Effectiveness of Scenedesmus sp. Biomass Grow and Nutrients Removal from Liquid Phase of Digestates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Microalgae Inoculum, Nutrient Medium Characteristics and Cultivation
2.3. Analytical Methods
2.4. Statistical Methods
3. Results and Discussion
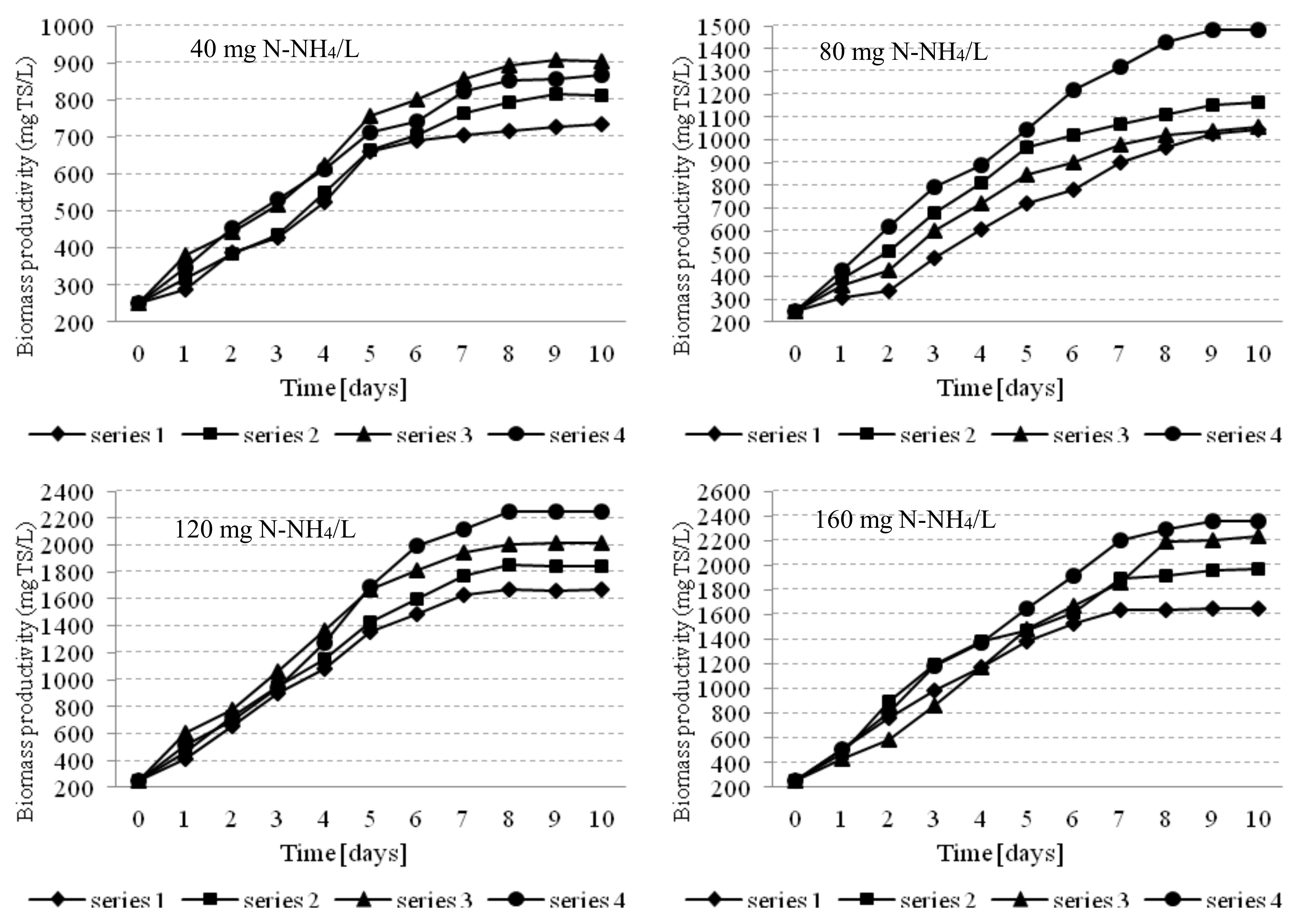
3.1. Microalgae Biomass Production
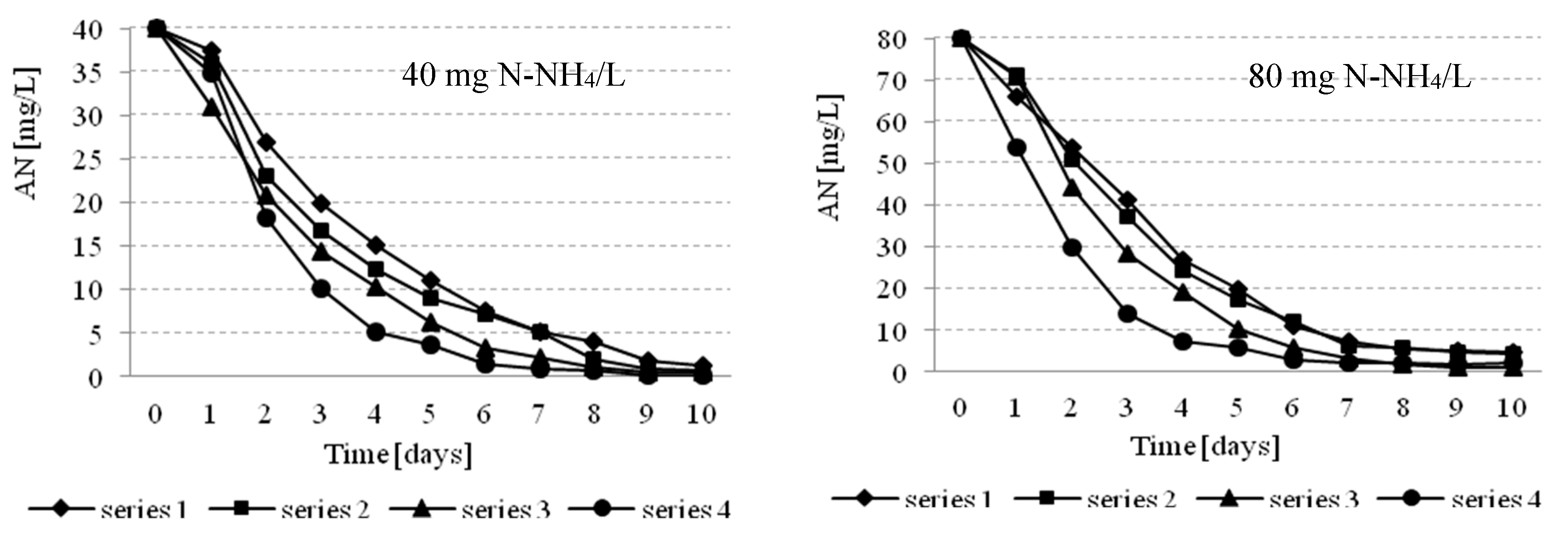
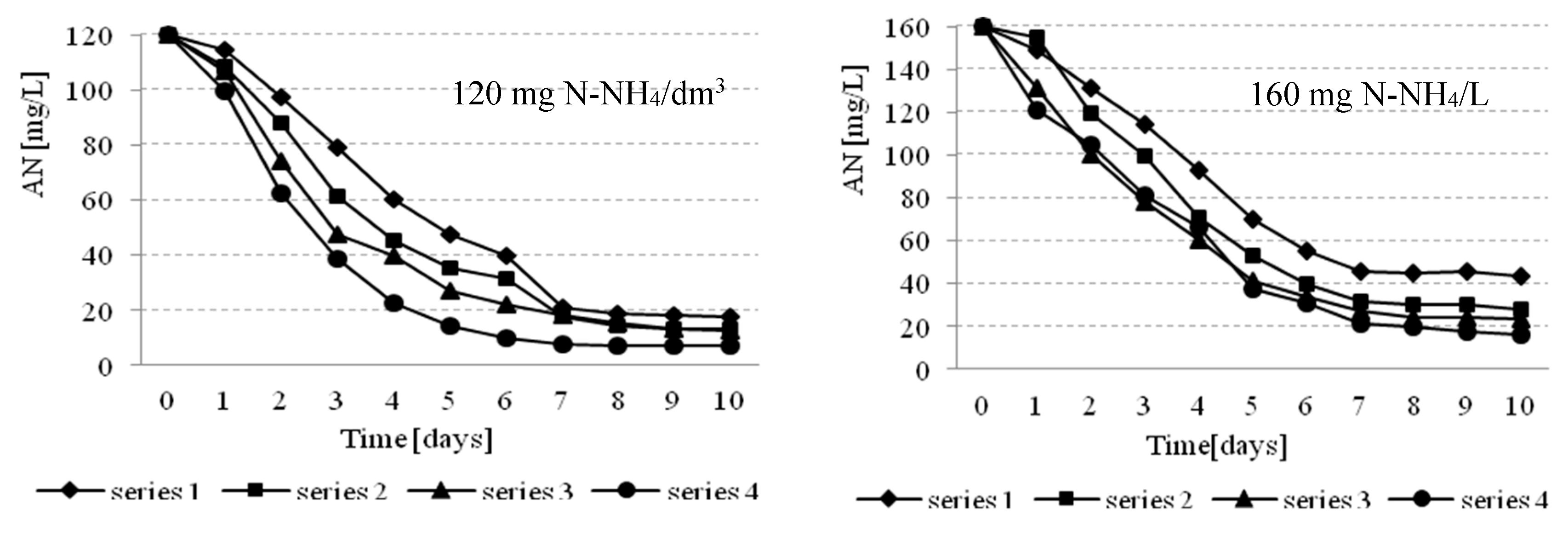
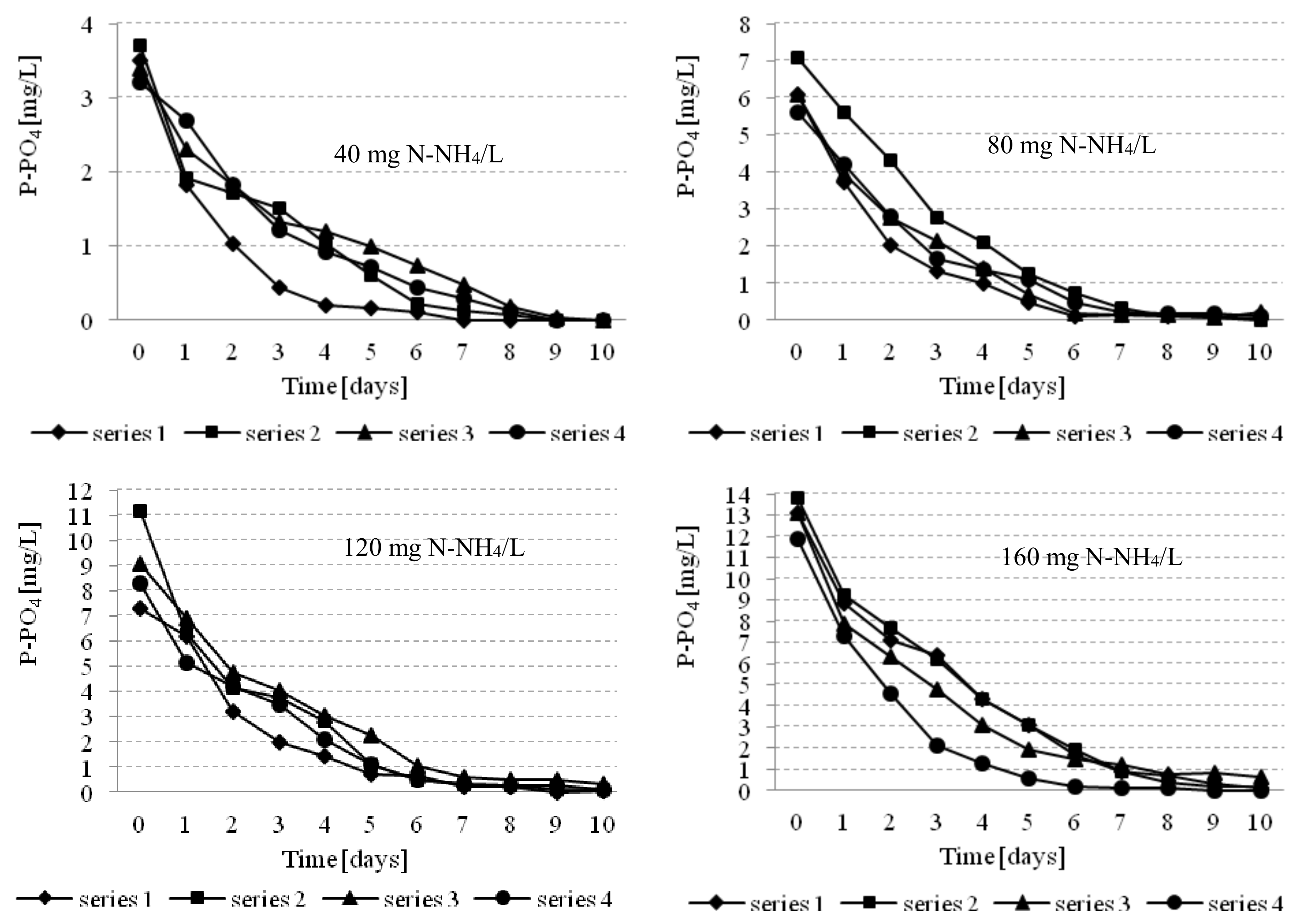
3.2. Effectiveness of Nutrients Removal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.; Aila, M.; Bangwal, D.P.; Kaul, S.; Garg, M.O. Algae based biorefinery—How to make sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M. Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J. Aquatic biomass (algae) as a future feed stock for bio-refineries: A review on cultivation, processing and products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wu, N.; Lan, C. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.; Dubois-Calero, N. Biofuels from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadib, T.; Oleskowicz-Popielc, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Rajeshwari, K.V.; Balakrishnan, M.; Kansal, A.; Lata, K.; Kishore, V.V.N. State-of-the-art of anaerobic digestion technology for industrial wastewater treatment. Renew. Sustain. Energy Rev. 2000, 4, 135–156. [Google Scholar] [CrossRef]
- Oswald, W.J. My sixty years in applied algology. J. Appl. Phycol. 2003, 15, 99–106. [Google Scholar] [CrossRef]
- Mùnoz, R.; Kollner, C.; Guieysse, B.; Mattiasson, B. Photosynthetically oxygenated salicylate biodegradation in a continuous stirred tank photobioreactor. Biotechnol. Bioeng. 2004, 87, 797–803. [Google Scholar] [CrossRef]
- Moheimani, R.M. The Culture of Coccolithophorid Alga for Carbon Dioxide Remediation. Ph.D. Thesis, Murdoch University, Perth, Australia, 2005. [Google Scholar]
- Mùnoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A.V. Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers. Manag. 2007, 48, 2169–2173. [Google Scholar] [CrossRef]
- Lundquist, T.J. Production of algae in conjunction with wastewater treatment. In NREL-AFOSR Workshop on Algal Oil for Jet Fuel Production; U.S. Department of Energy: Arlington, VA, USA, 2008. [Google Scholar]
- Cai, T.; Park, S.Y.; Racharaks, R.; Li, Y. Cultivation of Nannochloropsis salina using anaerobic digestion effluent as a nutrient source for biofuel production. Appl. Energy 2013, 108, 486–492. [Google Scholar] [CrossRef]
- Park, J.B.; Craggs, R.J.; Shilton, A.N. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Emdadi, D.; Berland, B. Variation in lipid class composition during batch growthof Nannochloropsissalina and Pavlova lutheri. Mar. Chem. 1989, 26, 215–225. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, B.; Latrille, E.; Steyer, J.P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, Y.-F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J. Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Gorecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef]
- Mùnoz, R.; Kollner, C.; Guieysse, B.; Mattiasson, B. Salicylate biodegradation by various algal–bacterial consortia under photosynthetic oxygenation. Biotechnol. Lett. 2003, 25, 1905–1911. [Google Scholar] [CrossRef]
- Lin, L.; Chan, G.Y.S.; Jiang, B.L.; Lan, C.Y. Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag. 2007, 27, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Yewalkar, S.N.; Dhumal, K.N.; Sainis, J.K. Chromium (VI)-reducing Chlorella spp. isolated from disposal sites of paper-pulp and electroplating industry. J. Appl. Phycol. 2007, 19, 459–465. [Google Scholar]
- Acuner, E.; Dilek, F.B. Treatment of tectilon yellow 2G by Chlorella vulgaris. Process Biochem. 2004, 39, 623–631. [Google Scholar] [CrossRef]
- Essam, T.; Magdy, A.A.; El Tayeb, O.; Mattiasson, B.; Guieysse, B. Solar-based detoxification of phenol and p-nitrophenol by sequential TiO2photocatalysis and photosynthetically aerated biological treatment. Water Res. 2007, 41, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Valderramaa, L.T.; Del Campoa, C.M.; Rodrigueza, C.M.; de-Bashan, L.E.; Bashan, Y. Treatment of recalcitrant wastewater from ethanol and citric acid production using the microalga Chlorella vulgaris and the macrophyteLemna minuscule. Water Res. 2002, 36, 4185–4192. [Google Scholar] [CrossRef]
- Tien, C.J. Biosorption of metal ions by freshwater algae with different surface characteristics. Process Biochem. 2002, 38, 605–613. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Coupled nutrient removal and biomass production with mixed algal culture: Impact of biotic and abiotic factors. Bioresour. Technol. 2012, 118, 469–476. [Google Scholar] [CrossRef]
- Cho, S.; Lee, N.; Park, S.; Yu, J.; Luong, T.T.; Oh, Y.K.; Lee, T. Microalgae cultivation for bioenergy production using wastewaters from a municipal WWTP as nutritional sources. Bioresour. Technol. 2013, 131, 515–520. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Li, Z.; Wang, W.; Tong, Y.; Wei, J. Microalgal cultivation in wastewater from the fermentation effluent in Riboflavin (B2) manufacturing for biodiesel production. Bioresour. Technol. 2013, 143, 499–504. [Google Scholar] [CrossRef]
- Gomez Villa, H.; Voltolina, D.; Nieves, M.; Pina, P. Biomass production and nutrient budget in outdoor cultures of Scenedesmus obliquus (chlorophyceae) in artificial wastewater, under the winter and summer conditions of Mazatla´ n, Sinaloa, Mexico. Vie Milieu 2005, 55, 121–126. [Google Scholar]
- Yun, Y.-S.; Lee, S.B.; Park, J.M.; Lee, C.-I.; Yang, J.-W. Carbon dioxide fixation by algal cultivation using wastewater nutrients. J. Chem. Technol. Biotechnol. 1997, 69, 451–455. [Google Scholar] [CrossRef]
| Series | Source of Nutrients | ||||
|---|---|---|---|---|---|
| Series 1 | Series 2 | Series 3 | Series 4 | ||
| Variant | Initial concentration of ammonia nitrogen (mg/L) | ||||
| 1 | 40 | 40 | 40 | 40 | |
| 2 | 80 | 80 | 80 | 80 | |
| 3 | 120 | 120 | 120 | 120 | |
| 4 | 160 | 160 | 160 | 160 | |
| Parameter | Unit | Series 1 | Series 2 | Series 3 | Series 4 |
|---|---|---|---|---|---|
| COD | mg O2/L | 9200 ± 470 | 8730 ± 340 | 7980 ± 510 | 7400 ± 220 |
| BOD5 | mg O2/L | 5400 ± 410 | 5430 ± 250 | 4840 ± 330 | 4130 ± 190 |
| TN | mg N/L | 1750 ± 190 | 1670 ± 130 | 1420 ± 110 | 1290 ± 120 |
| AN | mg N-NH4/L | 1120 ± 140 | 1280 ± 90 | 1010 ± 70 | 980 ± 80 |
| TP | mg P/L | 104 ± 21 | 99 ± 27 | 72 ± 17 | 68 ± 13 |
| P-PO4 | mg P-PO4/L | 77 ± 14 | 72 ± 21 | 51 ± 12 | 49 ± 11 |
| pH | - | 6.9 ± 0.1 | 6.9 ± 0.2 | 7.0 ± 0.2 | 7.0 ± 0.1 |
| Variant | Parameter | Unit | Series 1 | Series 2 | Series 3 | Series 4 |
|---|---|---|---|---|---|---|
| 1 | COD | mg O2/L | 319 ± 14 | 309 ± 32 | 277 ± 35 | 269 ± 17 |
| BOD5 | mg O2/L | 202 ± 9 | 193 ± 19 | 159 ± 21 | 121 ± 19 | |
| TN | mg N/L | 52 ± 7 | 61 ± 16 | 63 ± 7 | 54 ± 9 | |
| AN | mg N-NH4/L | 41 ± 5 | 40 ± 11 | 42 ± 12 | 41 ± 7 | |
| TP | mg P/L | 4.3 ± 1.2 | 5.2 ± 1.1 | 5.6 ± 1.1 | 4.9 ± 1.0 | |
| P-PO4 | mg P-PO4/L | 3.5 ± 1.1 | 3.7 ± 1.3 | 3.4 ± 0.9 | 3.2 ± 0.8 | |
| pH | - | 7.3 ± 0.1 | 7.2 ± 0.1 | 7.2 ± 0.1 | 7.2 ± 0.1 | |
| 2 | COD | mg O2/L | 607 ± 64 | 638 ± 48 | 581 ± 47 | 544 ± 31 |
| BOD5 | mg O2/L | 346 ± 31 | 306 ± 33 | 302 ± 31 | 345 ± 16 | |
| TN | mg N/L | 103 ± 17 | 123 ± 19 | 114 ± 22 | 109 ± 21 | |
| AN | mg N-NH4/L | 79 ± 12 | 83 ± 21 | 82 ± 17 | 74 ± 11 | |
| TP | mg P/L | 7.9 ± 1.4 | 9.2 ± 1.8 | 9.3 ± 1.4 | 8.3 ± 0.1 | |
| P-PO4 | mg P-PO4/L | 6.1 ± 2.0 | 7.1 ± 1.1 | 6.1 ± 2.5 | 5.6 ± 0.9 | |
| pH | - | 7.3 ± 0.2 | 7.2 ± 0.2 | 6.9 ± 0.1 | 7.1 ± 1.3 | |
| 3 | COD | mg O2/L | 997 ± 93 | 1002 ± 93 | 782 ± 102 | 738 ± 72 |
| BOD5 | mg O2/L | 688 ± 44 | 599 ± 41 | 361 ± 77 | 338 ± 38 | |
| TN | mg N/L | 161 ± 12 | 193 ± 26 | 192 ± 29 | 170 ± 20 | |
| AN | mg N-NH4/L | 120 ± 19 | 121 ± 23 | 119 ± 20 | 121 ± 13 | |
| TP | mg P/L | 9.3 ± 2.0 | 14.9 ± 3.4 | 13.9 ± 3.1 | 11.9 ± 2.6 | |
| P-PO4 | mg P-PO4/L | 7,3 ± 1.7 | 11.2 ± 3.1 | 9.1 ± 2.3 | 8.3 ± 1.9 | |
| pH | - | 7.1 ± 0.2 | 7.2 ± 0.2 | 7.0 ± 0.2 | 7.0 ± 0.5 | |
| 4 | COD | mg O2/L | 1204 ± 103 | 1310 ± 203 | 1137 ± 194 | 1040 ± 102 |
| BOD5 | mg O2/L | 697 ± 59 | 920 ± 143 | 591 ± 88 | 537 ± 57 | |
| TN | mg N/L | 214 ± 33 | 271 ± 41 | 252 ± 31 | 232 ± 23 | |
| AN | mg N-NH4/L | 163 ± 28 | 162 ± 23 | 160 ± 30 | 161 ± 12 | |
| TP | mg P/L | 17.3 ± 3.9 | 19.1 ± 4.1 | 20.3 ± 4.2 | 18.2 ± 3.8 | |
| P-PO4 | mg P-PO4/L | 13.1 ± 2.6 | 13.8 ± 3.9 | 13.1 ± 3.1 | 11.9 ± 2.2 | |
| pH | - | 7.1 ± 0.1 | 7.0 ± 0.1 | 7.0 ± 0.2 | 7.2 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisielewska, M.; Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Romanowska-Duda, Z.; Dudek, M. Effectiveness of Scenedesmus sp. Biomass Grow and Nutrients Removal from Liquid Phase of Digestates. Energies 2020, 13, 1432. https://doi.org/10.3390/en13061432
Kisielewska M, Zieliński M, Dębowski M, Kazimierowicz J, Romanowska-Duda Z, Dudek M. Effectiveness of Scenedesmus sp. Biomass Grow and Nutrients Removal from Liquid Phase of Digestates. Energies. 2020; 13(6):1432. https://doi.org/10.3390/en13061432
Chicago/Turabian StyleKisielewska, Marta, Marcin Zieliński, Marcin Dębowski, Joanna Kazimierowicz, Zdzisława Romanowska-Duda, and Magda Dudek. 2020. "Effectiveness of Scenedesmus sp. Biomass Grow and Nutrients Removal from Liquid Phase of Digestates" Energies 13, no. 6: 1432. https://doi.org/10.3390/en13061432







