1. Introduction
Biomass is an abundant source of carbon and is a widely used raw material for the production of bioenergy [
1]. The composition of biomass allows for the development of a large scale bioindustry that utilizes the various components present in lignocellulosic biomass, thereby enhancing the value of the biomass [
2]. Biomass is seen as a renewable source of energy and a highly available resource [
1]. Types of biomass include crop extracts, wood, and animal waste. Sugarcane bagasse is a by-product from the processing of sugarcane and a form of lignocellulosic biomass. Bagasse is a fibrous material obtained from the remnants of sugarcane stalks that are milled to extract their vital juice, which is used to produce sugar [
3]. The main constituent of the bagasse is cellulose, which is approximately 46%, while hemicellulose and lignin account for approximately 27% and 23% of the bagasse, respectively [
4]. Bagasse may be regarded as a feedstock for biofuel production due to its low cost and high availability [
5]. Sugarcane bagasse is widely used in industry as it can be converted into various valuable products such as biofuels (methanol, ethanol, etc.), paper, pulp, boards, biodegradable plastics, construction material, and certain chemicals [
6].
Bagasse may be stored for long periods and is usually used to produce heat and electricity through combustion [
7]. It may be used as a substrate for microbial production of products such as enzymes, organic acids, amino acids, protein rich animal feed, and as a source of carbon for the growth of filamentous fungi [
8]. Filamentous fungi are useful producers of enzymes due to the high production level of enzymes and the ease of cultivation [
8]. This type of fungi produces high levels of polysaccharide-degrading enzymes and are used to produce lignin modifying enzymes and industrial enzymes (e.g., amylases and cellulase) [
8]. These enzymes may be utilized in industrial processes to eliminate the use of extreme pH and high temperatures, while providing increased product purity [
8]. These enzymes also have a variety of biotechnological applications such as the production of food for animals and in the production of textiles, paper, and detergents [
8].
Biofuels may be regarded as the future of the fuel industry due to its net low greenhouse gas emissions during combustion when compared to the combustion of fossil fuels and the ability to produce biofuels with lignocellulosic material, which do not compete with food crops [
6]. Climate change as well as the effects of global warming have made biofuels an attractive option. Biofuels provide a renewable source of energy while reducing the effects on the environment significantly. Renewable biofuels are made through the use or conversion of biomass such as bagasse. Biomass can be converted to fuel sources in a variety of ways including physical conversion, thermal conversion, chemical conversion, and biochemical conversion [
9]. Depending on the conversion technology used, biomass can be utilized in the production of liquid bio-based fuels such as ethanol [
10]. The fuels produced from the conversion of biomass such as bio-ethanol and bio-diesel can be used in the transport sector. The use of these fuels will significantly reduce greenhouse gas emissions and reduce the demand placed on the processing and extraction of fossil fuels. Sugarcane bagasse plays an important role in the production of biofuels such as ethanol, however, it must undergo a multi-step process in order to be converted to biofuel [
11]. The four main steps of biofuel production are: biomass pretreatment, enzymatic hydrolysis, fermentation, and recovery (e.g., distillation) [
11]. Hence in the second generation biofuel scheme, pretreatment is a major step toward biofuel production, particularly ethanol.
Cellulose is generally the most desired component in lignocellulosic biomass, as it is a major component of plant matter and stores a large quantity of energy conserved by photosynthesis [
12]. It is considered a polymer of glucose, however, the cellulose is entangled in hemicellulose and covered by lignin [
13]. Cellulose found in biomass is a form of cellobiose, which consists of two glucose molecules [
14]. It may have a crystalline or non-crystalline structure and is insoluble in water [
14]. Hydrogen bonds aid in holding the crystalline structure together [
15]. Cellulose is also insoluble in dilute acid solutions at low temperatures [
14]. The solubility of cellulose is highly related to the degree of hydrolysis achieved [
14]. Cellulose is soluble in concentrated acids, but is at risk of undergoing degradation. Cellulose also has good solubility in alkaline compounds [
15]. At high temperatures, the polymer becomes soluble as there is enough energy to break the hydrogen bonds. For conversion of lignocellulosic biomass to biofuels such as ethanol, polymers such as cellulose must be broken down into the corresponding simple sugars so that microorganisms can process them. In order to access the cellulose and process it into its constituent sugars, the outer layer of lignin needs to be broken down.
Enzymatic hydrolysis is the process of using enzymes to break down cellulose into soluble glucose, which can then be used to produce paper, cotton textiles, and biofuels such as ethanol [
16]. Enzymatic hydrolysis strongly depends on the operating conditions of the process (such as temperature, solids loading, enzyme loading, and pH), enzyme consumption (specific activity, enzyme recycling strategies, and stability), and the effect of product inhibitor on enzyme catalysis [
17]. Normal enzymatic hydrolysis applied directly to raw lignocellulosic biomass is not effective in extracting the cellulose as the lignin remains intact and unaffected by the process [
18]. This is due to the complex structure of the lignocellulosic biomass, ensuring that it is very recalcitrant and resistant to enzyme attack [
1]. A combination of pretreatment with enzymatic hydrolysis processes is vital for the release of nutrients from the biomass. The pretreatment process therefore plays a vital role in weakening and breaking down the lignin layer. This enables the hydrolysis process to extract and break down the valuable cellulose into glucose. The parameters that influence effective pretreatment of lignocellulosic biomass are cellulose crystallinity, the presence of lignin, the surface area accessible to hydrolysis, and the presence of hemicellulose [
9]. A variety of pretreatment techniques are used in industry to alter the physical and chemical structure of the biomass and improve hydrolysis rates. Pretreatment is essential to reduce the overall cost for production because the cost of pretreatment is a significant factor affecting the selling price of the end product (e.g., ethanol) [
9]. There are a variety of pretreatment techniques, and each technique has its advantages and disadvantages.
Chemical pretreatment processes are initiated by chemical reactions to disrupt the biomass structure [
14]. Chemical pretreatment is characterized by the use of inorganic or organic compounds, which interact with the intrapolymer and interpolymer bonds of the cellulose, hemicellulose, and lignin to disrupt the structure of the biomass [
19]. Chemical pretreatment processes include acid hydrolysis, hydrothermal pretreatment, alkaline pretreatment, wet oxidation, and the use of deep eutectic solvents (DESs) [
14]. Chemical pretreatment methods involve the dissolution of part of the biomass (mainly lignin and hemicellulose) to make the cellulose more accessible for further processing such as enzymatic hydrolysis. Chemical processes usually yield high recovery of glucose at the end of the entire process [
14]. Deep eutectic solvents are a promising new class of solvents considered for the fractionation of lignocellulosic biomass and the dissolution of lignin, as lignin is highly soluble in deep eutectic solvents [
20]. These are primarily liquid eutectic mixtures that are formed by the hydrogen bonding interaction of two or three components [
20]. DESs are seen as green solvents and have a high potential for biomass processing as they are easily recycled and reused, biodegradable, easy to synthesize, have a low cost, and a low toxicity [
21]. Cellulose (to a certain degree) and lignin both have good solubility with dilute alkaline solutions. Sodium hydroxide is an alkaline solution used to aid delignification. Types of dilute alkaline solutions include aluminum hydroxide, aluminum oxide, magnesium hydroxide, lithium hydroxide, potassium carbonate, and sodium carbonate [
21].
Mercerization is an alkaline treatment method for cellulose fibers [
22]. Mercerization is a process whereby textiles (mainly cotton, which is made of cellulose fibers) are treated with a caustic solution (e.g., sodium hydroxide) to improve properties such as fiber strength, dye affinity, luster, and shrinkage resistance [
22]. It can also be used to improve the accessibility of cellulose and increases the effective surface area available for contact when processing lignocellulosic biomass [
22]. Following the treatment with sodium hydroxide, the fibers are treated with an acid or water to neutralize the fibers. The degree of modification of the cellulose depends on the length of treatment, the temperature, and concentration of the dilute alkaline solution [
23]. Alkaline pretreatment, using a variety of different chemical oxides and hydroxides of lithium, aluminum, magnesium, calcium, sodium, and potassium, is often used for the elimination of lignin. The alkaline pretreatments typically have longer processing times compared to acid pretreatment, and can form irrecoverable salts that are incorporated into the residual biomass.
Mechanical pretreatment involves the addition of a form of mechanical agitation to the biomass solution to break the structure of the biomass such as milling and grinding. Mechanical pretreatment processes result in a reduction in the particle size and an increase in pore size and specific surface area [
14]. It also decreases the cellulose crystallinity and the degree of polymerization [
9]. Steam explosion can be classified as a form of mechanical pretreatment, although there are also chemical actions involved. The biomass is subjected to high temperatures and pressures for a short period, and then the system is rapidly depressurized, which results in disruption of the fibrous structure [
14]. Another method of mechanical pretreatment is the application of ultrasonic irradiation to the biomass solution, which uses ultrasonic vibration to disrupt the chemical and physical structure of the biomass such as delignification and surface erosion [
24]. This disruption of the biomass structure occurs at different levels of interaction than chemical pretreatment processes and yields different products of transformation when compared to the chemical pretreatment methods.
Biological pretreatment involves the use of microorganisms such as white and brown rot-fungi to degrade lignin and hemicellulose in lignocellulosic biomass [
14]. The use of microorganisms modifies the chemical composition and structure by degrading the lignin to make the cellulose more accessible for enzyme digestion or dissolution [
25]. Biological pretreatments have mild operating conditions, usually no chemical requirements and low energy requirements [
14]. A major disadvantage of biological treatment with microorganisms is that the processing time varies and may take up to 60 days in some cases, therefore it is viewed as too long to be economically viable for biofuel production [
26]. Physiochemical pretreatment processes encompass a combination of physical changes and chemical reactions to disrupt the structure of the biomass [
14]. The lignocellulosic biomass is treated at high temperatures or pressures, with an inorganic compound, which causes the disruption of its recalcitrant structure [
26]. This type of pretreatment includes steam explosion, ammonia fiber explosion, and carbon dioxide explosion [
14]. These processes have a low energy requirement and decrease the cellulose crystallinity [
14].
Sonochemistry is regarded as the application of ultrasonic irradiation to physical and chemical processes to enhance and facilitate chemical reactions [
27]. The phenomenon of acoustic cavitation is the mechanism that causes sonochemical effects in solutions and liquids [
28]. Acoustic cavitation causes the formation, growth, and implosive collapse of bubbles in a liquid [
29]. This results in a pressure difference, which acts to overcome the adhesion and cohesion forces of the liquid or solution undergoing ultrasonication [
30]. The cavitation produced by the ultrasound releases large amounts of heat energy and mechanical energy, which enhances physical and chemical processes such as synthesis and catalysis [
30]. Acoustic cavitation occurs at low frequencies (20–100 kHz), where most of the energy from the ultrasound waves are dissipated into the medium [
31]. Sonochemical reactions undergo an increase in reaction rate and reaction output [
28]. When ultrasound is applied for biomass dissolution, there are both mechanical and chemical effects. On the mechanical side, microjetting and microstreaming occur. The collision of microjets, formed by acoustic cavitation, with particles result in shearing and pitting of the particle surfaces, increasing the surface area available for reaction with the liquid medium. Microstreaming disperses very fine particles and facilitates uniform dissolution [
32]. The energy added to the reaction medium by ultrasonic irradiation is also utilized locally to accelerate the chemical reaction of the dissolution agent (e.g., acid) and the biomass.
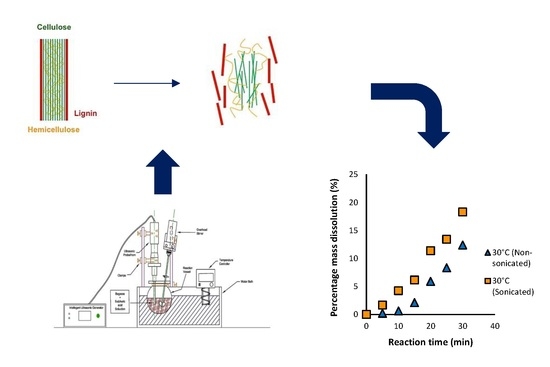
The primary hypothesis for this study was that ultrasonic irradiation improves the dissolution of biomass and that this effect is independent of temperature. The secondary hypothesis was that the ultrasonic irradiation will have a greater influence on performance than temperature when a low solid loading is employed since there would be increased contact for permeation of the acid into the biomass, and propensity for acoustic cavitation. The effect of ultrasonication on the dissolution of biomass during dilute acid hydrolysis was investigated in this work. The study considered dilute acid hydrolysis, since the corrosiveness of the reaction mixture is not as great as the concentrated acid pretreatment process, invariably reducing equipment costs. A low solid loading of biomass was used, which has not been reported in the literature, particularly with the application of ultrasound. Two key objectives were identified in this study. The first was to examine the effect of sonication on the dissolution of bagasse using varying reaction times and temperatures during dilute acid hydrolysis. The second objective was to compare the dissolution with and without the use of sonication and apply a simple kinetic model to the results obtained to further quantify the effect.