One-Dimensional Study on Hydrate Formation from Migrating Dissolved Gas in Sandy Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Materials and Experiment Procedure
3. Results
3.1. Pressure Variation
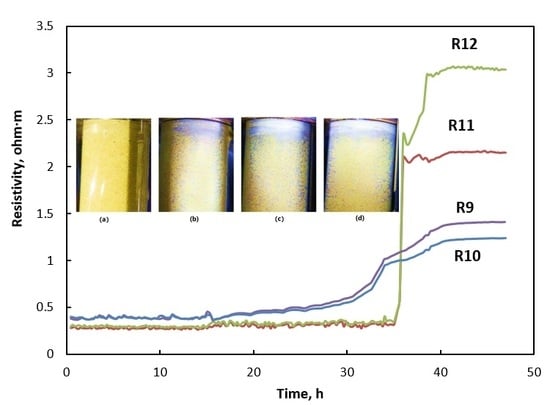
3.2. Electrical Resistivity and Hydrate Morphology
3.3. Hydrate Distribution
3.4. Permeability of Hydrate-Bearing Sediment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth-Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47, RG4003. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, Y.; Zhang, H.; Xiao, B.; Guo, X.; Wei, R.; Xu, L.; Sun, L.; Yu, B.; Leng, S.; et al. The status of exploitation techniques of natural gas hydrate. Chin. J. Chem. Eng. 2019, 27, 2133–2147. [Google Scholar] [CrossRef]
- Yin, Z.; Linga, P. Methane hydrates: A future clean energy resource. Chin. J. Chem. Eng. 2019, 27, 2026–2036. [Google Scholar] [CrossRef]
- Madden, M.E.; Ulrich, S.; Szymcek, P.; McCallum, S.; Phelps, T. Experimental formation of massive hydrate deposits from accumulation of CH4 gas bubbles within synthetic and natural sediments. Mar. Petrol. Geol. 2009, 26, 369–378. [Google Scholar] [CrossRef]
- Li, H.; Wei, N.; Jiang, L.; Zhao, J.; Cui, Z.; Sun, W.; Zhang, L.; Zhou, S.; Xu, H.; Zhang, X.; et al. Evaluation of experimental setup and procedure for rapid preparation of natural gas hydrate. Energies 2020, 13, 531. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.; Yee, D.; Linga, P.; Palmer, A.; Khoo, B.C.; Tan, T.S.; Rangsunvigit, P. Morphology of methane hydrate formation in porous media. Energy Fuels 2013, 27, 3364–3372. [Google Scholar] [CrossRef]
- Fandiño, O.; Ruffine, L. Methane hydrate nucleation and growth from the bulk phase: Further insights into their mechanisms. Fuel 2014, 117, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Ruffine, L. Exploring methane-hydrate formation and dissociation in geologic materials through laboratory experiments: Kinetic behavior and morphology. Fuel 2015, 141, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Xue, K.H.; Yang, L.; Zhao, J.F.; Li, Y.H.; Song, Y.C.; Yao, S. The study of flow characteristics during the decomposition process in hydrate-bearing porous media using magnetic resonance imaging. Energies 2019, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.T.; Li, N.; Sun, C.Y.; Chen, G.J.; Koh, C.A.; Sun, B.J. Hydrate formation in sediments from free gas using a one-dimensional visual simulator. Fuel 2017, 197, 298–309. [Google Scholar] [CrossRef]
- Hyndman, R.D.; Davis, E.E. A mechanism for the formation of methane hydrate and seafloor bottom simulating_reflectors. J. Geophy. Res. 1992, 97, 7025–7041. [Google Scholar] [CrossRef]
- Buffett, B.; Zatsepina, O. Formation of gas hydrate from dissolved gas in natural porous media. Mar. Geol. 2000, 164, 69–77. [Google Scholar] [CrossRef]
- Tohidi, B.; Anderson, R.; Clennell, M.B.; Burgass, R.W.; Biderkab, A.B. Visual observation of gas-hydrate formation and dissociation in synthetic porous media by means of glass micromodels. Geology 2001, 29, 867–870. [Google Scholar] [CrossRef]
- Spangenberg, E.; Kulenkampff, J.; Naumann, R.; Erzinger, J. Pore space hydrate formation in a glass bead sample from methane dissolved in water. Geophys. Res. Lett. 2005, 32, L24301. [Google Scholar] [CrossRef]
- Spangenberg, E.; Kulenkampff, J. Influence of methane hydrate content on electrical sediment properties. Geophys. Res. Lett. 2006, 33, L24315. [Google Scholar] [CrossRef]
- Waite, W.F.; Spangenberg, E. Gas hydrate formation rates from dissolved-phase methane in porous laboratory specimens. Geophys. Res. Lett. 2013, 40, 4310–4315. [Google Scholar] [CrossRef]
- Li, X.S.; Yang, B.; Zhang, Y.; Li, G.; Duan, L.P.; Wang, Y.; Chen, Z.Y.; Huang, N.S.; Wu, H.J. Experimental investigation into gas production from methane hydrate in sediment by depressurization in a novel pilot-scale hydrate simulator. Appl. Energy 2012, 93, 722–732. [Google Scholar] [CrossRef]
- Lin, Z.; Dong, H.; Fang, H.; Sun, J.; Wang, X. Sensitivity Analysis of Rock Electrical influencing factors of natural gas hydrate reservoir in permafrost region of Qilian Mountain, China. Energies 2019, 12, 4592. [Google Scholar] [CrossRef] [Green Version]
- Priegnitz, M.; Thaler, J.; Spangenberg, E.; Rucker, C.; Schicks, J.M. A cylindrical electrical resistivity tomography array for three-dimensional monitoring of hydrate formation and dissociation. Rev. Sci. Instrum. 2013, 84, 104502. [Google Scholar] [CrossRef] [PubMed]
- Archie, G.E. The electrical resistivity log as an aid in determining some reservoir characteristics. J. Pet. Sci. Technol. 1942, 5, 1–8. [Google Scholar] [CrossRef]
- Priest, J.A.; Rees, E.V.L.; Clayton, C.R.I. Influence of gas hydrate morphology on the seismic velocities of sands. J. Geophys. Res. 2009, 114, B1205. [Google Scholar] [CrossRef] [Green Version]
- Nimblett, J. Permeability evolution during the formation of gas hydrates in marine sediments. J. Geophys. Res. 2003, 108, B10. [Google Scholar] [CrossRef]
- Konno, Y.; Yoneda, J.; Egawa, K.; Ito, T.; Jin, Y.; Kida, M.; Suzuki, K.; Fujii, T.; Nagao, J. Permeability of sediment cores from methane hydrate deposit in the Eastern Nankai Trough. Mar. Petrol. Geol. 2015, 66, 487–495. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206. [Google Scholar] [CrossRef]
- Ren, X.; Guo, Z.; Ning, F.; Ma, S. Permeability of hydrate-bearing sediments. Earth-Sci. Rev. 2020, 202, 103100. [Google Scholar] [CrossRef]
- Kleinberg, R.L. Deep sea NMR: Methane hydrate growth habit in porous media and its relationship to hydraulic permeability, deposit accumulation, and submarine slope stability. J. Geophys. Res. 2003, 108, B9. [Google Scholar] [CrossRef]
- Duan, Z.H.; Mao, S.D. A thermodynamic model for calculating methane solubility, density and gas phase composition of methane-bearing aqueous fluids from 273 to 523 K and from 1 to 2000 bar. Geochim. Cosmochim. Acta 2006, 70, 3369–3386. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B.; Cheremisin, A. CO2 Capture by Injection of Flue Gas or CO2–N2 Mixtures into Hydrate Reservoirs: Dependence of CO2 Capture Efficiency on Gas Hydrate Reservoir Conditions. Environ. Sci. Technol. 2018, 52, 4324–4330. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B.; Cheremisin, A. Geological CO2 capture and storage with flue gas hydrate formation in frozen and unfrozen sediments: Method development, real time-scale kinetic characteristics, efficiency, and clathrate structural transition. ACS Sustain. Chem. Eng. 2019, 7, 5338–5345. [Google Scholar] [CrossRef]
- Okwananke, A.; Hassanpouryouzband, A.; Farahani, M.V.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B. Methane recovery from gas hydrate-bearing sediments: An experimental study on the gas permeation characteristics under varying pressure. J. Petrol. Sci. Eng. 2019, 180, 435–444. [Google Scholar] [CrossRef]









| Items | Value |
|---|---|
| Pressure | 7.4 MPa |
| Interface chamber temperature | 284 K |
| Sediment temperature | 280 K |
| Warm-bath temperature | 282 K |
| Sediment porosity | 0.39 |
| Flow rate | 105 cm3/min |
| Aqueous brine | 3.5 wt % |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Rehemituli, R.; Zhang, J.; Sun, C. One-Dimensional Study on Hydrate Formation from Migrating Dissolved Gas in Sandy Sediments. Energies 2020, 13, 1570. https://doi.org/10.3390/en13071570
Li N, Rehemituli R, Zhang J, Sun C. One-Dimensional Study on Hydrate Formation from Migrating Dissolved Gas in Sandy Sediments. Energies. 2020; 13(7):1570. https://doi.org/10.3390/en13071570
Chicago/Turabian StyleLi, Nan, Rezeye Rehemituli, Jie Zhang, and Changyu Sun. 2020. "One-Dimensional Study on Hydrate Formation from Migrating Dissolved Gas in Sandy Sediments" Energies 13, no. 7: 1570. https://doi.org/10.3390/en13071570