Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Station
2.2. Concept of Experimental Design
2.3. Materials
2.4. Analytical Methods and Statistical Procedures
3. Results and Discussion
3.1. Organic Compounds and Nutrient Removal
3.2. Biogas Yield and Its Composition
3.3. Biomass Growth
3.4. Energy Balance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ekanthalu, V.S.; Morscheck, G.; Narra, S.; Nelles, M. Hydrothermal Carbonization—A Sustainable Approach to Deal with the Challenges in Sewage Sludge Management. In Urban Mining and Sustainable Waste Management; Springer: Singapore, 2020; pp. 293–302. [Google Scholar]
- Smol, M.; Adam, C.; Preisner, M. Circular economy model framework in the European water and wastewater sector. J. Mater. Cycles Waste Manag. 2020, 22, 682–697. [Google Scholar] [CrossRef] [Green Version]
- Bot, F.; Plazzotta, S.; Anese, M. Chapter 16—Treatment of Food Industry Wastewater with Ultrasound: A Big Opportunity for the Technology. Ultrasound Adv. Food Process. Preserv. 2017, 391–408. [Google Scholar] [CrossRef]
- Bhatti, Z.A.; Maqbool, F.; Malik, A.H.; Mehmood, Q. UASB reactor startup for the treatment of municipal wastewater followed by advanced oxidation process. Braz. J. Chem. Eng. 2014, 31, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Schnürer, A.; Bohn, I.; Moestedt, J. Protocol for Start-Up and Operation of CSTR Biogas Processes. In Hydrocarbon and Lipid Microbiology Protocols; McGenity, T., Timmis, K., Nogales, B., Eds.; Springer Protocols Handbooks; Protocol for Start-Up and Operation of CSTR Biogas Processes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 171–200. [Google Scholar]
- Rajeshwari, K.V.; Balakrishnan, M.; Kansal, A.; Lata, K.; Kishore, V.V.N. State-of-the-art of anaerobic digestion technology for industrial wastewater treatment. Renew. Sustain. Energy Rev. 2000, 4, 135–156. [Google Scholar] [CrossRef]
- Nelson, M.J.; Nakhla, G.; Zhu, J. Fluidized-Bed Bioreactor Applications for Biological Wastewater Treatment: A Review of Research and Developments. Engineering 2017, 3, 330–342. [Google Scholar] [CrossRef]
- Goli, A.; Shamiri, A.; Khosroyar, S.; Talaiekhozani, A.; Sanaye, R.; Azizi, K. A Review on Different Aerobic and Anaerobic Treatment Methods in Dairy Industry Wastewater. J. Environ. Treat. Tech. 2019, 6, 113–141. [Google Scholar]
- Leyva-Díaz, J.C.; Monteoliva-García, A.; Martín-Pascual, J.; Munio, M.M.; García-Mesa, J.J.; Poyatos, J.M. Moving bed biofilm reactor as an alternative wastewater treatment process for nutrient removal and recovery in the circular economy model. Bioresour. Technol. 2020, 299, 122631. [Google Scholar] [CrossRef]
- Cravotto, G.; Binello, A.; Di Carlo, S.; Orio, L.; Wu, Z.L.; Ondruschka, B. Oxidative degradation of chlorophenol derivatives promoted by microwaves or power ultrasound: A mechanism investigation. Environ. Sci. Pollut. Res. 2010, 17, 674–687. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing-Review. Compos. Part A 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Rocky Flats Technology Summary. 2001. Available online: http://www.nttc.edu/env/Rock–Flats/RF–chap1.html (accessed on 25 March 2020).
- Remya, N.; Lin, J.G. Current status of microwave application in wastewater treatment—A review. Chem. Eng. J. 2011, 166, 797–813. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. 2004, 43–46, 6250–6284. [Google Scholar] [CrossRef]
- Gogate, P.R. Cavitation: An auxiliary technique in wastewater treatment schemes. Adv. Environ. Res. 2002, 6, 335–358. [Google Scholar] [CrossRef]
- Matouq, M.A.D.; Al-Anber, Z.A. The application of high frequency ultrasound waves to remove ammonia from simulated industrial wastewater. Ultrason. Sonochem. 2007, 14, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Sangave, C.P.; Pandit, B.A. Ultrasound pre-treatment for enhanced biodegradability of the distillery wastewater. Ultrason. Sonochem. 2014, 11, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Blume, T.; Neis, U. Improved wastewater disinfection by ultrasonic pre-treatment. Ultrason. Sonochem. 2004, 11, 333–336. [Google Scholar] [CrossRef]
- Kyllonen, H.; Pirkonen, P.; Nystrom, M.; Nuortila-Jokinen, J.; Gronroos, A. Experimental aspects of ultrasonically enhanced cross-flow membrane filtration of industrial wastewater. Ultrason. Sonochem. 2006, 13, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Seungmin, N.; Young-Uk, K.; Jeehyeong, K. Physiochemical properties of digested sewage sludge with ultrasonic treatment. Ultrason. Sonochem. 2007, 14, 281–285. [Google Scholar]
- Sangave, C.P.; Pandit, B.A. Ultrasound and enzyme assisted biodegradation of distillery wastewater. J. Environ. Manag. 2006, 80, 36–46. [Google Scholar] [CrossRef]
- Oz, N.A.; Uzun, A.C. Ultrasound pretreatment for enhanced biogas production from olive mill wastewater. Ultrason. Sonochem. 2015, 22, 565–572. [Google Scholar] [CrossRef]
- Mischopoulou, M.; Naidis, P.; Kalamaras, S.; Kotsopoulos, T.A.; Samaras, P. Effect of ultrasonic and ozonation pretreatment on methane production potential of raw molasses wastewater. Renew. Energy 2016, 96, 1078–1085. [Google Scholar] [CrossRef]
- Gude, V.G. Microwave-Meditated Biofuel Production, 1st ed.; CRC Press: Boca-Ratón, FL, USA, 2017. [Google Scholar]
- Amini, A.; Aponte-Morales, V.; Wang, M.; Dilbeck, M.; Lahav, O.; Zhang, Q. Cost-effective treatment of swine wastes through recovery of energy and nutrients. Waste Manag. 2017, 69, 508–517. [Google Scholar] [CrossRef]
- Ma, H.; Guo, Y.; Qin, Y.; Li, Y.Y. Nutrient recovery technologies integrated with energy recovery by waste biomass anaerobic digestion. Bioresour. Technol. 2018, 269, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Omil, F.; Garrido, J.M.; Arrojo, B.; Mendez, R. Anaerobic filter reactorperformance for the treatment of complex dairy wastewater AT industrial scale. Water Res. 2003, 37, 4099–4108. [Google Scholar] [CrossRef]
- Mendez, R.; Blazquez, R.; Lorenzo, F.; Lema, J.M. Anaerobic treatment of cheese whey: Start-up and operation. Water Sci. Technol. 1989, 21, 1857–1860. [Google Scholar] [CrossRef]
- Najafpour, G.D.; Hashemiyeh, B.A.; Asadi, M.; Ghasemi, M.B. Biological Treatment of Dairy Wastewater in an Upflow Anaerobic Sludge-Fixed Film Bioreactor American-Eurasian. J. Agric. Environ. Sci. 2008, 4, 251–257. [Google Scholar]
- Danalewich, J.R.; Papagiannis, T.G.; Belyea, R.L.; Tumbleson, M.E.; Raskin, L. Characterization of dairy waste streams, current treatment practices and potential for biological nutrient removal. Water Res. 1998, 32, 3555–3568. [Google Scholar] [CrossRef]
- Isik, M.; Sponza, D.T. Anaerobic/aerobic sequential treatment of a cotton textile mill wastewater. J. Chem. Technol. Biotechnol. 2004, 79, 1268–1274. [Google Scholar] [CrossRef]
- Sklyar, V.; Epov, A.; Gladchenko, M.; Danilovich, D.; Kalyuzhnyi, S. Combined biologic (anaerobic–aerobic) and chemical treatment of starch industry wastewater. Appl. Biochem. Biotechnol. 2003, 109, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, E.V.; Gajalakshmi, S.; Sanjeevi, R.; Jithesh, M.N.; Abbasi, S.A. Feasibility studies on the treatment of dairy wastewaters with upflow anaerobic sludge blanket reactors. Bioresour. Technol. 2004, 93, 209–212. [Google Scholar] [CrossRef]
- Raj, S.A.; Murthy, D.V.S. Comparison of the trickling filtermodels for the treatment of synthetic dairy wastewater. Bioprocess Eng. 1999, 21, 51–55. [Google Scholar] [CrossRef]
- Göblös, S.; Portörő, P.; Bordás, D.; Kálmán, M.; Kiss, I. Comparison of the effectivities of two-phase and single-phase anaerobic sequencing batch reactors during dairy wastewater treatment. Renew. Energy 2008, 33, 960–965. [Google Scholar] [CrossRef]
- Kotsyurbenko, O.R.; Friedrich, M.W.; Simankova, M.V.; Nozhevnikova, A.N.; Golyshin, P.N.; Timmis, K.N.; Conrad, R. Shift from acetoclastic to H2-dependent methanogenesis in a West Siberian peat bog at low pH values and isolation of an acidophilic Methanobacterium strain. Appl. Environ. Microbiol. 2007, 73, 2344–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–223. [Google Scholar] [CrossRef]
- Banik, S.; Sharma, A.; Dan, D. Effect of microwave on cell biology of Methanosarcina Barkeri. J. Mycopathol. Res. 2003, 41, 217–219. [Google Scholar]
- Parker, M.C.; Besson, T.; Lamare, S.; Legoy, M.D. Microwave radiation can increase the rate of enzyme catalysed reaction in organic media. Tetrahedron Lett. 1996, 37, 8383–8386. [Google Scholar] [CrossRef]
- Sanjay, G.; Sugunan, S. Enhanced pH and thermal stabilities of invertase immobilized on montmorillonite K-10. Food Chem. 2006, 94, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Sun, S.-Y.; Xia, Y.-M. The weakened 1,3-specificity in the consecutive microwave assisted enzymatic synthesis of glycerides. J. Mol. Catal. B Enzym. 2008, 55, 6–11. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shinb, S.G.; Hwangb, S. Effect of microwave irradiation on the disintegration and acidogenesis of municipal secondary sludge. Chem. Eng. J. 2009, 153, 145–150. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Kennedy, K.J.; Droste, R.L. Characterization of soluble organic of waste from sewage sludge before and after thermal pretreatment. Water Res. 2006, 40, 3725–3736. [Google Scholar] [CrossRef]
- Oz, N.A.; Yarimtepe, C.C. Ultrasound assisted biogas production from landfill leachate. Waste Manag. 2014, 34, 1165–1170. [Google Scholar] [CrossRef]
- Yarimtepe, C.C.; Oz, N.A. Investigation of the pretreatment effect of ultrasound on anaerobic sequencing batch reactor treating landfill leachate. WIT Transactions on Ecology and the Environment. Water Resour. Manag. IX 2017, 220, 93–98. [Google Scholar]
- Nivedha Ramanathan, R.M.; Balasubramanian, N.; Chithra, K. Biogas from confectionery wastewater with the application of ultrasound pre-treatment. Energy Sources Part A Recovery Util. Environ. Effects 2019. [Google Scholar] [CrossRef]
- Kavitha, S.; Rajesh Banu, J.; Kumar, G.; Kaliappan, S.; Yeom, I.T. Profitable ultrasonic assisted microwave disintegration of sludge biomass: Modelling of biomethanation and energy parameter analysis. Bioresour. Technol. 2018, 254, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lu, X.; Han, P.; Wang, Y. Ultrasonic treatment on activated sewage sludge from petro-plant for reduction. Ultrasonics 2006, 44, e397–e399. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, E.; Buczkowska, A.; Zamojska, A.; Szewczyk, K.W.; Ciosek, P. Monitoring of periodic anaerobic digestion with flow-through array of miniaturized ion-selective electrodes. Bioelectrochemistry 2010, 80, 87–93. [Google Scholar] [CrossRef]
- Begum, S.; Anupoju, G.R.; Sridhar, S.; Bhargava, S.K.; Jegatheesan, V.; Eshtiaghi, N. Evaluation of single and two stage anaerobic digestion of landfill leachate: Effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour. Technol. 2018, 251, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Seghezzo, L.; Zeeman, G.; Van Lier, J.B.; Hamelers, H.V.M.; Lettinga, G. A review: The Anaerobic treatment of sewage in UASB and EGSB reactors. Bioresour. Technol. 1998, 65, 175–190. [Google Scholar] [CrossRef]
- Ozay, Y.; Ünşar, E.K.; Işık, Z.; Yılmaz, F.; Dizge, N.; Perendeci, N.A.; Mazmanci, M.A.; Yalvac, M. Optimization of electrocoagulation process and combination of anaerobic digestion for the treatment of pistachio processing wastewater. J. Clean. Prod. 2018, 196, 42–50. [Google Scholar] [CrossRef]
- Gerek, E.E.; Yılmaz, S.; Koparal, A.S.; Gerek, Ö.N. Combined energy and removal efficiency of electrochemical wastewater treatment for leather industry. J. Water Process Eng. 2019, 30, 100382. [Google Scholar] [CrossRef]
- Hiwarkar, A.D.; Singh, S.; Srivastava, V.C.; Mall, I.D. Mineralization of pyrrole, a recalcitrant heterocyclic compound, by electrochemical method: Multi-response optimization and degradation mechanism. J. Environ. Manag. 2017, 198, 144–152. [Google Scholar] [CrossRef]
- Ribera-Pi, J.; Badia-Fabregat, M.; Calderer, M.; Polášková, M.; Svojitka, J.; Rovira, M.; Jubany, I.; Martínez-Lladó, X. Anaerobic Membrane Bioreactor (AnMBR) for the Treatment of Cheese Whey for the Potential Recovery of Water and Energy. Waste Biomass Valorization 2020, 11, 1821–1835. [Google Scholar] [CrossRef]
- Masłoń, A. Energy Consumption of Selected Wastewater Treatment Plants Located in South-Eastern Poland. Eng. Prot. Environ. 2017, 20, 331–342. [Google Scholar] [CrossRef]
- Longo, S.; d’Antoni, B.M.; Bongards, M.; Chaparro, A.; Cronrath, A.; Fatone, F.; Lema, J.M.; Mauricio-Iglesias, M.; Soares, A.; Hospido, A. Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art. And proposals for improvement. Appl. Energy 2016, 176, 1251–1268. [Google Scholar] [CrossRef]


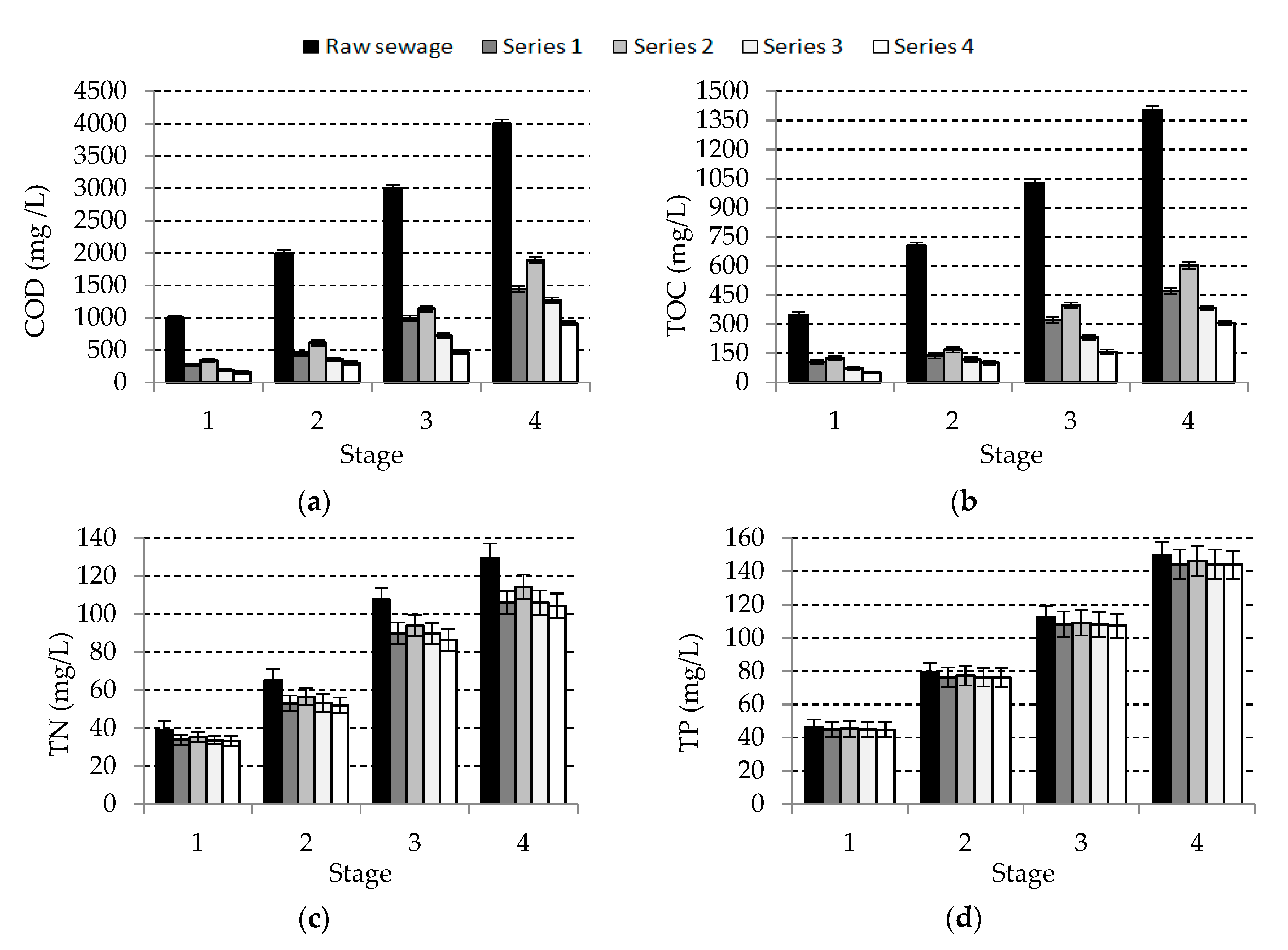
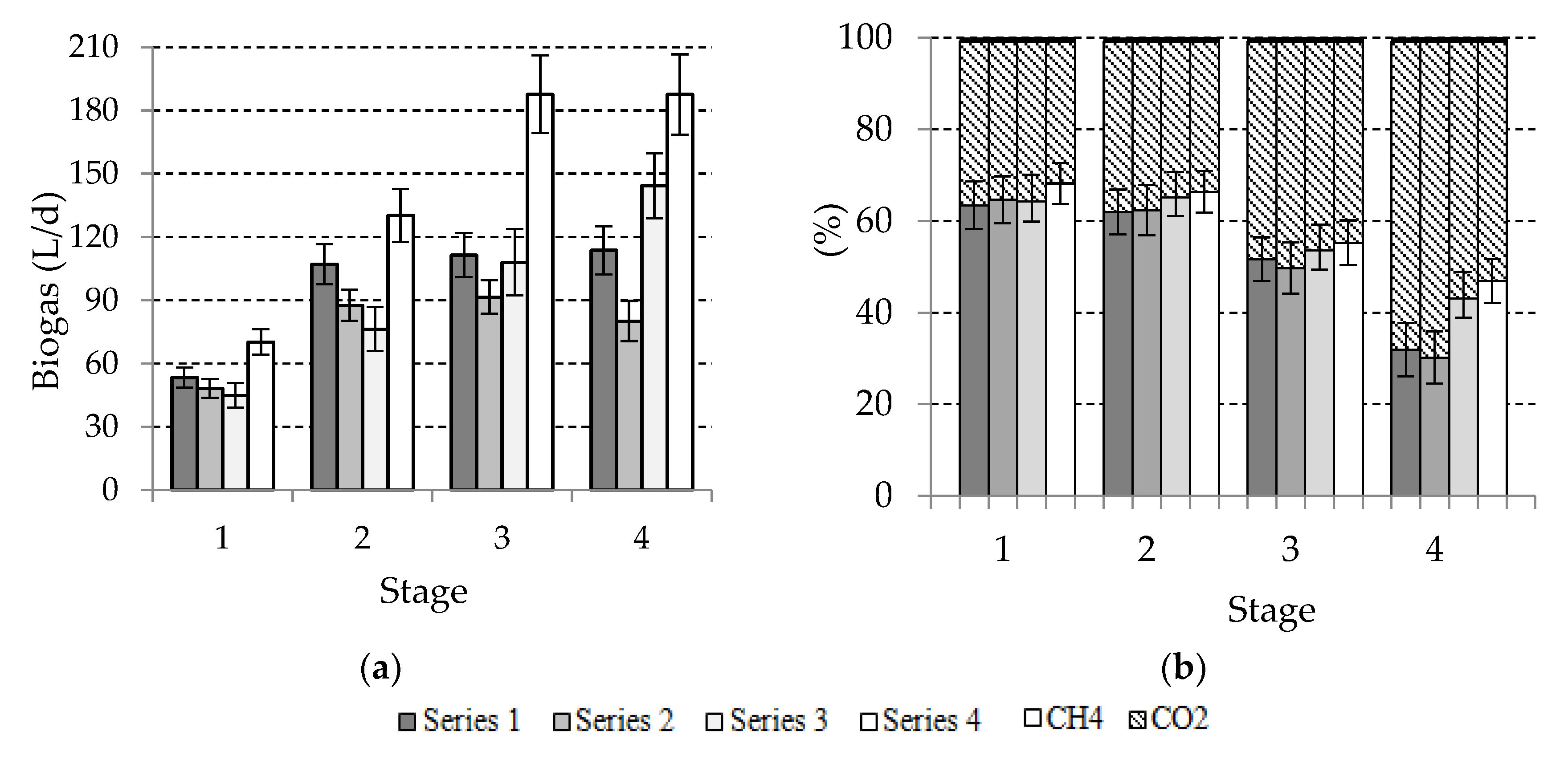
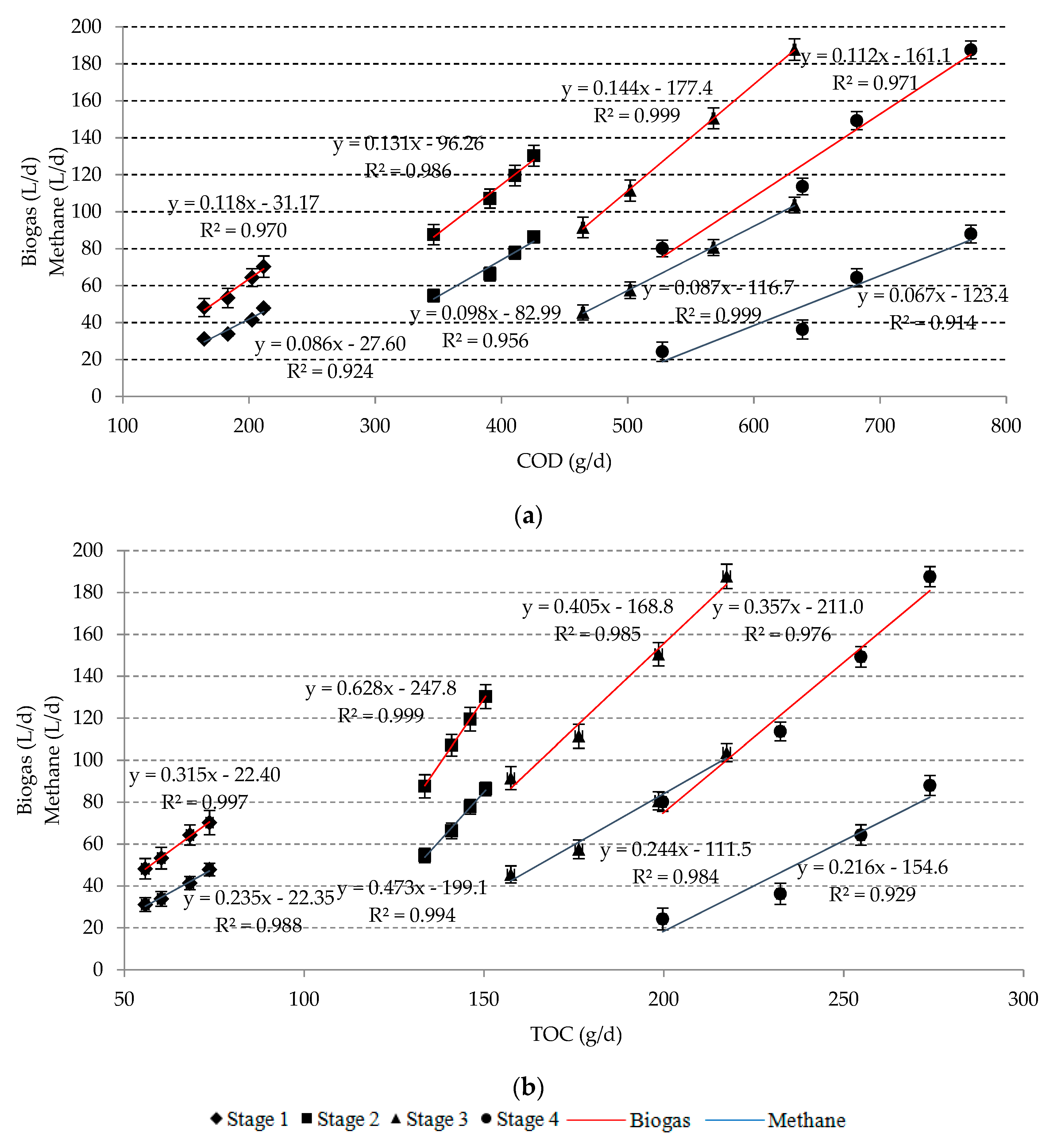

| Stage | Parameter | ||||
|---|---|---|---|---|---|
| COD (mg/L) | TOC (mg/L) | TP (mg/L) | TN (mg/L) | pH | |
| Raw Wastewater | 5138.4 ± 53.8 | 1780.9 ± 17.3 | 190.1 ± 7.2 | 164.3 ± 6.7 | 9.06 ± 0.24 |
| 1 | 1029 ± 20.5 | 347.3 ± 15.7 | 46.2 ± 4.7 | 39.1 ± 4.5 | 7.13 ± 0.25 |
| 2 | 2014 ± 40.4 | 703.2 ± 18.2 | 79.3 ± 5.8 | 65.3 ± 5.7 | 7.19 ± 0.22 |
| 3 | 3092 ± 50.2 | 1027.1 ± 20.1 | 112.4 ± 6.7 | 107.5 ± 6.4 | 7.09 ± 0.24 |
| 4 | 4046 ± 60.4 | 1402.3 ± 22.5 | 149.7 ± 7.9 | 129.4 ± 7.8 | 7.13 ± 0.21 |
| Parameter | Unit | Mean |
|---|---|---|
| Hydration | (%) | 95.6 ± 1.3 |
| Specific resistance to filtration | (m/kg) | 1.768 × 1015 ± 1.631 × 1014 |
| Capillary suction time | (s) | 1072 ± 175 |
| Total solids | (g/L) | 44.3 ± 4.9 |
| Mineral solids | (g/L) | 19.0 ± 1.3 |
| Volatile solids | (g/L) | 25.3 ± 3.6 |
| Filtrate COD | (mg/L) | 624.9 ± 84.0 |
| Orthophosphates in filtrate | (mg P-PO4/L) | 105.6 ± 24.0 |
| TN in filtrate | (mg TN/L) | 163.8 ± 17.5 |
| AN in filtrate | (mg N-NH4/L) | 144.2 ± 25.3 |
| pH | - | 7.75 ± 0.16 |
| Stage | Series | Removal Efficiency (%) | Load Removal (g/d) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| COD | TOC | TN | TP | COD | TOC | TN | TP | ||
| 1 | 1 | 73.26 ± 1.63 | 69.51 ± 1.77 | 13.46 ± 3.20 | 2.84 ± 0.33 | 183.15 ± 18.25 | 60.35 ± 7.13 | 1.32 ± 0.11 | 0.33 ± 0.05 |
| 2 | 65.78 ± 1.71 | 64.38 ± 1.35 | 9.67 ± 3.36 | 2.04 ± 0.38 | 164.45 ± 14.54 | 55.90 ± 6.41 | 0.95 ± 0.12 | 0.24 ± 0.06 | |
| 3 | 80.93 ± 1.26 | 78.66 ± 1.42 | 13.88 ± 4.07 | 2.93 ± 0.27 | 202.33 ± 21.22 | 68.30 ± 6.62 | 1.36 ± 0.23 | 0.34 ± 0.05 | |
| 4 | 84.64 ± 1.73 | 84.94 ± 0.56 | 14.52 ± 2.86 | 3.06 ± 0.11 | 211.60 ± 20.05 | 73.75 ± 6.81 | 1.42 ± 0.24 | 0.35 ± 0.07 | |
| 2 | 1 | 78.18 ± 1.09 | 80.22 ± 1.51 | 18.69 ± 0.61 | 3.72 ± 0.37 | 390.88 ± 25.11 | 141.03 ± 8.91 | 3.05 ± 0.61 | 0.74 ± 0.12 |
| 2 | 69.26 ± 1.52 | 76.00 ± 1.22 | 13.46 ± 0.61 | 2.68 ± 0.18 | 346.28 ± 23.24 | 133.60 ± 8.44 | 2.20 ± 0.53 | 0.53 ± 0.08 | |
| 3 | 82.15 ± 0.92 | 83.18 ± 1.29 | 18.42 ± 0.07 | 3.67 ± 0.13 | 410.73 ± 25.13 | 146.23 ± 9.02 | 3.01 ± 0.60 | 0.73 ± 0.13 | |
| 4 | 85.13 ± 1.05 | 85.62 ± 1.04 | 20.36 ± 0.62 | 4.05 ± 0.04 | 425.65 ± 24.21 | 150.53 ± 9.92 | 3.32 ± 0.63 | 0.80 ± 0.15 | |
| 3 | 1 | 66.93 ± 0.80 | 68.69 ± 0.76 | 16.44 ± 0.40 | 3.86 ± 1.14 | 501.95 ± 21.81 | 176.38 ± 10.11 | 4.42 ± 0.70 | 1.08 ± 0.21 |
| 2 | 61.94 ± 0.85 | 61.33 ± 0.67 | 12.68 ± 0.01 | 2.98 ± 1.01 | 464.55 ± 16.44 | 157.48 ± 10.21 | 3.41 ± 0.62 | 0.84 ± 0.18 | |
| 3 | 75.73 ± 0.84 | 77.33 ± 0.76 | 16.53 ± 0.14 | 3.88 ± 0.97 | 567.98 ± 19.23 | 198.58 ± 11.04 | 4.44 ± 0.71 | 1.09 ± 0.22 | |
| 4 | 84.28 ± 0.74 | 84.70 ± 0.82 | 19.55 ± 0.66 | 4.59 ± 0.68 | 632.13 ± 24.31 | 217.50 ± 12.03 | 5.25 ± 0.82 | 1.29 ± 0.25 | |
| 4 | 1 | 63.84 ± 0.55 | 66.31 ± 0.56 | 17.92 ± 0.22 | 3.57 ± 0.81 | 638.43 ± 26.42 | 232.48 ± 12.64 | 5.80 ± 0.80 | 1.33 ± 0.31 |
| 2 | 52.75 ± 0.42 | 56.98 ± 0.48 | 11.69 ± 0.28 | 2.33 ± 0.75 | 527.45 ± 25.24 | 199.75 ± 10.61 | 3.78 ± 0.63 | 0.87 ± 0.18 | |
| 3 | 68.15 ± 0.53 | 72.70 ± 0.33 | 18.12 ± 0.01 | 3.61 ± 0.75 | 681.53 ± 23.64 | 254.88 ± 13.92 | 5.86 ± 0.81 | 1.35 ± 0.40 | |
| 4 | 77.19 ± 0.43 | 78.17 ± 0.36 | 19.38 ± 0.15 | 3.86 ± 0.51 | 771.90 ± 23.53 | 274.05 ± 14.63 | 6.27 ± 0.73 | 1.44 ± 0.35 | |
| Stage | Series | Biogas Yield | |||
|---|---|---|---|---|---|
| L/g COD Added | L/g COD Removed | L/g TOC Added | L/g TOC Removed | ||
| 1 | 1 | 0.21 ± 0.01 | 0.29 ± 0.02 | 0.61 ± 0.07 | 0.88 ± 0.12 |
| 2 | 0.19 ± 0.01 | 0.29 ± 0.02 | 0.55 ± 0.08 | 0.86 ± 0.11 | |
| 3 | 0.26 ± 0.01 | 0.32 ± 0.02 | 0.74 ± 0.06 | 0.94 ± 0.13 | |
| 4 | 0.28 ± 0.02 | 0.33 ± 0.03 | 0.81 ± 0.09 | 0.95 ± 0.12 | |
| 2 | 1 | 0.21 ± 0.01 | 0.27 ± 0.03 | 0.61 ± 0.04 | 0.76 ± 0.11 |
| 2 | 0.18 ± 0.01 | 0.25 ± 0.02 | 0.50 ± 0.05 | 0.66 ± 0.10 | |
| 3 | 0.24 ± 0.01 | 0.29 ± 0.02 | 0.68 ± 0.07 | 0.82 ± 0.11 | |
| 4 | 0.26 ± 0.02 | 0.31 ± 0.02 | 0.74 ± 0.11 | 0.87 ± 0.12 | |
| 3 | 1 | 0.15 ± 0.01 | 0.22 ± 0.01 | 0.43 ± 0.06 | 0.63 ± 0.09 |
| 2 | 0.12 ± 0.01 | 0.20 ± 0.01 | 0.36 ± 0.04 | 0.58 ± 0.08 | |
| 3 | 0.20 ± 0.01 | 0.27 ± 0.01 | 0.59 ± 0.05 | 0.76 ± 0.08 | |
| 4 | 0.25 ± 0.02 | 0.30 ± 0.02 | 0.73 ± 0.07 | 0.86 ± 0.07 | |
| 4 | 1 | 0.11 ± 0.01 | 0.18 ± 0.01 | 0.32 ± 0.04 | 0.49 ± 0.06 |
| 2 | 0.08 ± 0.01 | 0.15 ± 0.01 | 0.23 ± 0.03 | 0.40 ± 0.05 | |
| 3 | 0.15 ± 0.01 | 0.22 ± 0.01 | 0.43 ± 0.04 | 0.59 ± 0.06 | |
| 4 | 0.19 ± 0.01 | 0.24 ± 0.01 | 0.54 ± 0.04 | 0.68 ± 0.08 | |
| Stage | Series | Ultrasounds (kWh/d) | Mixing (kWh/d) | Microwave Heating (kWh/d) | Total Energy Demand (kWh/d) | Daily Biogas Production (L/d) | CH4 Concentration (%) | Daily CH4 Production (L/d) | Energetic Value of CH4 (kWh/L) | Total Energy Production (kWh/d) | Energy Balance (kWh/d) | Load of COD Removed (kg/d) | kwh/kg COD Removed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 1.4 | 1.15 | 2.55 | 53.30 | 63.4 | 33.8 | 0.00917 | 0.310 | −2.240 | 0.183 | 12.230 |
| 2 | 0.38 | 2.93 | 48.18 | 64.6 | 31.1 | 0.285 | −2.645 | 0.164 | 16.084 | ||||
| 3 | 0.19 | 2.74 | 64.34 | 64.3 | 41.4 | 0.379 | −2.361 | 0.202 | 11.669 | ||||
| 4 | 0.076 | 2.626 | 70.25 | 68.1 | 47.8 | 0.439 | −2.187 | 0.211 | 10.336 | ||||
| 2 | 1 | 0 | 1.4 | 1.15 | 2.55 | 107.10 | 61.9 | 66.3 | 0.608 | −1.942 | 0.391 | 4.968 | |
| 2 | 0.38 | 2.93 | 87.61 | 62.3 | 54.6 | 0.500 | −2.430 | 0.346 | 7.017 | ||||
| 3 | 0.19 | 2.74 | 119.52 | 65.1 | 77.8 | 0.714 | −2.026 | 0.411 | 4.933 | ||||
| 4 | 0.076 | 2.626 | 130.25 | 66.3 | 86.4 | 0.792 | −1.834 | 0.423 | 4.309 | ||||
| 3 | 1 | 0 | 1.4 | 1.15 | 2.55 | 111.43 | 51.6 | 57.5 | 0.527 | −2.023 | 0.502 | 4.030 | |
| 2 | 0.38 | 2.93 | 91.52 | 49.7 | 45.5 | 0.417 | −2.513 | 0.464 | 5.410 | ||||
| 3 | 0.19 | 2.74 | 150.51 | 53.6 | 80.7 | 0.740 | −2.000 | 0.568 | 3.521 | ||||
| 4 | 0.076 | 2.626 | 187.74 | 55.2 | 103.6 | 0.950 | −1.676 | 0.632 | 2.651 | ||||
| 4 | 1 | 0 | 1.4 | 1.15 | 2.55 | 113.64 | 31.9 | 36.3 | 0.332 | −2.218 | 0.638 | 3.474 | |
| 2 | 0.38 | 2.93 | 80.17 | 30.2 | 24.2 | 0.222 | −2.708 | 0.527 | 5.134 | ||||
| 3 | 0.19 | 2.74 | 149.25 | 43.1 | 64.3 | 0.590 | −2.150 | 0.681 | 3.155 | ||||
| 4 | 0.076 | 2.626 | 187.57 | 46.9 | 88.0 | 0.807 | −1.819 | 0.772 | 2.357 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J. Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor. Energies 2020, 13, 2392. https://doi.org/10.3390/en13092392
Dębowski M, Zieliński M, Kisielewska M, Kazimierowicz J. Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor. Energies. 2020; 13(9):2392. https://doi.org/10.3390/en13092392
Chicago/Turabian StyleDębowski, Marcin, Marcin Zieliński, Marta Kisielewska, and Joanna Kazimierowicz. 2020. "Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor" Energies 13, no. 9: 2392. https://doi.org/10.3390/en13092392







