavoidable heat loss: | 180 | 5 | 149 | 26 | 206 |
| Elkem-Cooling water | 5 | 45 | 699 | −28 | −220 |
| Elkem-Heat loss | 350 | 5 | 50 | 17 | 136 |
| Ferroglobe-Heat in solids | 1200 | 5 | 31 | 37 | 291 |
| Ferroglobe-Cooling water | 5 | 45 | 88 | −4 | −28 |
| Ferroglobe-Heat loss | 200 | 60 | 130 | 18 | 144 |
| Mo Fjernvarme-Elkem | 70 | 95 | 548 | −14 | −108 |
| Mo Fjernvarme-Heat loss: Disposed heat | 95 | 5 | 49 | 4 | 35 |
| Mo Fjernvarme-Heat loss | 95 | 5 | 9 | 1 | 7 |
| Ranfjord fisk-Cooling water | 5 | 16 | 404 | −4 | −35 |
| MIP CO distribution network-Heat loss | 60 | 5 | 64 | 4 | 28 |
| Process Stream | Tstart (°C) | Tend (°C) | kW/C | MW | GWh |
|---|---|---|---|---|---|
| Aga-Heat loss | 50 | 5 | 111 | 5 | 39 |
| BitFury-Heat loss | 70 | 5 | 683 | 44 | 350 |
| Celsa-Off gas | 250 | 25 | 63 | 14 | 112 |
| Celsa-Cooling water | 10 | 20 | 3439 | −34 | −271 |
| Celsa-Heat loss: Solids& Gas | 250 | 5 | 80 | 20 | 154 |
| Elkem-Mo Fjernvarme: Off gas to district heating | 350 | 180 | 81 | 14 | 108 |
| Elkem-Waste heat recovery: Flue gas | 600 | 350 | 168 | 42 | 332 |
| Elkem-Waste heat recovery | 350 | 180 | 88 | 15 | 118 |
| Elkem-Off gas, unavoidable heat loss | 180 | 5 | 149 | 26 | 206 |
| Elkem-Cooling water | 5 | 45 | 699 | −28 | −220 |
| Elkem-Heat loss | 350 | 5 | 50 | 17 | 136 |
| Ferroglobe-Heat in solids | 1200 | 5 | 31 | 37 | 291 |
| Ferroglobe-Cooling water | 5 | 45 | 88 | −4 | −28 |
| Ferroglobe-Heat loss | 200 | 60 | 130 | 18 | 144 |
| Mo Fjernvarme-Elkem | 70 | 95 | 548 | −14 | −108 |
| Mo Fjernvarme-Heat loss: Disposed heat | 95 | 5 | 49 | 4 | 35 |
| Mo Fjernvarme-Heat loss | 95 | 5 | 9 | 1 | 7 |
| Ranfjord fisk-Cooling water | 45 | 5 | 111 | 4 | 35 |
| MIP CO distribution network-Heat loss | 60 | 5 | 64 | 4 | 28 |
| Elkem-CCS | 150 | 250 | 260 | −28 | −221 |
| Elkem-Biocarbon | 150 | 250 | 80 | −14 | −110 |
| Elkem-Heat to electricity | 45 | 420 | −13.5 | −106 |
| Source | Target | Energy Flow (GWh) |
|---|---|---|
| Electricity | Aga | 50 |
| Aga | Refrigerated product | 11 |
| Aga | Heat loss | 39 |
| Electricity | BitFury | 350 |
| BitFury | Heat loss | 350 |
| Electricity | Celsa | 352 |
| Coal/Char/Coke | Celsa | 49 |
| Gas/oil | Celsa | 54 |
| MIP CO nettverk | Celsa | 84 |
| Celsa | Off gas | 112 |
| Celsa | Cooling water | 271 |
| Celsa | Heat loss | 154 |
| Electricity | Elkem | 785 |
| Coal/Char/Coke | Elkem | 860 |
| Biocarbon (current) | Elkem | 49 |
| Gas/oil | Elkem | 2 |
| Elkem | Mo Fjernvarme | 108 |
| Elkem | Waste heat recovery | 332 |
| Elkem | Waste heat recovery | 118 |
| Elkem | Off gas, unavoidable heat loss | 206 |
| Elkem | Chemical energy in product | 576 |
| Elkem | Cooling water | 220 |
| Elkem | Heat loss | 136 |
| Electricity | Ferroglobe | 521 |
| Coal/Char/Coke | Ferroglobe | 599 |
| Ferroglobe | MIP CO nettverk | 174 |
| Ferroglobe | MIP CO nettverk | 174 |
| Ferroglobe | Chemical energy in product | 483 |
| Ferroglobe | Heat in solids | 291 |
| Ferroglobe | Cooling water | 28 |
| Ferroglobe | Heat loss | 144 |
| Electricity | Mo Fjernvarme | 3 |
| Elkem | Mo Fjernvarme | 108 |
| MIP CO nettverk | Mo Fjernvarme | 8 |
| Gas/oil | Mo Fjernvarme | 6 |
| Mo Fjernvarme | District heating | 83 |
| Mo Fjernvarme | Heat loss | 35 |
| Mo Fjernvarme | Heat loss | 7 |
| Elkem | Cooling water | 220 |
| Cooling water | Ranfjord fisk | 35 |
| Cooling water | Heat loss | 484 |
| Electricity | Ranfjord fisk | 5 |
| Cooling water | Ranfjord fisk | 35 |
| Ranfjord fisk | Heat loss | 40 |
| Ferroglobe | MIP CO nettverk | 174 |
| MIP CO nettverk | Celsa | 84 |
| MIP CO nettverk | Mo Fjernvarme | 8 |
| MIP CO nettverk | SMA Minerals | 42 |
| MIP CO nettverk | CO Fakling MIP | 42 |
| Heat | MIP CO nettverk | 28 |
| MIP CO nettverk | Heat loss | 28 |
| Electricity | SMA Minerals | 5 |
| MIP CO nettverk | SMA Minerals | 42 |
| SMA Minerals | Heat loss | 46 |
| Elkem | Waste heat recovery | 332 |
| Elkem | Waste heat recovery | 118 |
| Waste heat recovery | Biocarbon Production | 110 |
| Waste heat recovery | Carbon capture | 221 |
| Waste heat recovery | Electricity out | 21 |
| Waste heat recovery | Heat loss | 97 |
| Biomass | Biocarbon Production | 231 |
| Biocarbon Production | Biocarbon | 146 |
| Biocarbon Production | Bio oil | 81 |
| Waste heat recovery | Biocarbon Production | 110 |
| Biocarbon Production | Heat loss | 114 |
| Electricity | Carbon capture | 30 |
| Waste heat recovery | Carbon capture | 221 |
| Carbon capture | Heat loss | 251 |
Appendix B. Quantifying the Surplus Heat
Appendix B.1. General Assumptions
| Energy Carrier | Heating Value (MJ/kg) [53] |
|---|---|
| Diesel | 45.6 |
| Electrode mass | 26.0 |
| Anthracite (coal) | 32.5 |
| Coke | 26.0 |
| Petroleum coke | 31.3 |
| Propane | 50.4 |
| CO | 10.1 |
| Light fuel oil | 44.0 |
| Residual oil | 39.5 |
| Biocarbon | 29.6 |
| Hydrogen | 120 |
Appendix B.2. Celsa
Appendix B.3. Elkem
Appendix B.4. Ferroglobe
Appendix B.5. Other Actors
References
- International Energy Agency (IEA). Tracking Industry Technical Report; IEA: Paris, France, 2019. [Google Scholar]
- Fischedick, M.; Roy, J.; Abdel-Aziz, A.; Acquaye, A.; Allwood, J.; Ceron, J.; Geng, Y.; Kheshgi, H.; Lanza, A.; Perczyk, D.; et al. Chapter 10: Industry. In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 138–160. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Chertow, M.R. Industrial Symbiosis: Literature and Taxonomy. Annu. Rev. Energy Environ. 2000, 25, 313–337. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Tian, J.; Chertow, M.; Chen, L. Exploring Greenhouse Gas-Mitigation Strategies in Chinese Eco-Industrial Parks by Targeting Energy Infrastructure Stocks. J. Ind. Ecol. 2018, 22, 106–120. [Google Scholar] [CrossRef]
- Røyne, F.; Hackl, R.; Ringström, E.; Berlin, J. Environmental Evaluation of Industry Cluster Strategies with a Life Cycle Perspective: Replacing Fossil Feedstock with Forest-Based Feedstock and Increasing Thermal Energy Integration. J. Ind. Ecol. 2018, 22, 694–705. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Pu, G.; Qi, H. Integrated assessment of exergy, energy and carbon dioxide emissions in an iron and steel industrial network. Appl. Energy 2016, 183, 430–444. [Google Scholar] [CrossRef]
- Yu, B.; Li, X.; Shi, L.; Qian, Y. Quantifying CO2 emission reduction from industrial symbiosis in integrated steel mills in China. J. Clean. Prod. 2015, 103, 801–810. [Google Scholar] [CrossRef]
- Johansson, M.T.; Söderström, M. Options for the Swedish steel industry—Energy efficiency measures and fuel conversion. Energy 2011, 36, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Rene, E.R.; Choi, S.M.; Chiu, A.S. Strategies for sustainable development of industrial park in Ulsan, South Korea—From spontaneous evolution to systematic expansion of industrial symbiosis. J. Environ. Manag. 2008, 87, 1–13. [Google Scholar] [CrossRef]
- Gibbs, D.; Deutz, P. Reflections on implementing industrial ecology through eco-industrial park development. J. Clean. Prod. 2007, 15, 1683–1695. [Google Scholar] [CrossRef]
- Mattila, T.; Lehtoranta, S.; Sokka, L.; Melanen, M.; Nissinen, A. Methodological Aspects of Applying Life Cycle Assessment to Industrial Symbioses. J. Ind. Ecol. 2012, 16, 51–60. [Google Scholar] [CrossRef]
- Saidani, M.; Yannou, B.; Leroy, Y.; Cluzel, F.; Kendall, A. A taxonomy of circular economy indicators. J. Clean. Prod. 2019, 207, 542–559. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.H.; Kim, S.H.; Yoon, S.G.; Park, S. Optimization of a waste heat utilization network in an eco-industrial park. Appl. Energy 2010, 87, 1978–1988. [Google Scholar] [CrossRef]
- Buoro, D.; Casisi, M.; De Nardi, A.; Pinamonti, P.; Reini, M. Multicriteria optimization of a distributed energy supply system for an industrial area. Energy 2013, 58, 128–137. [Google Scholar] [CrossRef]
- Yeo, Z.; Masi, D.; Low, J.S.C.; Ng, Y.T.; Tan, P.S.; Barnes, S. Tools for promoting industrial symbiosis: A systematic review. J. Ind. Ecol. 2019, 23, 1087–1108. [Google Scholar] [CrossRef] [Green Version]
- Dhole, V.; Linnhoff, B. Total site targets for fuel, co-generation, emissions, and cooling. Comput. Chem. Eng. 1993, 17, 101–109. [Google Scholar] [CrossRef]
- Linnhoff, B.; Townsend, D.; Hewitt, G.; Thomas, B.; Guy, A.; Marsland, R. A User Guide on Process Integration for the Efficient Use of Energy; Institution of Chemical Engineers: Rugby, UK, 1983. [Google Scholar]
- Klemeš, J.; Dhole, V.; Raissi, K.; Perry, S.; Puigjaner, L. Targeting and design methodology for reduction of fuel, power and CO2 on total sites. Appl. Therm. Eng. 1997, 17, 993–1003. [Google Scholar] [CrossRef]
- Matsuda, K.; Hirochi, Y.; Tatsumi, H.; Shire, T. Applying heat integration total site based pinch technology to a large industrial area in Japan to further improve performance of highly efficient process plants. Energy 2009, 34, 1687–1692. [Google Scholar] [CrossRef]
- Hackl, R.; Harvey, S.; Andersson, E. Total Site Analysis (TSA) Stenungsund Technical Report; Chalmers University of Technology: Gothenburg, Sweden, 2010. [Google Scholar]
- Norwegian Environment Agency. The Norwegian PRTR—Industry. 2018. Available online: https://www.norskeutslipp.no/en/Industrial-activities/?SectorID=600 (accessed on 1 February 2020).
- Gundersen, T. A Process Integration PRIMER. IEA Tutorial on Process Integration; SINTEF Energy Research: Trondheim, Norway, 2000. [Google Scholar]
- Børset, M.T.; Wilhelmsen, Ø.; Kjelstrup, S.; Burheim, O.S. Exploring the potential for waste heat recovery during metal casting with thermoelectric generators: On-site experiments and mathematical modeling. Energy 2017, 118, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Schei, A.; Tuset, J.; Tveit, H. Production of High Silicon Alloys; Tapir: Trondheim, Norway, 1997. [Google Scholar]
- Tangstad, M. Chapter 6—Ferrosilicon and Silicon Technology. In Handbook of Ferroalloys; Gasik, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 179–220. [Google Scholar] [CrossRef]
- Kero, I.; Grådahl, S.; Tranell, G. Airborne Emissions from Si/FeSi Production. JOM 2017, 69, 365–380. [Google Scholar] [CrossRef] [Green Version]
- Wittgens, B.; Panjwani, B.; Pettersen, T.; Jensen, R.; Ravary, B.; Hjertenes, D.O. SCORE Staged combustion for energy Recovery in Ferro-alloy Industries—Experimental Verification. In Proceedings of the Infacon XV, Cape Town, South Africa, 25–28 February 2018. [Google Scholar]
- Pettersen, T.; Wittgens, B.; Berglihn, O.T.; Panjwani, B.; Ravary, B.; Myrhaug, E. Reduced environmental impact and increased energy and material recovery in the ferroalloy industry through staged combustion of flue gas. In Proceedings of the 2018 Sustainable Industrial Processing Summit and Exhibition, Rio de Janeiro, Brasil, 4–7 November 2018; Volume 1, p. 18. [Google Scholar]
- Takla, M.; Kamfjord, N.; Tveit, H.; Kjelstrup, S. Energy and exergy analysis of the silicon production process. Energy 2013, 58, 138–146. [Google Scholar] [CrossRef]
- Ladam, Y.; Tangstad, M.; Ravary, B. Energy mapping of industrial ferroalloy plants. In Proceedings of the Thirteenth International Ferroalloys Congress Efficient technologies in Ferroalloy Industry, Almaty, Kazahstan, 9–12 June 2013; pp. 919–925. [Google Scholar]
- Børset, M.; Kolbeinsen, L.; Tveit, H.; Kjelstrup, S. Exergy based efficiency indicators for the silicon furnace. Energy 2015, 90, 1916–1921. [Google Scholar] [CrossRef]
- Martin, C.R. PYroMat—Thermodynamic Properties in Python; The Pennsylvania State University, Altoona College: Altoona, PA, USA, 2018; version 2.0.10. [Google Scholar]
- National Institute of Standards and Technology, US Department of Commerce. NIST Chemistry WebBook. 2018. Available online: https://webbook.nist.gov/chemistry/ (accessed on 1 February 2020).
- Chase, M. NIST-JANAF Thermochemical Tables (Journal of Physical and Chemical Reference Data Monographs); American Institute of Physics: College Park, MD, USA, 1998; pp. 1–1951. [Google Scholar]
- Romera, J.J.G. IAPWS—Python Libray for IAPWS Standard Calculation of Water and Steam Properties. Version 1.5.2; 2017; Available online: https://pypi.org/project/iapws (accessed on 1 February 2020).
- Husebye, J.; Brunsvold, A.L.; Roussanaly, S.; Zhang, X. Techno Economic Evaluation of Amine based CO2 Capture: Impact of CO2 Concentration and Steam Supply. Energy Procedia 2012, 23, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Technology Centre Mongstad. About TCM. 2018. Available online: http://www.tcmda.com/en/About-TCM/ (accessed on 1 February 2020).
- Gorset, O.; Knudsen, J.N.; Bade, O.M.; Askestad, I. Results from Testing of Aker Solutions Advanced Amine Solvents at CO2 Technology Centre Mongstad. Energy Procedia 2014, 63, 6267–6280. [Google Scholar] [CrossRef] [Green Version]
- McGlashan, N.R.; Marquis, A.J. Availability analysis of post-combustion carbon capture systems: Minimum work input. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2007, 221, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, J.N.; Andersen, J.; Jensen, J.N.; Biede, O. Evaluation of process upgrades and novel solvents for the post combustion CO2 capture process in pilot-scale. Energy Procedia 2011, 4, 1558–1565. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, M.; Kempegowda, R.S.; Skreiberg, Ø.; Wang, L.; Løvås, T. Techno-Economics of Biocarbon Production Processes under Norwegian Conditions. Energy Fuels 2017, 31, 14338–14356. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Tranberg, B.; Corradi, O.; Lajoie, B.; Gibon, T.; Staffell, I.; Andresen, G.B. Real-time carbon accounting method for the European electricity markets. Energy Strat. Rev. 2019, 26, 100367. [Google Scholar] [CrossRef]
- Asplan Viak. Nordisk Strøm Blir Renere. 2016. Available online: https://www.asplanviak.no/aktuelt/2016/02/03/nordiskstroem-blir-renere/ (accessed on 1 February 2020).
- Watson, F. Factbox: EU CO2 Price Hits 11-Year High. 2019. Available online: https://www.spglobal.com/platts/en/marketinsights/latest-news/electric-power/071119-factbox-eu-co2-price-hits-11-year-high (accessed on 1 February 2020).
- Eurostat. Electricity Price Statistics. 2019. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Electricity_price_statistics#Electricity_prices_for_non-household_consumers (accessed on 1 February 2020).
- Statistics Norway. Electricity Prices. 2019. Available online: https://www.ssb.no/energi-og-industri/statistikker/elkraftpris/kvartal/ (accessed on 1 February 2020).
- Valero Capilla, A.; Valero Delgado, A. Thanatia: The Destiny of the Earth’s Mineral Resources. A Thermodynamic Cradle-to-cradle Assessment; World Scientific: Singapore, 2014. [Google Scholar]
- Magnanelli, E.; Berglihn, O.T.; Kjelstrup, S. Exergy-Based Performance Indicators for Industrial Practice. Int. J. Energy Res. 2018, 42, 3989–4007. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/er.4123 (accessed on 1 February 2020). [CrossRef] [Green Version]
- Monsen, B.; Grønli, M.; Nygaard, L.; Tveit, H. The Use of Biocarbon in Norwegian Ferroalloy Production. In Proceedings of the INFACON IX, Quebec City, QC, Canada, 3–6 June 2001; Volume 10, p. 10. [Google Scholar]
- Riva, L.; Nielsen, H.K.; Skreiberg, Ø.; Wang, L.; Bartocci, P.; Barbanera, M.; Bidini, G.; Fantozzi, F. Analysis of optimal temperature, pressure and binder quantity for the production of biocarbon pellet to be used as a substitute for coke. Appl. Energy 2019, 256, 113933. [Google Scholar] [CrossRef]
- Engineering Toolbox. Fuels—Higher and Lower Calorific Values. 2003. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 1 February 2020).
- MIP. Celsas Hot Charging Ready for Start Up. 2015. Available online: https://www.mip.no/en/2015/celsas-hot-charging-readyfor-start-up/ (accessed on 1 February 2020).
- Tunc, M.; Camdali, U.; Arasil, G. Energy Analysis of the Operation of an Electric-Arc Furnace at a Steel Company in Turkey. Metallurgist 2015, 59, 489–497. [Google Scholar] [CrossRef]
- Hjartarson, H. Waste Heat Utilization at Elkem Ferrosilicon Plant in Iceland. Master’s Thesis, University of Iceland, Reykjavík, Iceland, 2009. [Google Scholar]
- Kolbeinsen, L.; Lindstad, T.; Tveit, H.; Bruno, M.; Nygaard, L. Energy Recovery in the Norwegian Ferro Alloy Industry. In Proceedings of the INFACON 7, Trondheim, Norway, 11–14 June 1995; pp. 165–177. [Google Scholar]
- MIP. Kraft i Bruk fra Vann til Verk. 2012. Available online: https://www.mip.no/2012/kraft-i-bruk-fra-vann-til-verk/ (accessed on 1 February 2020).
- Enova. Mo Industripark: Grønn Industriklynge Med Verdensambisjoner. 2017. Available online: https://www.enova.no/bedrift/industri-og-anlegg/historier/mo-industripark-gronn-industriklynge-med-verdensambisjoner/ (accessed on 1 February 2020).
- Larssen, T.A.; Tangstad, M.; Kero, I. Energy Distribution in HC FeMn and SiMn—Energy vs. Exergy Analyses. In Proceedings of the Infacon XV, Cape Town, South Africa, 25–28 February 2018. [Google Scholar]
- Norsk Fjernvarme. Mo i Rana. 2019. Available online: https://www.fjernkontrollen.no/mo-i-rana/ (accessed on 1 February 2020).
- Norsk Fjernvarme. Dumper Spillvarme Påhavet. 2019. Available online: http://fjernvarmedagene.no/index.php?pageID=29&openLevel=4&cid=4395 (accessed on 1 February 2020).
- Helgeland Kraft. Helgeland Kraft Inngår Kraftavtale med Norges Største Datasenter. 2019. Available online: https://www.helgelandkraft.no/konsern/forside/om-helgeland-kraft/nyheter-og-aktuelt/bitfury/ (accessed on 1 February 2020).
- Ulriksen, A.; Gabor, J. Mo Industrial Park. 2017. Available online: https://industrysummit.fi/wp-content/uploads/2017/10/Susanne-M.-Naevermo-Sand.pdf (accessed on 1 February 2020).
- Bjørndal, T.; Holte, E.A.; Hilmarsen, O.; Tusvik, A. Analyse av Lukka Oppdrett av Laks-Landbasert og I sjø: Produksjon, Økonomi og Risiko. Technical Report; NTNU, SINTEF, SNF: Trondheim, Norway, September 2018. [Google Scholar]
- MIP. Lindegruppens Nordligste Luftgassfabrikk. 2014. Available online: https://www.mip.no/2014/lindegruppens-nordligste566luftgassfabrikk/ (accessed on 1 February 2020).







| Actor/infrastrucucture | Explanation |
| Elkem Rana | Ferrosilicon |
| Ferroglobe Mangan | Silicomanganese and ferromanganese |
| Celsa | Reinforcing steel (from steel scrap) |
| SMA Minerals | Quicklime and calcined dolomite |
| Ranfjord fiskeprodukter | Land-based aquaculture |
| Aga | Industrial gases (cryogenic) |
| Bitfury | Data centre |
| MIP AS | Utility and property company |
| Syn-gas grid | CO-rich gas from Ferroglobe is utilised in other companies |
| Mo Fjernvarme | District heating from recovered surplus heat |
| Cooling water | Flow-through from nearby reservoirs |
| Parameter | Value | |
|---|---|---|
| Charge gas composition (vol.%) | CH4: 9, CO: 57.6, SiO: 3.7, H2: 6.4, H2O: 23.3, N2: rest | |
| Charge gas temperature | 1500 | °C |
| Specific charge gas generation | 287.5 | Nm3/(h*MW) |
| Specific electricity consumption | 11.7 | MWh/t Me |
| Specific carbon consumption | 1.7 | kg/kg Me |
| Max inlet temperature to WHRU | 750 | °C |
| Recirculated flue gas temperature from WHRU | 150 | °C |
| Heat loss from SAF | 1500 | kW |
| Design Parameter | Value |
|---|---|
| Flue gas inlet-outlet temperature (°C) | 750–150 |
| Flue gas heat loss (%) | 2 |
| Steam pressure (bar) | 40 |
| Superheated steam temperature (°C) | 420 |
| Saturated steam temperature (°C) | 250 |
| Steam turbine isentropic efficiency (%) | 75 |
| Turbine exit temperature and pressure (°C), (bar) | 45.8, 0.1 |
| Generator overall efficiency (%) | 98 |
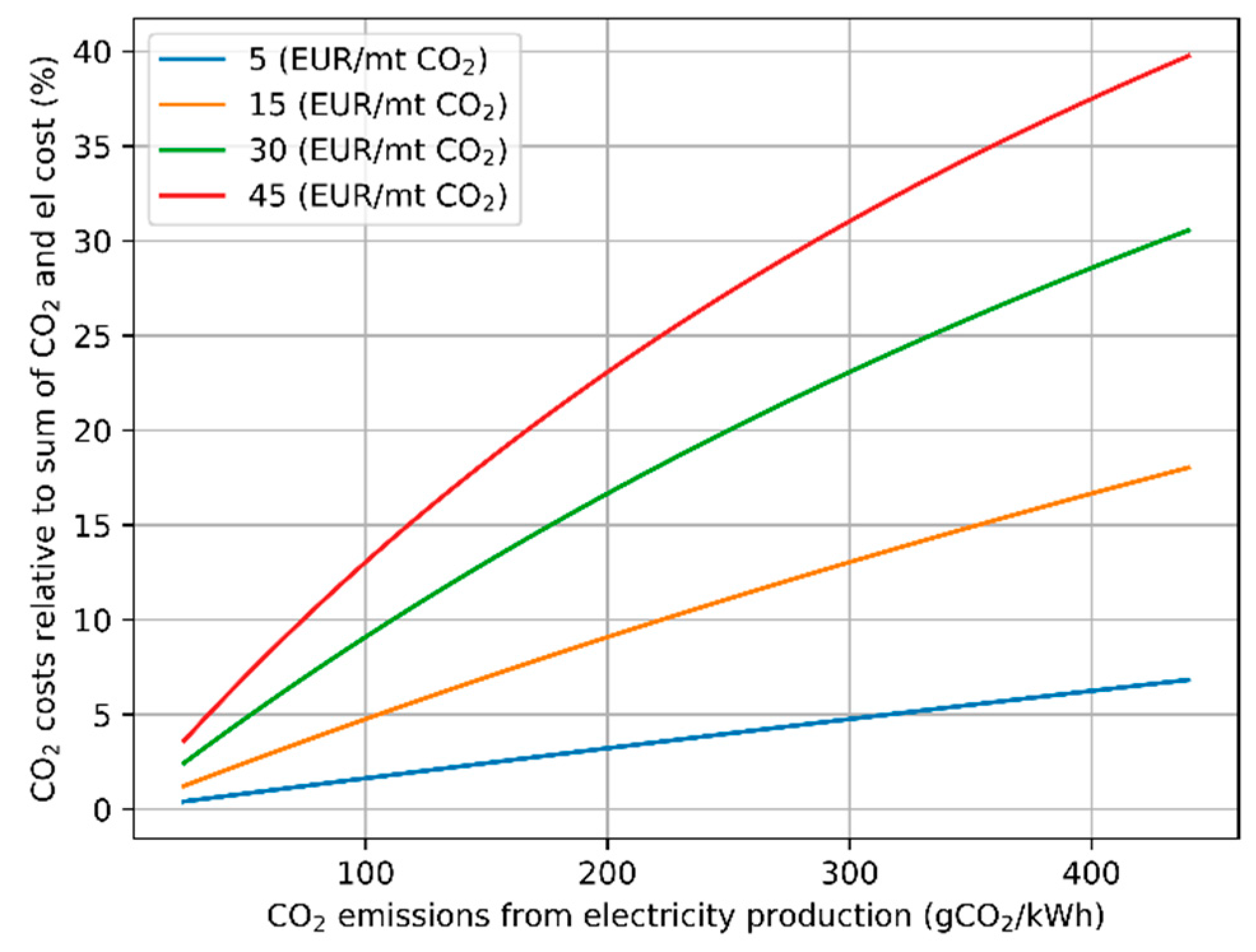
| Parameter | Range | |
|---|---|---|
| Electricity price from grid | 0.03–0.1 | EUR/kWh |
| CO2 emission price | 5–30 | EUR/t CO2 |
| CO2 emissions from electricity production | 25–440 | g CO2-eq/kWh |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettersen, T.; Dæhlin, E.; Eidem, P.A.; Berglihn, O.T. Investigating the Potential for Increased Energy Utilisation and Reduced CO2 Emissions at Mo Industrial Park. Energies 2020, 13, 4627. https://doi.org/10.3390/en13184627
Pettersen T, Dæhlin E, Eidem PA, Berglihn OT. Investigating the Potential for Increased Energy Utilisation and Reduced CO2 Emissions at Mo Industrial Park. Energies. 2020; 13(18):4627. https://doi.org/10.3390/en13184627
Chicago/Turabian StylePettersen, Torbjørn, Emil Dæhlin, Per Anders Eidem, and Olaf Trygve Berglihn. 2020. "Investigating the Potential for Increased Energy Utilisation and Reduced CO2 Emissions at Mo Industrial Park" Energies 13, no. 18: 4627. https://doi.org/10.3390/en13184627







