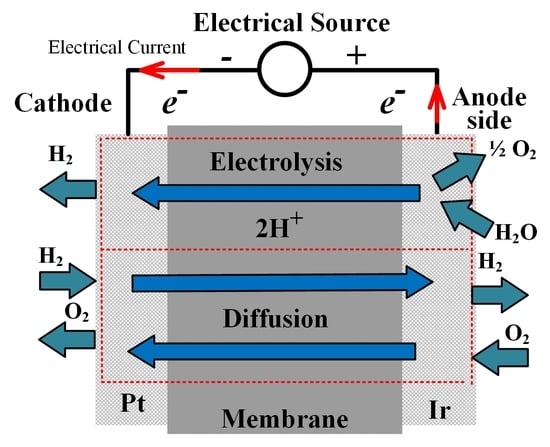
1. Introduction
To face the depletion of fossil fuels (i.e., oil, natural gas) and the reduction of the greenhouse gas emission, decarbonized hydrogen production pathways based on renewable energy sources (i.e., wind energy, photovoltaic energy, hydro energy) and water electrolysis are considered attractive and promising solutions for a sustainable future [
1,
2]. In these pathways, generated and stored hydrogen can be employed for different applications such as transportation, energy storage, power-to-gas, and industry [
1,
3]. The water electrolysis process consists of employing electricity generated from renewable energy sources to split pure water into oxygen and hydrogen [
4]. It is performed by electrolyzers. Currently, different types of electrolyzer exist depending on the type of electrolyte used and charge carrier such as alkaline, proton exchange membrane (PEM) and solid oxide (SO) [
5,
6]. Nevertheless, alkaline and PEM are the only technologies that can be found in the market; the SO technology is still in the research and development phase because of its recent introduction [
4]. Since the principle of alkaline water electrolysis is well known (first introduced more than 200 years ago), this technology has been deployed all over the world, meeting the requirements for power-to-gas and industrial applications [
7,
8]. Indeed, the available stack power can reach a few megawatts. On one hand, the main advantages of this technology are low-cost catalysts, having a higher lifetime, and gas purity. On the other hand, this technology features several disadvantages in terms of low current density, flexibility, ohmic loss, and operating pressure [
7]. Their penetration in markets of great potential is limited to their partial load range. Generally, it is included between 40–100% but some manufacturers such as NEL have developed alkaline electrolyzers with a partial load range of 15–100% [
2]. The partial load range is an important issue when coupling electrolyzers with renewable energy sources to ensure decarbonized hydrogen production. Since renewable energy sources are very dynamic sources, the electrolyzers must be able to absorb the energy during dynamic operation and even at low wind speed [
9]. However, the limited partial load range and low current densities of alkaline electrolyzers make them less efficient during dynamic operation. In comparison, PEM electrolyzers feature high current densities (above 2 A.cm
−2), as well as a high power density, small footprint, high efficiency, very thin membrane (i.e., 25–254 µm), high-pressure operation, and fast response to dynamic operations, which makes it perfectly fit when connecting with renewable energy sources [
4,
5]. However, this technology suffers from high cost due to the use of costly catalyst materials (e.g., iridium, platinum) both at the anode and the cathode; this slows down large scale use and market penetration compared to alkaline electrolyzers [
4,
5].
In electrolyzers, to evaluate their performance from the amount of hydrogen produced and energy efficiency point of view, the Faraday’s efficiency, commonly called current efficiency, is a challenging issue [
10]. In fact, it allows for describing the fraction between the amount of hydrogen generated and the theoretical hydrogen amount which could be generated according to the electrical energy input [
10]. It is important to point out that Faradaic losses are due to shunt currents introduced by the design of bipolar plate materials of electrolyzers. These shunt currents bypass into current collectors in the electrolyzer stack [
11]. They can be evaluated by the ratio between measured and theoretical gas flow rate. The gas flow rate measurement is carried out through a bubble flowrate meter [
12]. In the literature, many papers have been reported to investigate and predict shunt currents in electrochemical devices such as batteries [
13,
14] and electrolyzers [
11,
12,
15].
The first investigations on Faraday’s efficiency have been introduced for alkaline water electrolysis in the 1990s by Hug et al. [
15] and Ulleberg [
16]. They have demonstrated that the Faradaic losses increase at low current densities due to lower electrolyte resistance. From experiments, they have developed empirical expressions to model the Faraday’s efficiency based on the input current and temperature change. A growth in temperature causes a lower electrical resistance since the electrolyte is sensitive to temperature change [
10]. As a result, the Faraday’s efficiency decreases when increasing the temperature. In [
17], the authors analyzed the effects of operating temperature and membrane thickness on the Faraday’s efficiency for a PEM electrolyzer. Nevertheless, they have not developed a model to assess Faraday’s efficiency. Based on the first works introduced in [
15,
16], Faraday’s efficiency has been reported in several works for modeling purposes both for alkaline and PEM electrolyzers [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. Nonetheless, this model is only valid for alkaline electrolyzers and cannot be employed to the PEM electrolyzer because of their different materials used for their manufacturing (e.g., membrane). In general, the proposed models for alkaline electrolyzers are characterized by some parameters that have to be identified based on the electrolyzer, the temperature appears explicitly as a variable, differently the pressure is assumed as constant; as a consequence, it is hidden inside the parameters.
From the current state-of-the-art, it can be highlighted that a few works have been carried out on the Faraday’s efficiency investigation for PEM electrolyzers. Besides, no models depending on the hydrogen pressure have been proposed either for alkaline or PEM electrolyzers. Hence, the objective of this article is to assess the effects of the input current and hydrogen pressure on the Faraday’s efficiency. This analysis aims to describe the behavior of the eletrolyzer in the low-density current zone; here varying the current the temperature shows low variations since losses are reduced, and that contrarily, the influence of the pressure of the efficiency curve is relevant. The investigation is based on experiments carried out with a commercial PEM electrolyzer. The obtained results have enabled developing a simple empirical expression valid for the PEM stack electrolyzer studied to model the Faraday’s efficiency based on the current and hydrogen pressure change. The comparison between the experiments and the proposed model demonstrate the exactness of the model in reproducing the Faraday’s efficiency according to the operating conditions. Faraday’s efficiency model can be helpful to determine the energy efficiency of the electrolyzer based on the cell voltage efficiency. Cell voltage efficiency can be assessed by dividing the thermoneutral voltage (i.e., 1.291 V) by the voltage of each cell. It quantifies the effects of cell polarization. In the literature, Faraday’s efficiency is usually considered equal to 1 and consequently, the energy efficiency does not take into consideration gas crossover losses.
This article is divided into four sections. After this introduction emphasizing the current state-of-the-art and reasons to carry out this work,
Section 2 summarizes the main contributions of the investigation and modeling of the Faraday’s efficiency of alkaline and PEM electrolyzers. Then, in
Section 3, the realized test bench to carry out experiments is presented. Besides, different tests at different hydrogen pressure are performed to analyze their effects on the Faraday’s efficiency for modeling purposes. Finally, in
Section 4, a discussion is provided to compare the experiments and the developed model and then conclusions about its effectiveness. Furthermore, future challenges in enhancing the Faraday’s efficiency are given.
2. Research Background
The performance of the electrolyzers are strongly linked with the hydrogen flow rate.
, and energy efficiency,
, which are expressed by the following expression [
10]:
Faraday’s efficiency defines the fraction between the generated quantity of hydrogen (H2) and the theoretical hydrogen quantity which could be produced according to the electrical energy input.
The electrolyzer current is the same in each of the electrolysis cells and consequently, the calculated gas volume is produced in each of the cells. Hence, the theoretical hydrogen quantity must be multiplied by the number of cells nc.
Faraday’s efficiency influences the variation of the hydrogen for given current variation. When this curve asymptotically tends to the unitary value, the derivative is approximately null and a current variation around the operating point produces the same hydrogen variation. Contrarily, when the efficiency drops, as in the low current zone, the same increase or decrease of the current produces different changes on the hydrogen. This phenomenon is emphasized when the supply current is obtained by rectifiers since the DC current contains a low-frequency ripple superimposed.
From Equations (1) and (2), it can be noted that the Faraday’s efficiency affects both hydrogen flow rate and energy efficiency. By inserting Equation (1) into Equation (2), the following expression is obtained:
The terms appearing in the brackets are considered constants, whereas the variables are the Faraday’s efficiency and the stack voltage. Faradaic losses are particularly distinct at low current densities. For this reason, energy efficiency is strongly affected by the stack voltage. The higher the stack voltage, the lower the energy efficiency.
According to the second Faraday’s law of electrolysis and the general gas equation, the theoretical hydrogen quantity can be determined:
On the one hand, the Faraday’s efficiency is greater than the energy efficiency (2), since it only takes into consideration the losses due to the gas crossover [
10]. On the other hand, energy efficiency takes into consideration additional losses such as ohmic losses and joule losses both at the anode and cathode. Since the 1990s, a few works have been published in the literature on the analysis and the modeling of Faraday’s efficiency [
10,
15,
16]. The works are mainly focused on alkaline electrolyzers and two empirical models have been reported in the 1990s to model Faraday’s efficiency based on the input current of the electrolyzer and temperature. The first empirical model was developed by Hug et al. [
15] in 1993; whereas the second model by Ulleberg [
16] in 1998. The first empirical model developed by Hug et al. [
15] includes five parameters as follows:
This empirical model can provide a good accuracy of Faraday’s efficiency, whatever the temperature and the current. Experimental data at different temperatures are required to determine the five parameters of the model.
By comparison, Ulleberg first proposed an empirical model on seven parameters described by [
16]:
where
A is the active area in (m
2) of the electrolyzer. To evaluate the seven parameters, experimental tests have been carried out at different temperatures (i.e., 40 °C, 60 °C, and 80 °C) on an HYSOLAR alkaline electrolyzer (10 kW, 5 bar, 0.05 m
2, 25 cells). The obtained parameters are reported in [
16]. To highlight the effect of the temperature on the Faraday’s efficiency, it has been plotted according to the current density at different temperatures in
Figure 1.
It can be emphasized that the higher the temperature, the lower the Faraday’s efficiency since the electrolyte resistance is lower, leading up to higher crossover currents. This model has been employed in [
28] to evaluate the hydrogen flow rate based on the temperature and current.
The model (11) has been then simplified by Ulleberg with only four parameters:
The four parameters can be evaluated based on experimental data at different temperatures.
Finally, in [
10], the Faraday’s efficiency depends on two parameters for a given temperature:
The parameters of this model have been reported for a PHOEBUS electrolyzer (26 kW, 7 bar, T = 80 °C, 0.25 m
2, 21 cells) and an HYSOLAR alkaline electrolyzer (10 kW, 5 bar, T = 80 °C) and are summarized in [
10,
16].
From these parameters reported for both alkaline electrolyzers, Faraday’s efficiency has been plotted versus the current density in
Figure 2. It can be underlined that both Faraday’s efficiencies are similar at low current densities but from a current density of 20 mA.cm
−2, the HYSOLAR alkaline electrolyzer shows a higher Faraday’s efficiency than the PHOEBUS alkaline electrolyzer. It can be explained by the fact that the gas pressure of the HYSOLAR electrolyzer is smaller than that of the PHOEBUS electrolyzer. The consequence of gas pressure on Faraday’s efficiency has been introduced in previous works [
29,
30,
31] where an increase in gas pressure decreases Faraday’s efficiency.
It should be remarked that even if the Faraday’s efficiency is often sketched at constant temperature, it does not represent the real operation of the electrolyzer. In fact, higher currents imply an increase in the temperature due to the losses.
The model (8) has been used in [
26,
27] to obtain the hydrogen flow rate for a given temperature.
Conversely, based on the work carried out by Ulleberg in [
16], many authors have employed this following expression to determine the Faraday’s efficiency at a given temperature (40 °C) [
18,
19,
20,
21,
22,
23,
24]:
This model has been obtained from experiments performed on a PHOEBUS electrolyzer (26 kW, 7 bar, T = 40 °C, 0.25 m
2, 21 cells) [
10]. The Faraday’s efficiency given by Equation (9) has been plotted according to the current density in
Figure 3.
Finally, in [
17,
29,
30,
31], compared to results reported in [
10,
15,
16] for alkaline electrolyzers, the authors have shown that the temperature slightly affects the Faraday’s efficiency of PEM electrolyzers. However, membrane thickness and gas pressure have a strong influence on the Faraday’s efficiency. On the one hand, the greater the gas pressure, the smaller the Faraday’s efficiency. On the other hand, the thinner the membrane thickness, the higher the crossover currents. As a result, membrane thickness and operating pressures are challenging issues to improve the efficiency of PEM electrolyzers [
29,
30,
31].
4. Discussion and Future Challenges
The first investigations on Faraday’s efficiency of an alkaline electrolyzer were reported in the 1990s. These studies have enabled modeling the effect of current density and temperature on the Faraday’s efficiency by using empirical equations. Based on the current literature, Ulleberg’s model is often used to assess the hydrogen flow rate and energy efficiency for a provided temperature. Recently, several studies have been reported to demonstrate the effect of membrane thickness and gas pressure on Faraday’s efficiency. Temperature, membrane thickness, and gas pressure are relevant data affecting the performance of the electrolyzers. However, no models have been proposed on recent electrolyzers, that demonstrate technical improvements compared to the electrolyzers in the 1990s. For this reason, this preliminary work offers a new contribution about the modeling of Faraday’s efficiency according to current density and hydrogen pressure change.
The obtained empirical model is simple and includes a few parameters, which can be obtained by employing a least-square algorithm. The comparison between the experimental measurements and the developed model demonstrates the accuracy of the model in reproducing Faraday’s efficiency according to current density and hydrogen pressure change. However, due to the use of a small-scale electrolyzer, the hydrogen pressure is set to 10 bar; whereas some commercial electrolyzer models can produce pressurized hydrogen up to 30 bar. At that pressure, the faradaic losses are higher due to higher crossover currents as shown in the scientific literature [
25,
26,
27]. In this case, the model proposed is still valid but a new identification of the parameters is required. To highlight the contribution of this work, a comparative summary reported in
Table 5 has been realized to summarize the main characteristics of the reported models in the literature and the proposed model.
The method proposed in this paper is a “black box” approach in which the main parameters, such as current, pressure, and temperature are considered, taken at the electrolyzer’s external terminals. These parameters are supposed to be uniform inside the electrolyzer; for example, the current density is obtained by dividing the current for the surface. Of course, it is an approximation; however, it is useful to achieve the proposed model. In general, a nonuniform distribution of the current worsens the performance; hot-spots can be created with consequent over-heating. Besides, if the flow is disturbed by parasitic paths, energy conversion efficiency into hydrogen molecules is lowered. The Faraday’s efficiency is influenced by the nonuniformity of the distribution of the current and potential as explained in [
12] and of shunt current [
11] depending on the presence of separators and electrodes shape. The knowledge of these phenomena requires a study taking into account the internal model of the electrochemical reactor. Some endeavors are proposed by literature; the current distribution can be improved by a suitable choice of flow paths [
34,
35]; these studies showed that it is possible to devise a good model of flows inside electrochemical reactors, and it is confirmed by experimental validation [
36]. It is important to underline that the above-mentioned studies can be used for general problems taking into account concentration or conductivity variations and helps the designer in scale-up situations. The Faraday’s efficiency can be further improved by combining the proposed “black-box” approach and the analysis presented in the literature.
In the future, to face hydrogen storage and refueling issues, the production of hydrogen at high-pressure is a key issue. Indeed, it allows for making hydrogen storage easier in tanks and improving its refueling in fuel cell electric vehicles. Furthermore, it avoids the use of an external compressor, responsible for increasing the overall cost and decreasing energy efficiency. As a result, since higher operating pressure leads up to the decrease of Faraday’s efficiency, technical improvements in designing electrolyzers must be brought [
31].