Process and Energy Intensification of Glycerol Carbonate Production from Glycerol and Dimethyl Carbonate in the Presence of Eggshell-Derived CaO Heterogeneous Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Catalyst Preparation
2.3. Catalyst Characterizations
2.4. Evaluation of Catalytic Efficiency
2.4.1. Conventional Process
2.4.2. Process Intensification Using a Kitchen Countertop Blender
2.5. Product Analysis
2.6. Energy Consumption and Yield Efficiency
3. Results and Discussion
3.1. Characteristics of Catalyst
3.2. Effect of Stirring Speed of Conventional Magnetic Stirrer on Mass Transfer Limitation
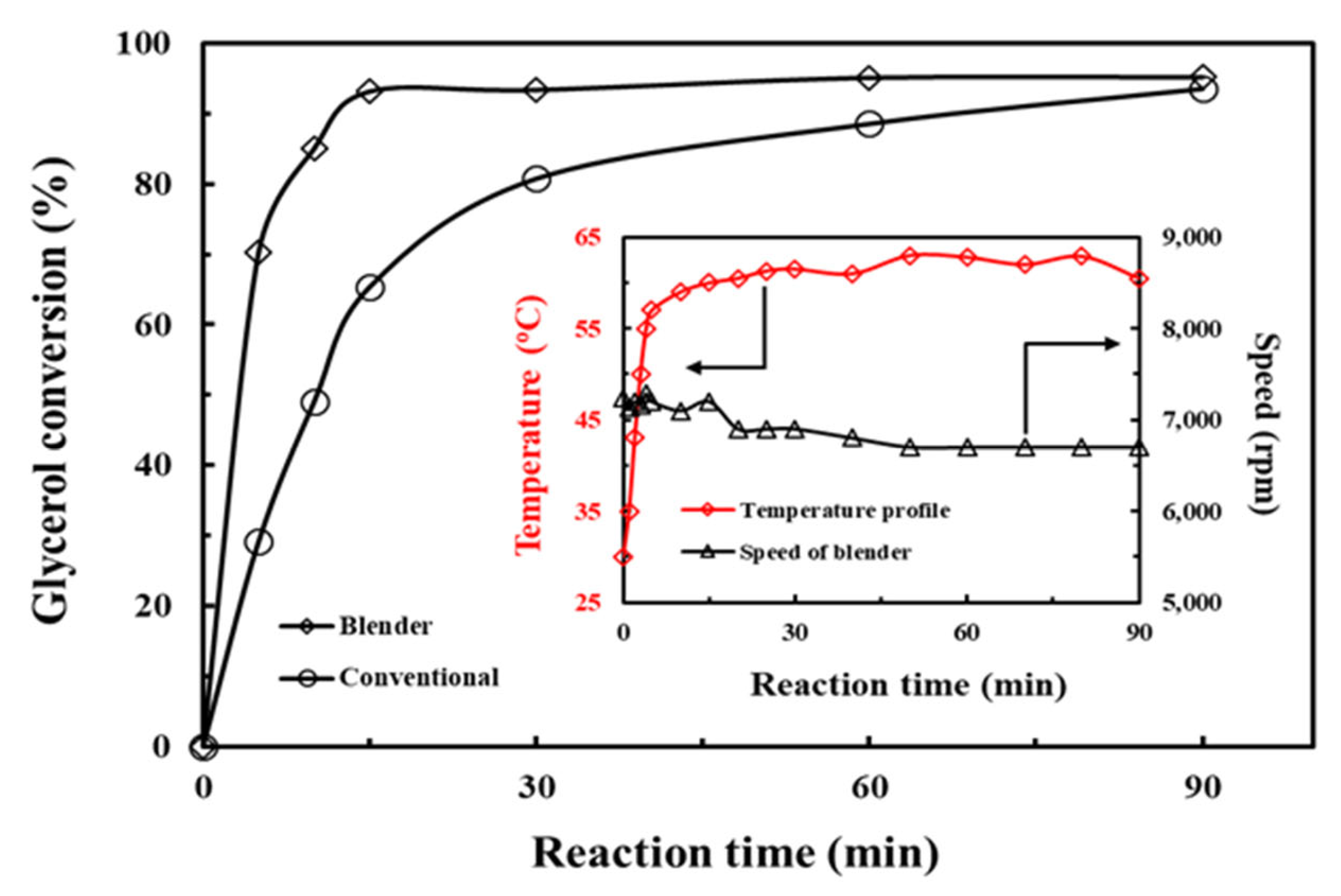
3.3. Performance Comparison between Kitchen Countertop Blender and Conventional Process
3.4. Effect of Molar Ratio of DMC to Glycerol
3.5. Effect of Catalyst Loading
3.6. Comparison of High Speed of Kitchen Countertop Blender with Other Intensification Processes Using CaO as a Catalyst
| Reactor System | Heat Source | Advantages | Disadvantages/Limitations | Reaction Condition | YGC or XGly (%) | Yield Eff. (g/kJ) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| DMC: Gly | CaO load. (wt %) | T (°C) | t (min) | |||||||
| Conventional | ||||||||||
| Atmospheric pressure reactor | electrical hot plate | - | - Long reaction time and/or high catalyst loading - Low yield efficiency | 2 a | 1 | 65 | 120 | YGC = 84.3 | 0.023 | [20] |
| Atmospheric pressure reactor | electrical hot plate | 5 | 6.1 | 75 | 90 | YGC = 64.1 | 0.018 | [20] | ||
| Autogenously pressure reactor | electrical hot plate | 4 | 5 | 95 | 80 | XGly = 97.0 | 0.003 | [36] | ||
| Atmospheric pressure reactor | electrical hot plate | 2.5 | 4.8 | 60 | 120 | YGC = 94.0 | 0.064 | This work | ||
| Process intensification | ||||||||||
| Microwave reactor | microwave irradiation | - Improve heat transfer rate - Low energy consumption - Short reaction time | - Inability to penetrate in large reaction volumes [36] | 4 | 5 | 95 b | 15 | YGC = 90.1 | 0.118 | [36] |
| Microwave reactor | microwave irradiation | 4 | 1 | < 200 c | 0.83 | YGC = 92.1 | 0.125 | [36] | ||
| Microwave reactor | microwave irradiation | 2 a | 1 | 65 | 5 | YGC = 93.4 | 0.766 | [20] | ||
| Ultrasonic reactor | ultrasonic irradiation | - Fast heating time - Improve mass transfer | - Difficult for using in Large-scale [39] - High cost ultrasound equipment [21] | 3 a | 5.4 | 70 | 90 | YGC = 95.4 | n/a | [40] |
| Batch distillation tower | electric heating mantle | - Stoichiometric feed ratio is possible | - High energy consumption [23] - Benzene as azeotropic agent is needed | 1 | 1.2 | 85 | n/a | YGC = 98.0 | n/a | [22] |
| Reactive and Extractive distillation | electric heating mantle | - When CaO reused, the reduction of catalytic activity is less than the conventional process | - Difficult to control and scale-up | 4 | 3 | 85 | n/a | YGC = 99.0 | n/a | [23] |
| Supercritical tube reactor | electric heating jacket | - Absence catalyst - Simple to separation due to the absence of the catalyst | - Severe operating condition - High cost of operating equipment | 10 | - | 300 | 15 | YGC = 98.0 | n/a | [24] |
| Countertop blender | in situ heat generation | - Intensive mixing, improve mass transfer - No external heat source as in situ heat generation and thus rapid heat transfer rate - Enhance reaction rate | - Balance heat to maintain the desired temperature | 2.5 | 2.4 | 60 | 15 | YGC = 91.3 | 2.391 | This work |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pali, H.S.; Sharma, A.; Singh, Y.; Kumar, N. Sal biodiesel production using Indian abundant forest feedstock. Fuel 2020, 273, 117781. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.; Belsué, M. A Brief Review on Industrial Alternatives for the Manufacturing of Glycerol Carbonate, a Green Chemical. Org. Process. Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Okoye, P.; Hameed, B. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Ji, Y. Recent Development of Heterogeneous Catalysis in the Transesterification of Glycerol to Glycerol Carbonate. Catalysts 2019, 9, 581. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl. Catal. A Gen. 2016, 513, 9–18. [Google Scholar] [CrossRef]
- AL-Kurdhani, J.; Wang, H.; Elhaj, E. Glycerol Carbonylation with CO2 to Producing the Glycerol Carbonate over Metal Oxide Nanoparticle Catalyst and the Influence of Both the Calcination Temperature of the Catalyst and the Reaction Parameters. J. Mater. Sci. Eng. 2019, 8, 2. [Google Scholar]
- Hu, J.; Li, J.; Gu, Y.; Guan, Z.; Mo, W.; Ni, Y.; Li, T.; Li, G. Oxidative carbonylation of glycerol to glycerol carbonate catalyzed by PdCl2 (phen)/KI. Appl. Catal. A Gen. 2010, 386, 188–193. [Google Scholar] [CrossRef]
- Casiello, M.; Monopoli, A.; Cotugno, P.; Milella, A.; Dell’Anna, M.M.; Ciminale, F.; Nacci, A. Copper (II) chloride-catalyzed oxidative carbonylation of glycerol to glycerol carbonate. J. Mol. Catal. A Chem. 2014, 381, 99–106. [Google Scholar] [CrossRef]
- Fernandes, G.P.; Yadav, G.D. Selective glycerolysis of urea to glycerol carbonate using combustion synthesized magnesium oxide as catalyst. Catal. Today 2018, 309, 153–160. [Google Scholar] [CrossRef]
- Kondawar, S.E.; Mane, R.B.; Vasishta, A.; More, S.B.; Dhengale, S.D.; Rode, C.V. Carbonylation of glycerol with urea to glycerol carbonate over supported Zn catalysts. Appl. Petrochem. Res. 2017, 7, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wu, Y.; Pan, D.; Cai, F.; Xiao, G.M. High-efficiency and low-cost Li/ZnO catalysts for synthesis of glycerol carbonate from glycerol transesterification: The role of Li and ZnO interaction. Appl. Catal. A Gen. 2017, 532, 77–85. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Sun, P.; Xu, L. Disposable baby diapers waste derived catalyst for synthesizing glycerol carbonate by the transesterification of glycerol with dimethyl carbonate. J. Clean. Prod. 2019, 211, 330–341. [Google Scholar] [CrossRef]
- Parameswaram, G.; Srinivas, M.; Babu, B.H.; Prasad, P.S.S.; Lingaiah, N. Transesterification of glycerol with dimethyl carbonate for the synthesis of glycerol carbonate over Mg/Zr/Sr mixed oxide base catalysts. Catal. Sci. Technol. 2013, 3, 3242–3249. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.-L.; Lam, M.K.; Chen, W.-H. Organic Carbonate Production Utilizing Crude Glycerol Derived as By-Product of Biodiesel Production: A Review. Energies 2020, 13, 1483. [Google Scholar] [CrossRef] [Green Version]
- Malyaadri, M.; Jagadeeswaraiah, K.; Prasad, P.S.; Lingaiah, N. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over Mg/Al/Zr catalysts. Appl. Catal. A Gen. 2011, 401, 153–157. [Google Scholar] [CrossRef]
- Algoufi, Y.; Hameed, B.J.F. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over K-zeolite derived from coal fly ash. Fuel Process. Technol. 2014, 126, 5–11. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: Effects of influencing parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Algoufi, Y.; Kabir, G.; Hameed, B. Synthesis of glycerol carbonate from biodiesel by-product glycerol over calcined dolomite. J. Taiwan Inst. Chem. Eng. 2017, 70, 179–187. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. Microwave-assisted transesterification of industrial grade crude glycerol for the production of glycerol carbonate. Chem. Eng. J. 2016, 284, 469–477. [Google Scholar] [CrossRef]
- Waghmare, G.; Vetal, M.D.; Rathod, V.K. Ultrasound assisted enzyme catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Ultrason. Sonochemistry 2015, 22, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T. Coupling reaction and azeotropic distillation for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Chem. Eng. Process. Process. Intensif. 2010, 49, 530–535. [Google Scholar] [CrossRef]
- Wang, H.; Pang, L.; Yang, C.; Liu, Y. Production of glycerol carbonate via reactive distillation and extractive distillation: An experimental study. Chin. J. Chem. Eng. 2015, 23, 1469–1474. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Esterification of glycerol from biodiesel production to glycerol carbonate in non-catalytic supercritical dimethyl carbonate. SpringerPlus 2016, 5, 923. [Google Scholar] [CrossRef] [Green Version]
- Kamjam, M.; Wongjaikham, W.; Wongsawaeng, D.; Ratnisai, V.; Ngaosuwan, K.; Kiatkittipong, W.; Hosemann, P.; Assabumrungrat, S. Continuous biodiesel production based on hand blender technology for sustainable household utilization. J. Clean. Prod. 2021, 297, 126737. [Google Scholar] [CrossRef]
- Wongsawaeng, D.; Ngaosuwan, K.; Kiatkittipong, W.; Laosuttiwong, T.; Chanthon, N.; Assabumrungrat, S. Simple and effective technology for sustainable biodiesel production using high-power household fruit blender. J. Clean. Prod. 2019, 237, 117842. [Google Scholar] [CrossRef]
- Praikaew, W.; Kiatkittipong, W.; Aiouache, F.; Najdanovic-Visak, V.; Termtanun, M.; Lim, J.W.; Lam, S.S.; Kiatkittipong, K.; Laosiripojana, N.; Boonyasuwat, S.; et al. Mechanism of CaO catalyst deactivation with unconventional monitoring method for glycerol carbonate production via transesterification of glycerol with dimethyl carbonate. Int. J. Energy Res. 2021. under review. [Google Scholar]
- Maddikeri, G.L.; Gogate, P.R.; Pandit, A.B. Intensified synthesis of biodiesel using hydrodynamic cavitation reactors based on the interesterification of waste cooking oil. Fuel 2014, 137, 285–292. [Google Scholar] [CrossRef]
- Praikaew, W.; Kiatkittipong, W.; Kiatkittipong, K.; Laosiripojana, N.; Viriya-empikul, N.; Boonyaduwat, S.; Aiouache, F.; Najdanovic-Visak, V.; Assabumrungrat, S. Synthesis of glycerol carbonate from dimethyl carbonate and glycerol using CaO derived from eggshells. MATEC Web Conf. EDP Sci. 2018, 192, 03045. [Google Scholar] [CrossRef]
- Khemthong, P.; Luadthong, C.; Nualpaeng, W.; Changsuwan, P.; Tongprem, P.; Viriya-Empikul, N.; Faungnawakij, K. Industrial eggshell wastes as the heterogeneous catalysts for microwave-assisted biodiesel production. Catal. Today 2012, 190, 112–116. [Google Scholar] [CrossRef]
- Viriya-Empikul, N.; Krasae, P.; Nualpaeng, W.; Yoosuk, B.; Faungnawakij, K. Biodiesel production over Ca-based solid catalysts derived from industrial wastes. Fuel 2012, 92, 239–244. [Google Scholar] [CrossRef]
- Esteban, J.; Blanco, A.; Fuente, E.; Ladero, M.; Garcia-Ochoa, F. Phenomenological kinetic model of the synthesis of glycerol carbonate assisted by focused beam reflectance measurements. Chem. Eng. J. 2015, 260, 434–443. [Google Scholar] [CrossRef]
- Nakhchi, M.; Esfahani, J.A. Numerical investigation of different geometrical parameters of perforated conical rings on flow structure and heat transfer in heat exchangers. Appl. Therm. Eng. 2019, 156, 494–505. [Google Scholar] [CrossRef]
- Mohod, A.V.; Gogate, P.R.; Viel, G.; Firmino, P.; Giudici, R. Intensification of biodiesel production using hydrodynamic cavitation based on high speed homogenizer. Chem. Eng. J. 2017, 316, 751–757. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. Chemical equilibrium of glycerol carbonate synthesis from glycerol. J. Chem. Thermodyn. 2011, 43, 731–736. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Okoye, U.; Li, S.; Tian, C. Microwave-assisted transesterification of glycerol with dimethyl carbonate over sodium silicate catalyst in the sealed reaction system. Energy Convers. Manag. 2018, 164, 543–551. [Google Scholar] [CrossRef]
- Gharat, N.; Rathod, V.K. Ultrasound assisted enzyme catalyzed transesterification of waste cooking oil with dimethyl carbonate. Ultrason. Sonochem. 2013, 20, 900–905. [Google Scholar] [CrossRef]
- Bolívar-Diaz, C.; Calvino-Casilda, V.; Rubio-Marcos, F.; Fernández, J.; Bañares, M.A. New concepts for process intensification in the conversion of glycerol carbonate to glycidol. Appl. Catal. B Environ. 2013, 129, 575–579. [Google Scholar] [CrossRef]
- Zhao, Z.; Xue, Y.; Xu, G.; Chen, D.; Zhou, J.; Liu, P.; Han, S.; Lin, H. Reaction conditions of ultrasound-assisted production of biodiesel: A review. Int. J. Energy Res. 2017, 41, 1081–1095. [Google Scholar] [CrossRef]
- Lo, P.; Leong, S.; Tan, C. Investigation on the effect of ultrasonic-assisted transesterification for green synthesis of glycerol carbonate from crude glycerol. IOP Conf. Ser. Mater. Sci. Eng. 2020, 943, 012011. [Google Scholar] [CrossRef]


 reaction time = 15 min,
reaction time = 15 min,  reaction time = 120 min.
reaction time = 120 min.
 reaction time = 15 min,
reaction time = 15 min,  reaction time = 120 min.
reaction time = 120 min.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praikaew, W.; Kiatkittipong, W.; Aiouache, F.; Najdanovic-Visak, V.; Ngaosuwan, K.; Wongsawaeng, D.; Lim, J.W.; Lam, S.S.; Kiatkittipong, K.; Laosiripojana, N.; et al. Process and Energy Intensification of Glycerol Carbonate Production from Glycerol and Dimethyl Carbonate in the Presence of Eggshell-Derived CaO Heterogeneous Catalyst. Energies 2021, 14, 4249. https://doi.org/10.3390/en14144249
Praikaew W, Kiatkittipong W, Aiouache F, Najdanovic-Visak V, Ngaosuwan K, Wongsawaeng D, Lim JW, Lam SS, Kiatkittipong K, Laosiripojana N, et al. Process and Energy Intensification of Glycerol Carbonate Production from Glycerol and Dimethyl Carbonate in the Presence of Eggshell-Derived CaO Heterogeneous Catalyst. Energies. 2021; 14(14):4249. https://doi.org/10.3390/en14144249
Chicago/Turabian StylePraikaew, Wanichaya, Worapon Kiatkittipong, Farid Aiouache, Vesna Najdanovic-Visak, Kanokwan Ngaosuwan, Doonyapong Wongsawaeng, Jun Wei Lim, Su Shiung Lam, Kunlanan Kiatkittipong, Navadol Laosiripojana, and et al. 2021. "Process and Energy Intensification of Glycerol Carbonate Production from Glycerol and Dimethyl Carbonate in the Presence of Eggshell-Derived CaO Heterogeneous Catalyst" Energies 14, no. 14: 4249. https://doi.org/10.3390/en14144249










