Local Green Power Supply Plants Based on Alcohol Regenerative Gas Turbines: Economic and Environmental Aspects
Abstract
:1. Introduction
- lower carbon dioxide emissions;
- energy diversification;
- strengthening energy security;
- slowing the depletion of fossil fuels.
2. Literature Review
3. Materials and Methods
3.1. Steam Reforming
3.2. Fuels
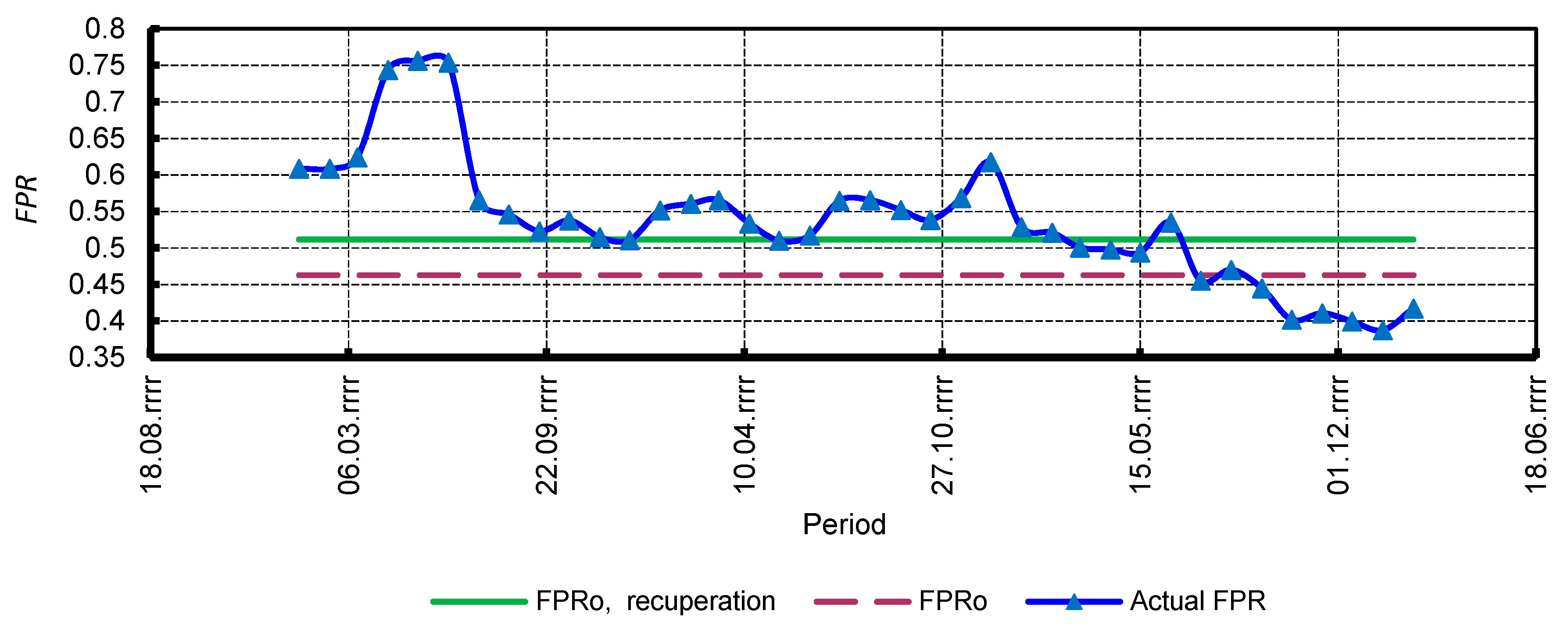
3.3. Efficiency Indicators
- lower heating values of primary alcohol fuels and syngas;
- syngas to alcohol lower heating value ratio;
- energy efficiency of conversion;
- gas turbine engine efficiency.
3.4. Carbon Dioxide Emission Indicator
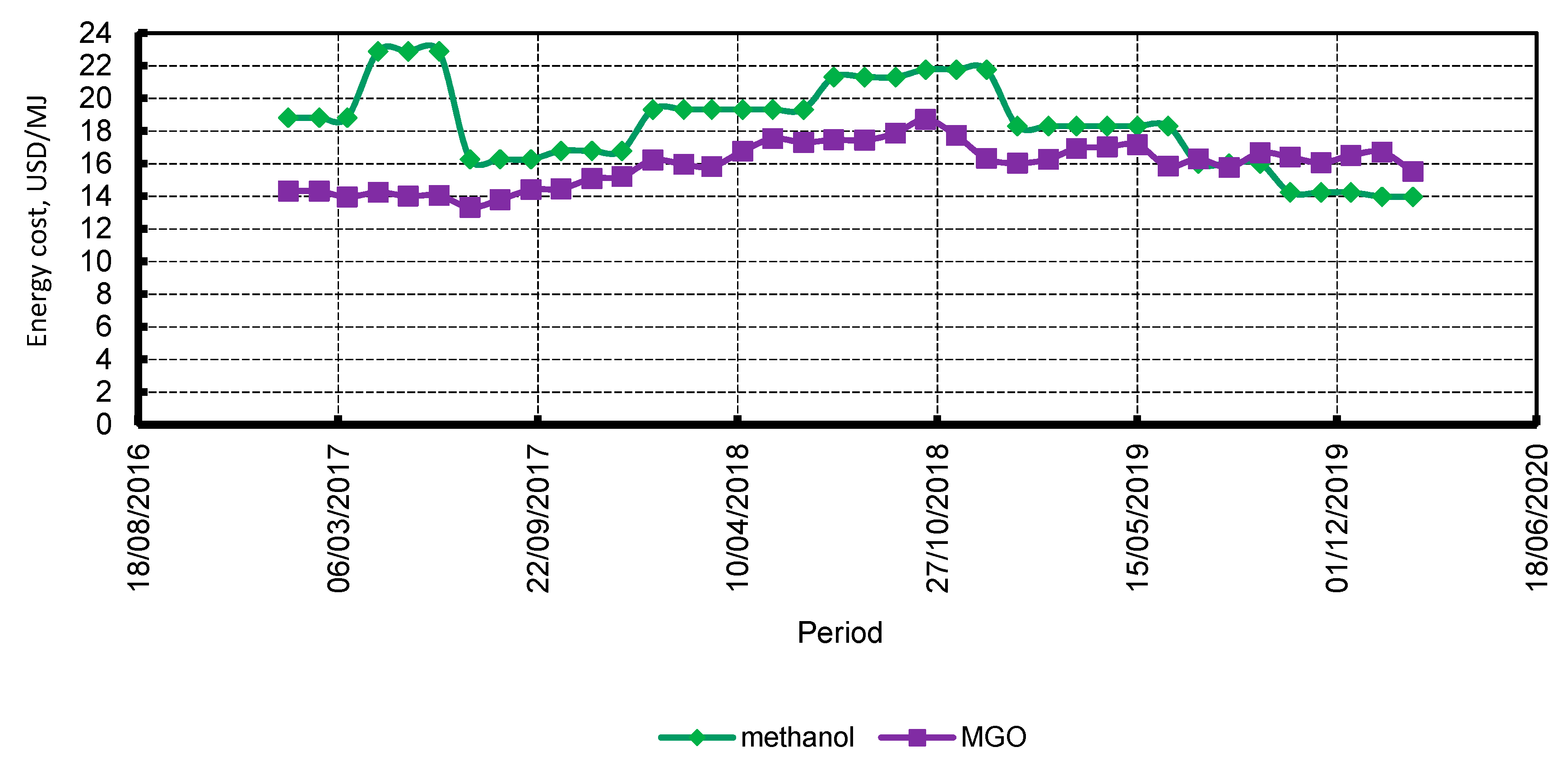
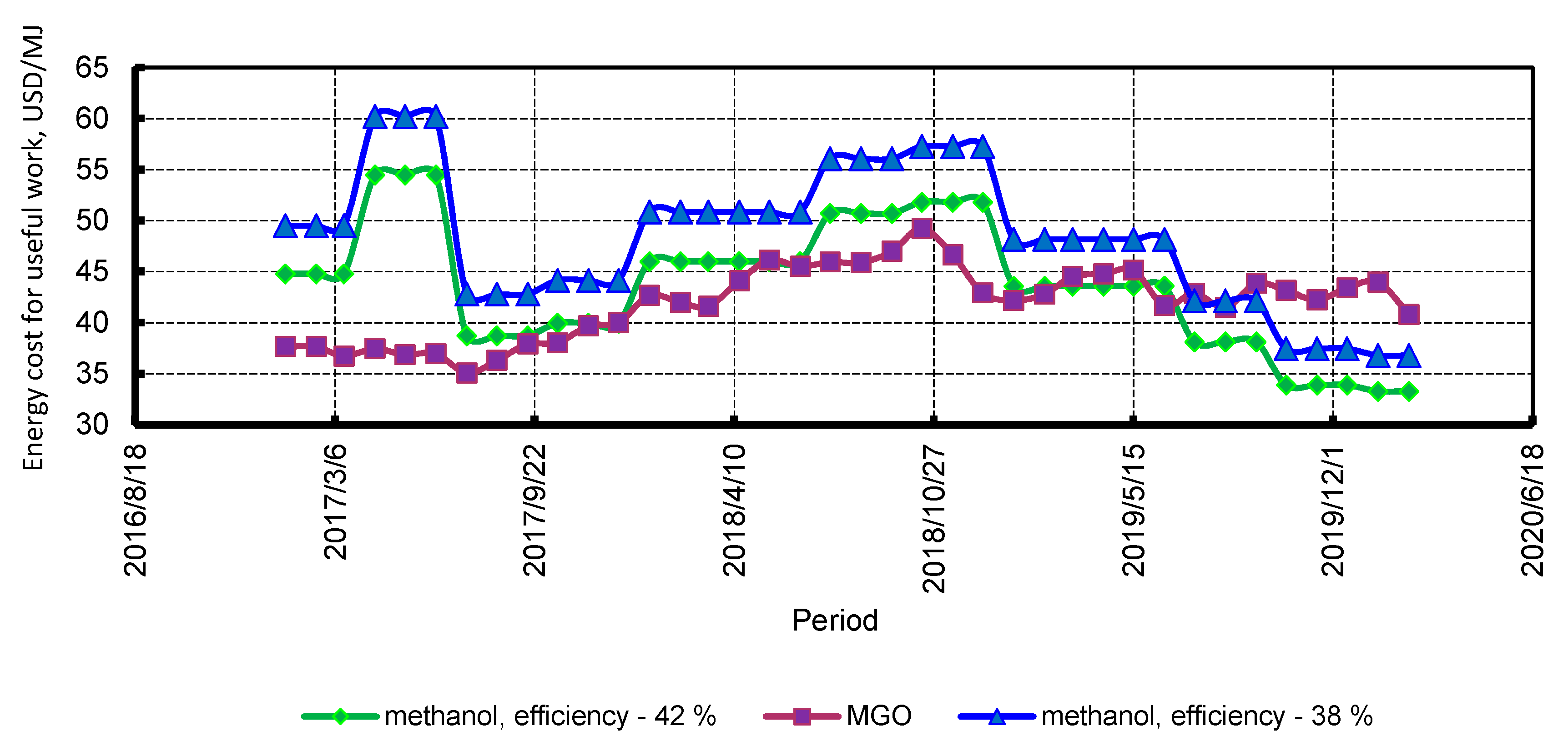
3.5. Economic Assessment
4. Results
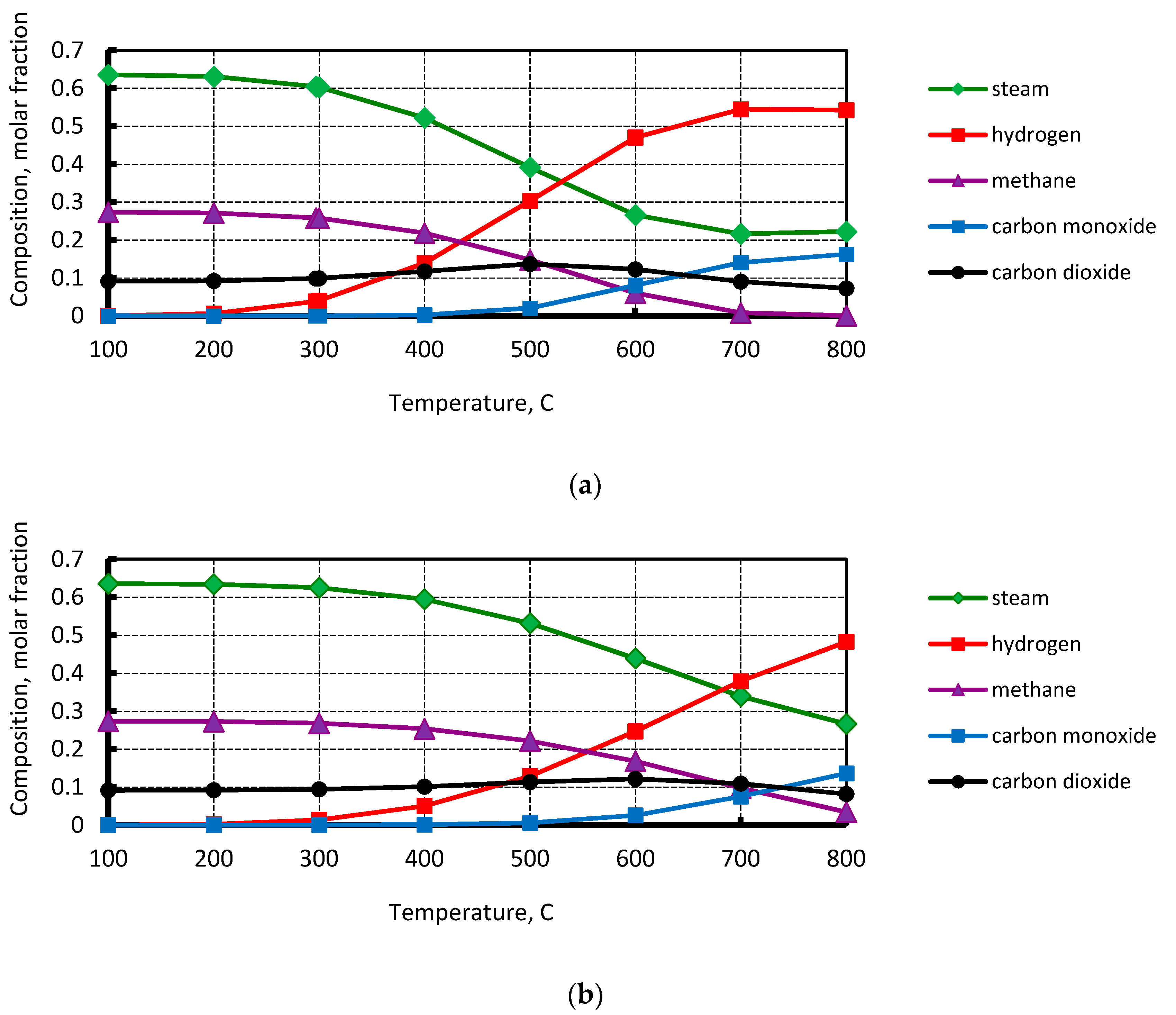
4.1. Steam Alcohol Conversion
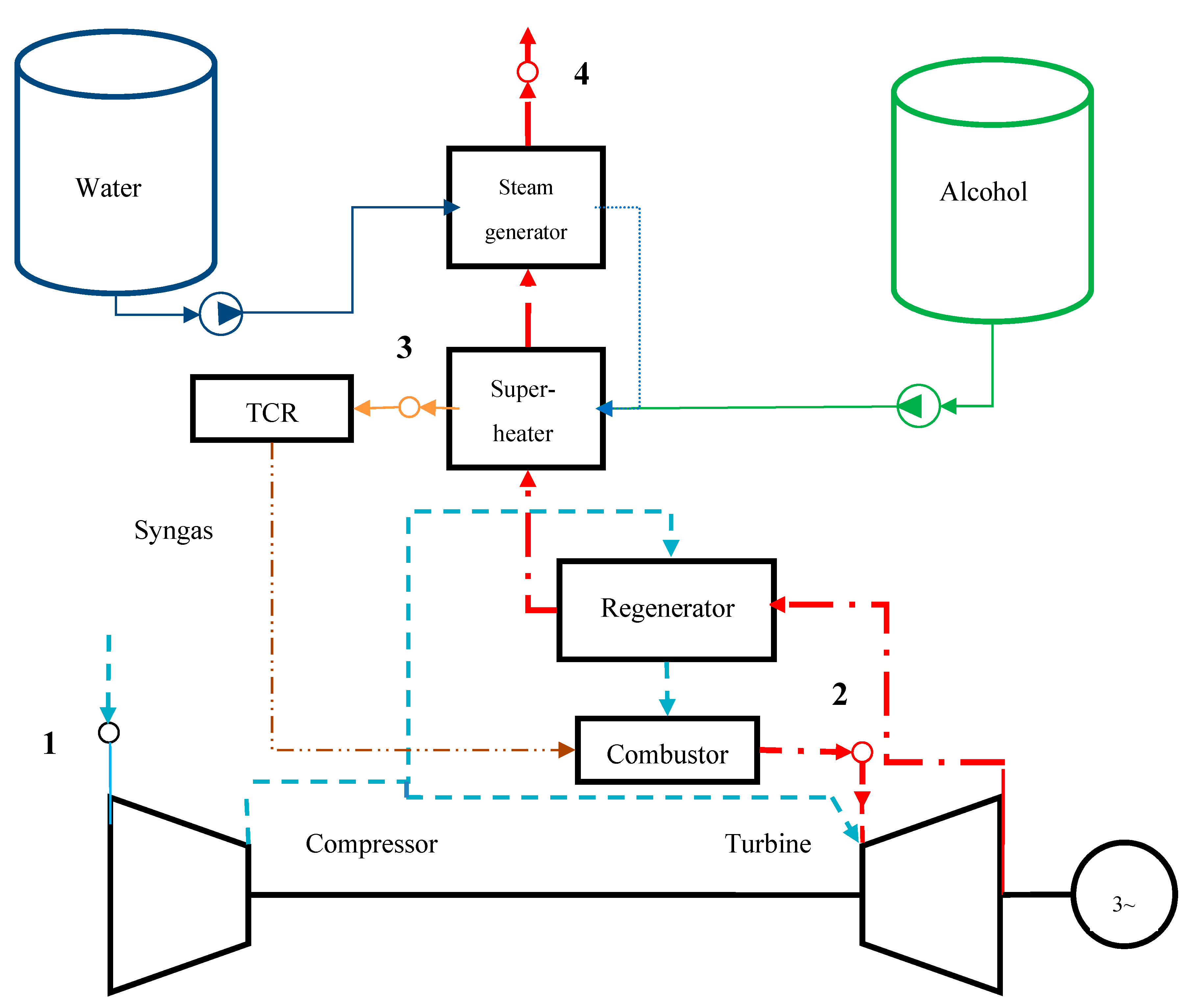
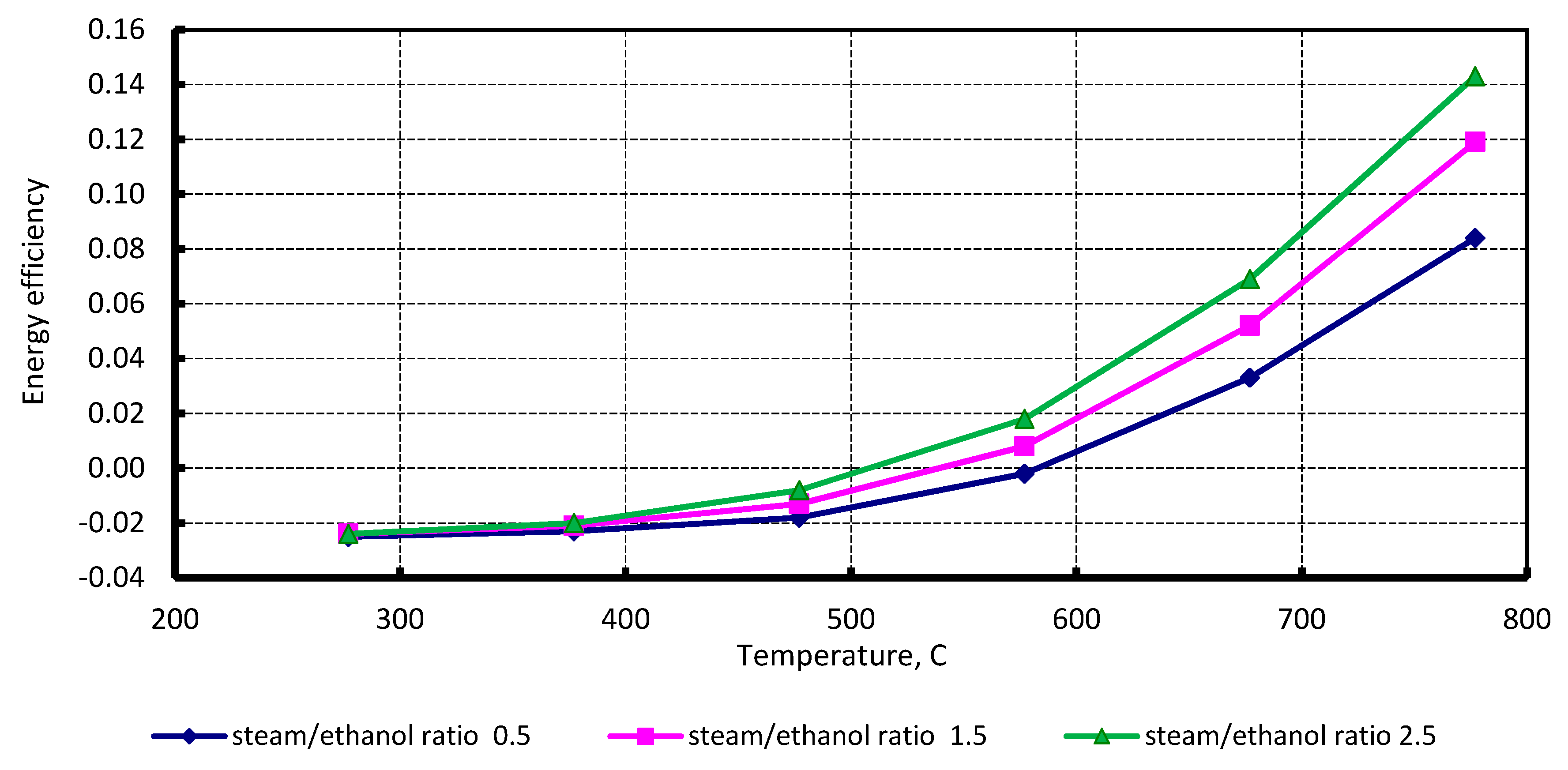
4.2. Regenerative Engine
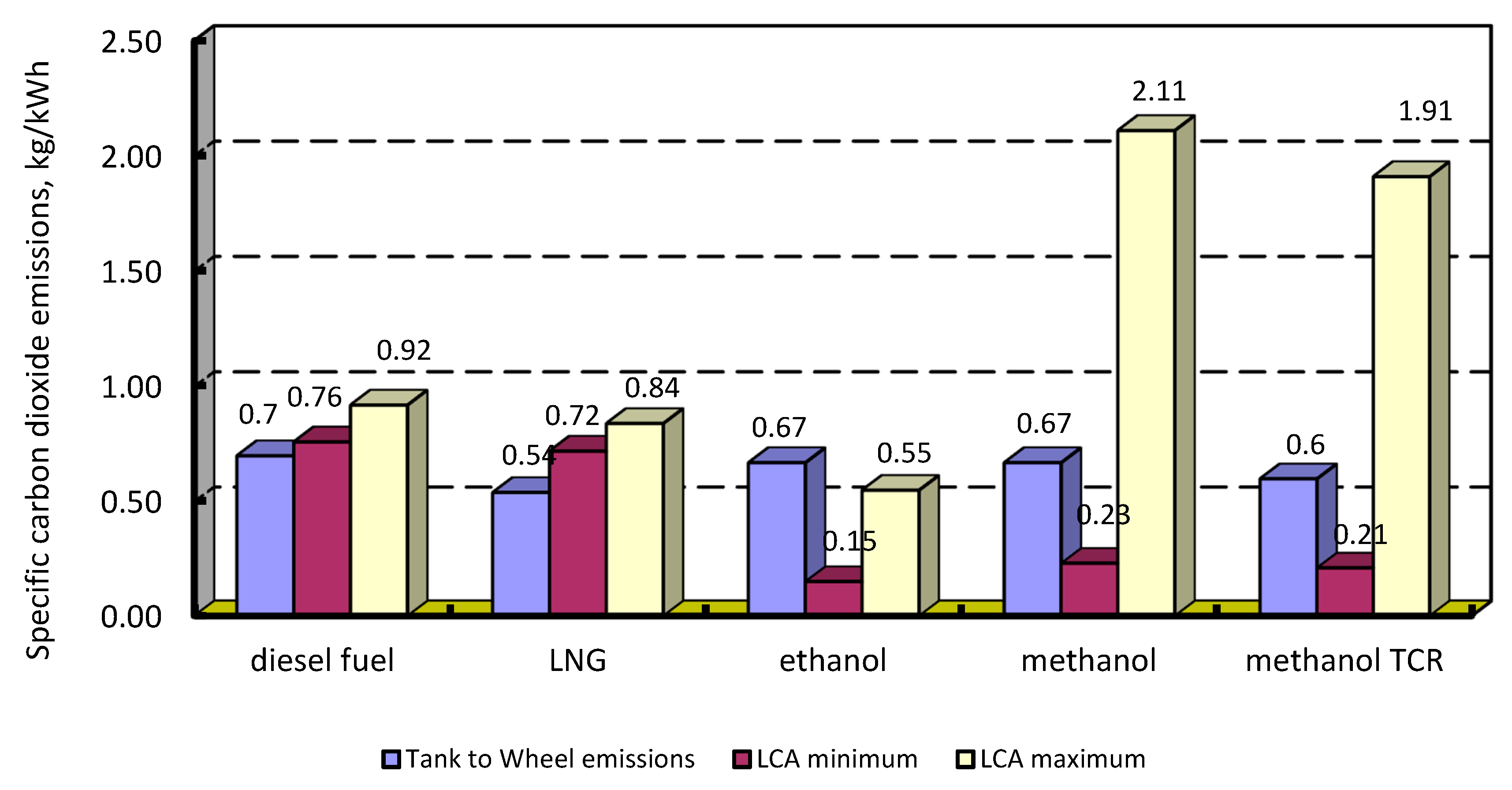
4.3. Carbon Dioxide Emission
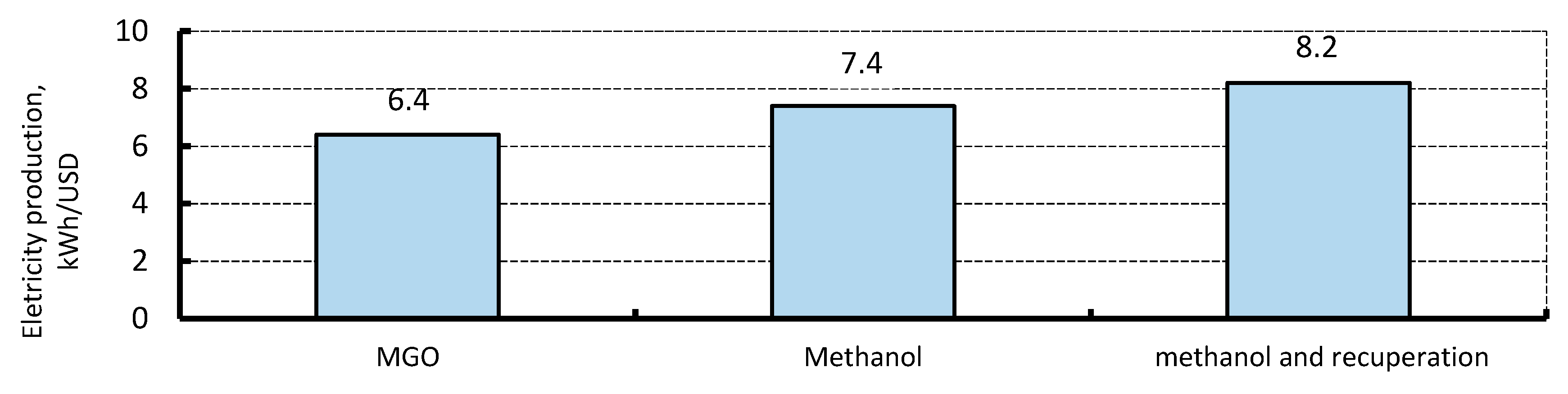
4.4. Economic Assessment
4.5. Combined Heat and Power Plant
- the load factor was varied from 60% to 100%;
- the price ratios were assumed of 4, 5.6 (the base case), and 7.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- BP Energy Outlook: 2019 Edition. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2019.pdf (accessed on 20 January 2020).
- Yoon, S.K.; Kim, M.S.; Kim, H.J.; Choi, N.J. Effects of canola oil biodiesel fuel blends on combustion, performance, and emissions reduction in a common rail diesel engine. Energies 2014, 7, 8132–8149. [Google Scholar] [CrossRef] [Green Version]
- Qasim, M.; Ansari, T.M.; Hussain, M. Combustion, performance, and emission evaluation of a diesel engine with biodiesel like fuel blends derived from a mixture of Pakistani waste canola and waste transformer oils. Energies 2017, 10, 1023. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yao, C.; Wang, Q.; Han, G.; Dou, Z.; Wei, H.; Wang, B.; Liu, M.; Wu, T. Study of cylinder-to-cylinder variation in a diesel engine fueled with diesel/methanol dual fuel. Fuel 2016, 170, 67–76. [Google Scholar] [CrossRef]
- European Union. Directive 2009/28/EC of the European Parliament and of the Council, 23 April 2009. Available online: http://eur-lex.europa.eu/legal-content/PL/ALL/?uri=celex%3A32009L0028/ (accessed on 12 January 2020).
- IMO Train the Trainer (TTT) Course on Energy Efficient Ship Operation. Module 2—Ship Energy Efficiency Regulations and Related Guidelines. 2016. Available online: http://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/IMO-Train-the-Trainer-Course.aspx (accessed on 20 January 2020).
- IPCC—Intergovernmental Panel on Climate Change. Fourth Assessment Report; UN IPCC: Geneva, Switzerland, 2007; Available online: https://www.ipcc.ch/assessment-report/ar4/ (accessed on 20 January 2020).
- Remme, U. Energy Technology Perspectives 2010: Scenarios & Strategies to 2050; OECD/IEA: Paris, France, 2010. [Google Scholar]
- Ge, J.C.; Kim, M.S.; Yoon, S.K.; Choi, N.J. Effects of pilot injection timing and EGR on combustion, performance and exhaust emissions in a common rail diesel engine fueled with a canola oil biodiesel-diesel blend. Energies 2015, 8, 7312–7325. [Google Scholar] [CrossRef] [Green Version]
- Atmanli, A.; Yilmaz, N. A comparative analysis of n-butanol/diesel and 1-pentanol/diesel blends in a compression ignition engine. Fuel 2018, 234, 161–169. [Google Scholar] [CrossRef]
- Yilmaz, N.; Atmanli, A.; Vigil, F.M. Quaternary blends of diesel, biodiesel, higher alcohols and vegetable oil in a compression ignition engine. Fuel 2018, 212, 462–469. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Pyrc, M.; Sobiepanski, M. Investigation on combustion process and emissions characteristic in direct injection diesel engine powered by wet ethanol using blend mode. Fuel Process. Technol. 2016, 149, 86–95. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Pyrc, M.; Sobiepanski, M. A comparative study of co-combustion process of diesel-ethanol and biodiesel-ethanol blends in the direct injection diesel engine. Appl. Therm. Eng. 2017, 117, 155–163. [Google Scholar] [CrossRef]
- Kuszewski, H. Experimental investigation of the effect of ambient gas temperature on the auto-ignition properties of ethanol–diesel fuel blends. Fuel 2018, 214, 26–38. [Google Scholar] [CrossRef]
- Tutak, W.; Lukács, K.; Szwaja, S.; Bereczky, Á. Alcohol–diesel fuel combustion in the compression ignition engine. Fuel 2015, 154, 196–206. [Google Scholar] [CrossRef]
- Tutak, W. Bioethanol E85 as a fuel for dual fuel diesel engine. Energy Convers. Manag. 2014, 86, 39–48. [Google Scholar] [CrossRef]
- Poran, A.; Tartakovsky, L. Energy efficiency of a direct injection internal combustion engine with high-pressure methanol steam reforming. Energy 2015, 88, 506–514. [Google Scholar] [CrossRef]
- Cherednichenko, O.; Serbin, S. Analysis of Efficiency of the Ship Propulsion System with Thermochemical Recuperation of Waste Heat. J. Mar. Sci. Appl. 2018, 17, 122–130. [Google Scholar] [CrossRef]
- Cherednichenko, O. Analysis of efficiency of diesel-gas turbine power plant with thermo-chemical heat recovery. MOTROL Comm. Mot. Energ. Agric. 2015, 17, 25–28. [Google Scholar]
- Matveev, I.; Serbin, S. Investigation of a reverse-vortex plasma assisted combustion system. In Proceedings of the ASME 2012 Heat Transfer Summer Conference, Rio Grande, Puerto Rico, USA, 8–12 July 2012; pp. 133–140. [Google Scholar] [CrossRef]
- Serbin, S. Features of liquid-fuel plasma-chemical gasification for diesel engines. IEEE Trans. Plasma Sci. 2006, 34, 2488–2496. [Google Scholar] [CrossRef]
- Serbin, S.I.; Matveev, I.B.; Goncharova, M.A. Plasma Assisted Reforming of Natural Gas for GTL. Part I. IEEE Trans. Plasma Sci. 2014, 42, 3896–3900. [Google Scholar] [CrossRef]
- Chakravarthy, V.K.; Daw, C.S.; Pihl, J.A.; Conklin, J.C. Study of the theoretical potential of thermochemical exhaust heat recuperation for internal combustion engines. Energy Fuels 2010, 24, 1529–1537. [Google Scholar] [CrossRef]
- Tartakovsky, L.; Baibikov, V.; Gutman, M.; Mosyak, A.; Veinblat, M. Performance Analysis of SI Engine Fuelled by Ethanol Steam Reforming Products; 2011-01-1992; SAE Technical Paper: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Tlili, I.; Hamadneh, N.; Khan, W.A. Thermodynamic analysis of MHD heat and mass transfer of nanofluids past a static wedge with Navier slip and convective boundary conditions. Arab. J. Sci. Eng. 2019, 44, 1255–1267. [Google Scholar] [CrossRef]
- Khan, M.N.; Tlili, I.; Khan, W.A. Thermodynamic optimization of new combined gas/steam power cycles with HRSG and heat exchanger. Arab. J. Sci. Eng. 2017, 42, 4547–4558. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, W.A.; Tlili, I. Forced convection of nanofluid flow across horizontal elliptical cylinder with constant heat flux boundary condition. J. Nanofluids 2019, 8, 386–393. [Google Scholar] [CrossRef]
- Gonca, G. The effects of turbine design parameters on the thermo-ecologic performance of a regenerated gas turbine running with different fuel kinds. Appl. Therm. Eng. 2018, 137, 419–429. [Google Scholar] [CrossRef]
- Musmar, S.A.; Razavinia, N.; Mucciardi, F.; Tlili, I. Performance analysis of a new waste heat recovery system. Int. J. Therm. Environ. Eng. 2016, 12, 47–52. [Google Scholar] [CrossRef]
- Wahab, A.; Ibrahim, T. The effects of the temperatures on the performance of the gas turbine. In The Latest Methods of Construction Design; Springer: Berlin, Germany, 2016; pp. 185–193. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, X.; Hong, H.; Han, W. An Innovative Gas Turbine Cycle with Methanol-Fueled Chemical-Looping Combustion. J. Eng. Gas Turbines Power 2009, 131, 061701. [Google Scholar] [CrossRef]
- Pan, F.; Zheng, H.; Liu, Q.; Yang, R. Design and performance calculations of chemically recuperated gas turbine on ship. Proc. Inst. Mech. Eng. Part A J. Power Energy 2013, 227, 908–918. [Google Scholar] [CrossRef]
- Al-Saraf, A.S.J.; Al-Jumaily, S.A.R. The Power Enhancement of a Mini-Gas Turbine by Adding Ethanol to the Compressor Inlet Air. Al-Khwarizmi Eng. J. 2013, 9, 54–64. [Google Scholar]
- Ethanol Power Plant, Minas Gerais. 21 January 2010. Available online: https://www.power-technology.com/projects/ethanol-power-plant/ (accessed on 21 January 2020).
- Moliere, M.; Vierling, M.; Aboujaib, M.; Patil, P.; Eranki, A.; Campbell, A. Gas Turbines in Alternative Fuel Applications: Bio-Ethanol Field Test. In Proceedings of the ASME Turbo Expo 2009: Power for Land, Sea, and Air, Orlando, FL, USA, 8–12 June 2009. [Google Scholar] [CrossRef]
- Eilat Power Plant, Unit No. 3 Preliminary Report GT Performance with Methanol Firing After Retrofit. Preliminary Report. Eilat Power Plant. April 2014. Available online: https://g2xenergy.com/images/downloads/eilat-simple-cycle-pratt-and-whitney-ft4c.pdf (accessed on 21 January 2020).
- Kalinichenko, A.; Havrysh, V.; Atamanyuk, I. The Acceptable Alternative Vehicle Fuel Price. Energies 2019, 12, 3889. [Google Scholar] [CrossRef] [Green Version]
- Tartakovsky, L.; Baibikov, V.; Veinblat, M. Comparative Performance Analysis of SI Engine Fed by Ethanol and Methanol Reforming Products; 2013-01-2617; SAE Technical Paper: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Choi, K.S.; Kim, H.M.; Dorr, J.L.; Yoon, H.C.; Erickson, P.A. Equilibrium model validation through the experiments of methanol autothermal reformation. Int. J. Hydrog. Energy 2008, 33, 7039–7047. [Google Scholar] [CrossRef]
- Stamatis, A.; Vinni, C.; Bakalis, D.; Tzorbatzoglou, F.; Tsiakaras, P. Exergy Analysis of an Intermediate Temperature Solid Oxide Fuel Cell-Gas Turbine Hybrid System Fed with Ethanol. Energies 2012, 5, 4268–4287. [Google Scholar] [CrossRef]
- Kandepu, R.; Imsland, L.; Foss, B.A.; Stiller, C.; Thorud, B.; Bolland, O. Modeling and control of a SOFC-GT-based autonomous power system. Energy 2007, 32, 406–417. [Google Scholar] [CrossRef]
- Haseli, Y.; Dincer, I.; Naterer, G.F. Thermodynamic analysis of a combined gas turbine power system with a solid oxide fuel cell through exergy. Thermochim. Acta 2008, 480, 1–9. [Google Scholar] [CrossRef]
- Carapellucci, R. A unified approach to assess performance of different techniques for recovering exhaust heat from gas turbines. Energy Convers. Manag. 2009, 50, 1218–1226. [Google Scholar] [CrossRef]
- Cocco, D.; Tola, V.; Cau, G. Performance evaluation of chemically recuperated gas turbine (CRGT) power plants fuelled by di-methyl-ether (DME). Energy 2006, 31, 1446–1458. [Google Scholar] [CrossRef]
- Verkhivker, G.; Kravchenko, V. The use of chemical recuperation of heat in a power plant. Energy 2004, 29, 379–388. [Google Scholar] [CrossRef]
- Sá, S.; Sousa, J.M.; Mendes, A. Steam reforming of methanol over a CuO/ZnO/Al2O3 catalyst part II: A carbon membrane reactor. Chem. Eng. Sci. 2011, 66, 5523–5530. [Google Scholar] [CrossRef]
- Mingheng, L.; Duraiswamy, K.; Knobbe, M. Adsorption enhanced steam reforming of methanol for hydrogen generation in conjunction with fuel cell: Process design and reactor dynamics. Chem. Eng. Sci. 2012, 67, 26–33. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Casanovas, A.; Divins, N.J.; Rejas, A.; Bosch, R.; Llorca, J. Finding a suitable catalyst for on-board ethanol reforming using exhaust heat from an internal combustion engine. Int. J. Hydrog. Energy 2017, 42, 13681–13690. [Google Scholar] [CrossRef] [Green Version]
- Tartakovsky, L.; Baibikov, V.; Veinblat, M. Modeling Methanol Steam Reforming for Internal Combustion Engine. Energy Power 2014, 4, 50–56. [Google Scholar] [CrossRef]
- We Power the World with Innovative Gas Turbines. Siemens Gas Turbine Portfolio. Available online: https://assets.new.siemens.com/siemens/assets/api/uuid:10f4860b140b2456f05d32629d8d758dc00bcc30/gas-turbines-siemens-interactive.pdf (accessed on 23 January 2020).
- Gas Turbine Engines for Gas Pipelines. Zorya-Mashproekt. Available online: https://www.epicos.com/sites/default/files//gas_turbine_engines_for_gas_pipelines.pdf (accessed on 23 January 2020).
- Kawasaki Gas Turbine Generator Sets. Available online: https://global.kawasaki.com/en/energy/pdf/Green_Brochure.pdf (accessed on 23 January 2020).
- Mercury 50. Recuperated Gas Turbine Generator Set. Available online: http://s7d2.scene7.com/is/content/Caterpillar/CM20150710-52396-21070 (accessed on 23 January 2020).
- Colin, R. The WR-21 Intercooled Recuperated Gas Turbine Engine—Integration into Future Warships. In Proceedings of the International Gas Turbine Congress 2003, Tokyo, Japan, 2–7 November 2003; Available online: https://nippon.zaidan.info/seikabutsu/2003/00916/pdf/igtc2003tokyo_os203.pdf (accessed on 23 January 2020).
- Matveev, I.; Waschilenko, N.; Serbin, S.; Goncharova, N. Integrated Plasma Coal Gasification Power Plant. IEEE Trans. Plasma Sci. 2013, 41, 3195–3200. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Boles, A.M. Thermodynamics: An Engineering Approach, 8th ed.; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- Poran, A.; Tartakovsky, L. Influence of methanol reformate injection strategy on performance, available exhaust gas enthalpy and emissions of a direct-injection spark ignition engine. Int. J. Hydrog. Energy 2017, 42, 15652–15668. [Google Scholar] [CrossRef]
- Hoffman, W.; Wong, V.; Cheng, W. A New Approach to Ethanol Utilization: High Effciency and Low NOx in an Engine Operating on Simulated Reformed Ethano; 2008-01-2415; SAE Technical Paper: Warrendale, PA, USA, 2008. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, R.; Huang, S.; Chen, W.; Shi, Q. Ni/Y2O3-Al2O3 catalysts for hydrogen production from steam reforming of ethanol at low temperature. J. Rare Earths 2012, 30, 683–690. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, W.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production by steam reforming of ethanol over an Ir/CeO2 catalyst: Reaction mechanism and stability of the catalyst. Int. J. Hydrog. Energy 2008, 33, 4377–4386. [Google Scholar] [CrossRef]
- Fayyazbakhsh, A.; Pirouzfar, V. Comprehensive overview on diesel additives to reduce emissions, enhance fuel properties and improve engine performance. Renew. Sustain. Energy Rev. 2017, 74, 891–901. [Google Scholar] [CrossRef]
- Yusri, I.M.; Mamat, R.; Najafi, G.; Razman, A.; Awad, O.I.; Azmi, W.H.; Ishak, W.F.W.; Shaiful, A.I.M. Alcohol based automotive fuels from first four alcohol family in compression and spark ignition engine: A review on engine performance and exhaust emissions. Renew. Sustain. Energy Rev. 2017, 77, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.S.; Nataraj, G.; Arulselvan, S. A comprehensive assessment on the effect of high octane fuels induction on engine’s combustion behaviour of a Mahua oil based dual fuel engine. Fuel 2017, 199, 176–184. [Google Scholar] [CrossRef]
- Yates, A.; Bell, A.; Swarts, A. Insights relating to the autoignition characteristics of alcohol fuels. Fuel 2010, 89, 83–93. [Google Scholar] [CrossRef]
- Goncharuk, A.G.; Havrysh, V.I.; Nitsenko, V.S. National features for alternative motor fuels market. Int. J. Energy Technol. Policy 2018, 14, 226–249. [Google Scholar] [CrossRef]
- LNG Carriers with ME-GI Engine and—High Pressure Gas Supply System. MAN Diesel & Turbo. 5510-0026-04ppr September 2014. Available online: https://marine.mandieselturbo.com/docs/librariesprovider6/technical-papers/lng-carriers-with-high-pressure-gas-supply-system.pdf?sfvrsn=16 (accessed on 23 January 2020).
- Tartakovsky, L.; Sheintuch, M. Fuel reforming in internal combustion engines. Prog. Energy Combust. Sci. 2018, 67, 88–114. [Google Scholar] [CrossRef]
- Poran, A.; Tartakovsky, L. Performance and emissions of a direct injection internal combustion engine devised for joint operation with a high-pressure thermochemical recuperation system. Energy 2017, 124, 214–226. [Google Scholar] [CrossRef]
- Larfeldt, J.; Andersson, M.; Larsson, A.; Moell, D. Hydrogen Co-Firing in Siemens Low NOX Industrial Gas Turbines. In Proceedings of the POWER-GEN Europe, Cologne, Germany, 27–29 June 2017; Available online: https://pdfs.semanticscholar.org/37fd/8e07212bf1e60f6db535d6e422b11880b816.pdf (accessed on 23 January 2020).
- Bancalari, E.; Chan, P.; Diakunchak, I.S. Advanced Hydrogen Gas Turbine Development Program. In Proceedings of the ASME Turbo Expo 2007: Power for Land, Sea, and Air, Montreal, QC, Canada, 14–17 May 2007; Volume 2: Turbo Expo 2007, pp. 977–987. [Google Scholar] [CrossRef]
- Nose, M.; Kawakami, T.; Araki, H.; Senba, N.; Tanimura, S. Hydrogen-fired Gas Turbine Targeting Realization of CO2-free Society. Mitsubishi Heavy Ind. Technol. Rev. 2018, 55, 1–7. [Google Scholar]
- Larfeldt, J. Applying Simulation and Additive Manufacturing to the Development of Next Generation of Hydrogen Combustion. 2017. Available online: http://www.international-bc-online.org/wp-content/uploads/2018/04/2_-Hydrogen_cofiring_Larfeldt_13april2018.pdf (accessed on 23 January 2020).
- ANSYS. Chemkin Theory Manual 17.0. Reaction Design. 2016. Available online: https://www.ems.psu.edu/~radovic/ChemKin_Theory_PaSR.pdf (accessed on 23 January 2020).
- Gagliano, A.; Nocera, F.; Bruno, M.; Cardillo, G. Development of an equilibrium-based model of gasification of biomass by Aspen Plus. Energy Procedia 2017, 111, 1010–1019. [Google Scholar] [CrossRef]
- Cherednichenko, O.K. Peculiarities of application of methanol conversion products in a ship gas turbine plants with thermochemical regeneration of waste heat. Aerosp. Eng. Technol. 2019, 3, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Havrysh, V.; Kalinichenko, A.; Hruban, V. Heat Recovery Systems for Agricultural Vehicles: UtilizationWays and Their Efficiency. Agriculture 2018, 8, 199. [Google Scholar] [CrossRef]
- Eriksson, M.; Ahlgren, S. LCAs of Petrol and Diesel—A Literature Review; Swedish University of Agricultural Science: Uppsala, Sweden, 2013; Available online: https://pub.epsilon.slu.se/10424/17/ahlgren_s_and_eriksson_m_130529.pdf (accessed on 12 February 2020).
- Gupta, P.; Zhuge, W.; Luo, S.; Ma, F. The well-to-wheel analysis of hydrogen enriched compressed natural gas for heavy-duty vehicles using life cycle approach to a fuel cycle. Int. J. Low-Carbon Technol. 2019, 14, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Roman-White, S.; Rai, S.; Littlefield, J.; Cooney, G.; Skone, T.J. Life Cycle Greenhouse Gas Perspective on Exporting Liquefied Natural Gas from the United States: 2019 Update; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2019. [Google Scholar]
- Ingham, A. Reducing the Carbon Intensity of Methanol for Use as a Transport Fuel. Johns. Matthey Technol. Rev. 2017, 61, 297–307. [Google Scholar] [CrossRef]
- Kajaste, R.; Hurme, M.; Oinas, P. Methanol-Managing greenhouse gas emissions in the production chain by optimizing the resource base. AIMS Energy 2018, 6, 1074–1102. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Life cycle assessment of energy and GHG emissions during ethanol production from grass straws using various pretreatment processes. Int. J. Life Cycle Assess. 2012, 17, 388–401. [Google Scholar] [CrossRef]
- ADI Analytics, Energy Insight and Consulting. Methanol for Power Generation: A White Paper; ADI Analytics, Energy Insight and Consulting: Katy, TX, USA, 2017. [Google Scholar]
- Kalinichenko, A.; Havrysh, V. Environmentally Friendly Fuel Usage: Economic Margin of Feasibility. Ecol. Chem. Eng. S 2019, 26, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Global Average Bunker Price. Available online: https://shipandbunker.com/prices/av/global/av-glb-global-average-bunker-price#MGO (accessed on 10 February 2020).
- Methanex Posts Regional Contract Methanol Prices for North America, Europe and Asia. Available online: https://www.methanex.com/our-business/pricing (accessed on 10 February 2020).
- Balussou, D. An Analysis of Current and Future Electricity Production from Biogas in Germany. Ph.D. Thesis, Karlsruhe Institute of Technology, Karlsruhe, Germany, 2018. Available online: https://publikationen.bibliothek.kit.edu/1000084909 (accessed on 12 February 2020).
- Kondratenko, Y.P.; Kozlov, O.V.; Kondratenko, G.V.; Atamanyuk, I.P. Mathematical Model and Parametrical Identification of Ecopyrogenesis Plant Based on Soft Computing Techniques. In Complex Systems: Solutions and Challenges in Economics, Management and Engineering; Berger-Vachon, C., Gil Lafuente, A., Kacprzyk, J., Kondratenko, Y., Merigó, J., Morabito, C., Eds.; Springer: Cham, Switzerland, 2018; Volume 125, pp. 201–233. [Google Scholar] [CrossRef]



| Properties | Unit | Diesel | Methanol | Ethanol |
|---|---|---|---|---|
| Density | kg/m3 | 840 | 796 | 789 |
| Boiling point | K | 453–643 | 338 | 351 |
| Lower heating value | MJ/kg | 42.5 | 19.67 | 26.9 |
| Stoichiometric air–fuel ratio | - | 14.6 | 6.45 | 9.06 |
| Heat of vaporization | kJ/kg | 243 | 1100 | 840 |
| Viscosity | cSt | 4.59 | 0.65 | 1.52 |
| Auto-ignition temperature | K | 503 | 736 | 698 |
| Carbon content by mass | % | 85 | 37.5 | 52.2 |
| Hydrogen content by mass | % | 15 | 12.5 | 13 |
| Oxygen content by mass | % | 0 | 50 | 34.8 |
| Specific carbon dioxide emission | g/MJ | 73.33 | 68.44 | 70.99 |
| Parameters | Unit | Value |
|---|---|---|
| Output | MW | 3.4 |
| Air-flow | kg/s | 16 |
| Pressure ratio | - | 7 |
| Heat exchange thermal ratio | - | 0.85 |
| Exhaust gas temperature | K | 600 |
| Turbine inlet temperature | K | 1270 |
| Initial temperature | K | 288 |
| Points | Temperature, K | Pressure, MPa | Mass Flow, kg/s |
|---|---|---|---|
| 1 | 288 | 0.101 | 16.00 |
| 2 | 1251 | 0.672 | 12.17 |
| 3 | 584 | 1.000 | 1.09 |
| 4 | 451 | 0.101 | 17.09 |
| Parameters | Unit | MGO | Methanol | Methanol (Recuperation) |
|---|---|---|---|---|
| Rated power of the gas turbine | kW | 3400 | 3400 | 3400 |
| Air-flow | kg/s | 16 | 16 | 16 |
| Engine efficiency | % | 38 | 38 | 42 |
| Specific fuel consumption | g/kWh | 222,910 | 481,631 | 435,761 |
| Fuel consumption | kg/h | 757,895 | 1,637,546 | 1,481,589 |
| kg/s | 0.211 | 0.455 | 0.412 | |
| Water flow | kg/s | 0 | 0 | 0.69 |
| Exhaust gas temperature | K | 600 | 600 | 451 |
| Outlet boiler temperature | K | 438 | 333 | 333 |
| Thermal power | kW | 2626.11 | 4393.45 | 2017.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherednichenko, O.; Havrysh, V.; Shebanin, V.; Kalinichenko, A.; Mentel, G.; Nakonieczny, J. Local Green Power Supply Plants Based on Alcohol Regenerative Gas Turbines: Economic and Environmental Aspects. Energies 2020, 13, 2156. https://doi.org/10.3390/en13092156
Cherednichenko O, Havrysh V, Shebanin V, Kalinichenko A, Mentel G, Nakonieczny J. Local Green Power Supply Plants Based on Alcohol Regenerative Gas Turbines: Economic and Environmental Aspects. Energies. 2020; 13(9):2156. https://doi.org/10.3390/en13092156
Chicago/Turabian StyleCherednichenko, Oleksandr, Valerii Havrysh, Vyacheslav Shebanin, Antonina Kalinichenko, Grzegorz Mentel, and Joanna Nakonieczny. 2020. "Local Green Power Supply Plants Based on Alcohol Regenerative Gas Turbines: Economic and Environmental Aspects" Energies 13, no. 9: 2156. https://doi.org/10.3390/en13092156






