Review of Wood Biomass Cyclone Burner
Abstract
:1. Introduction
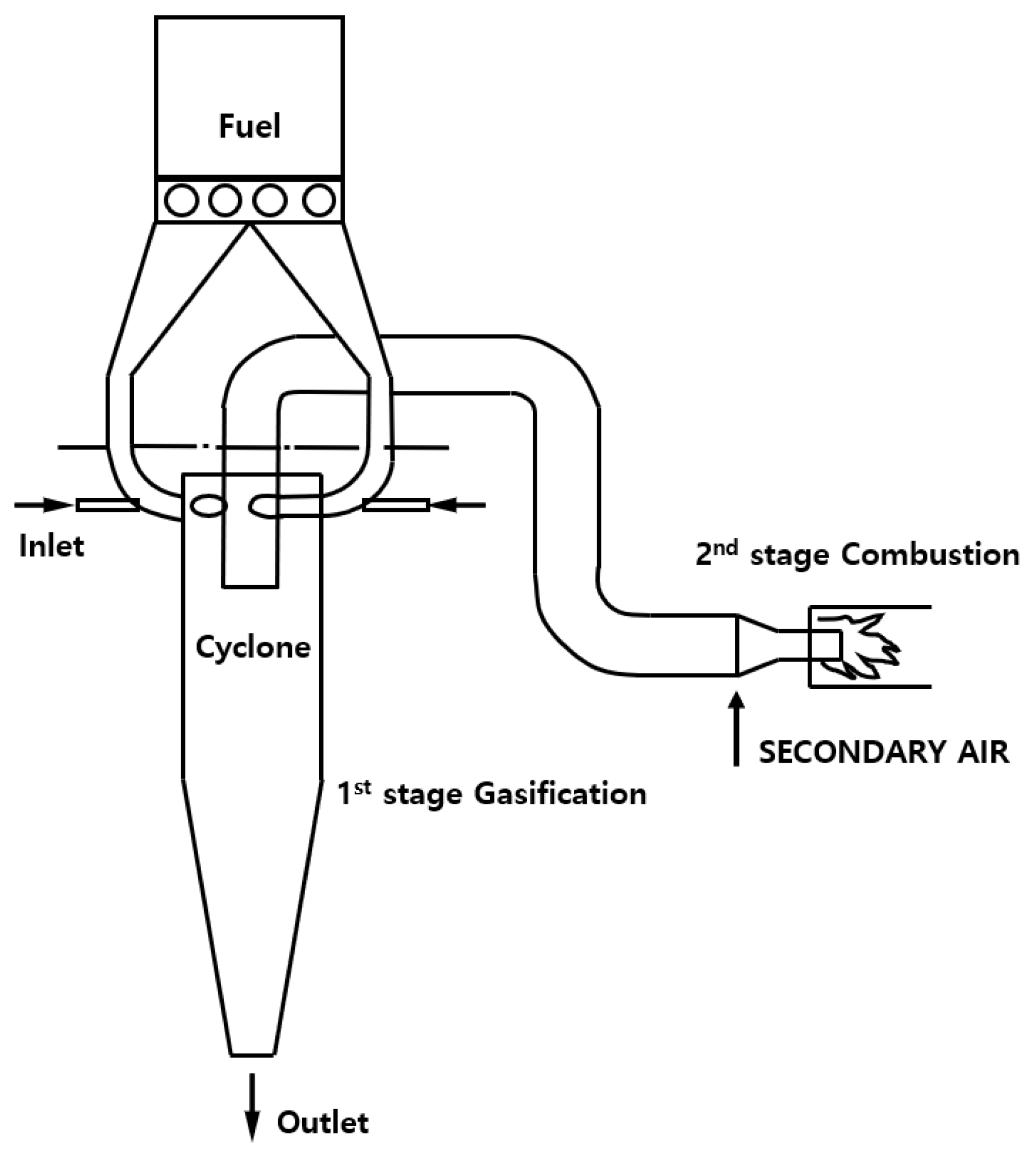
2. Cyclone Burner Technology
- G∅ = axial flux of angular momentum;
- Gx = axial flux of linear momentum;
- De = exit diameter of chamber;
- Do = chamber diameter;
- At = cross sectional area of the tangential inlet.
- Ti = inlet gas temperature [Kelvin];
- To = outlet gas temperature [Kelvin].
- = mass flow rate of the chamber;
- = mass flow rate of the tangential inlet;
- = cross sectional area of the chamber;
- = cross sectional area of the tangential inlet.
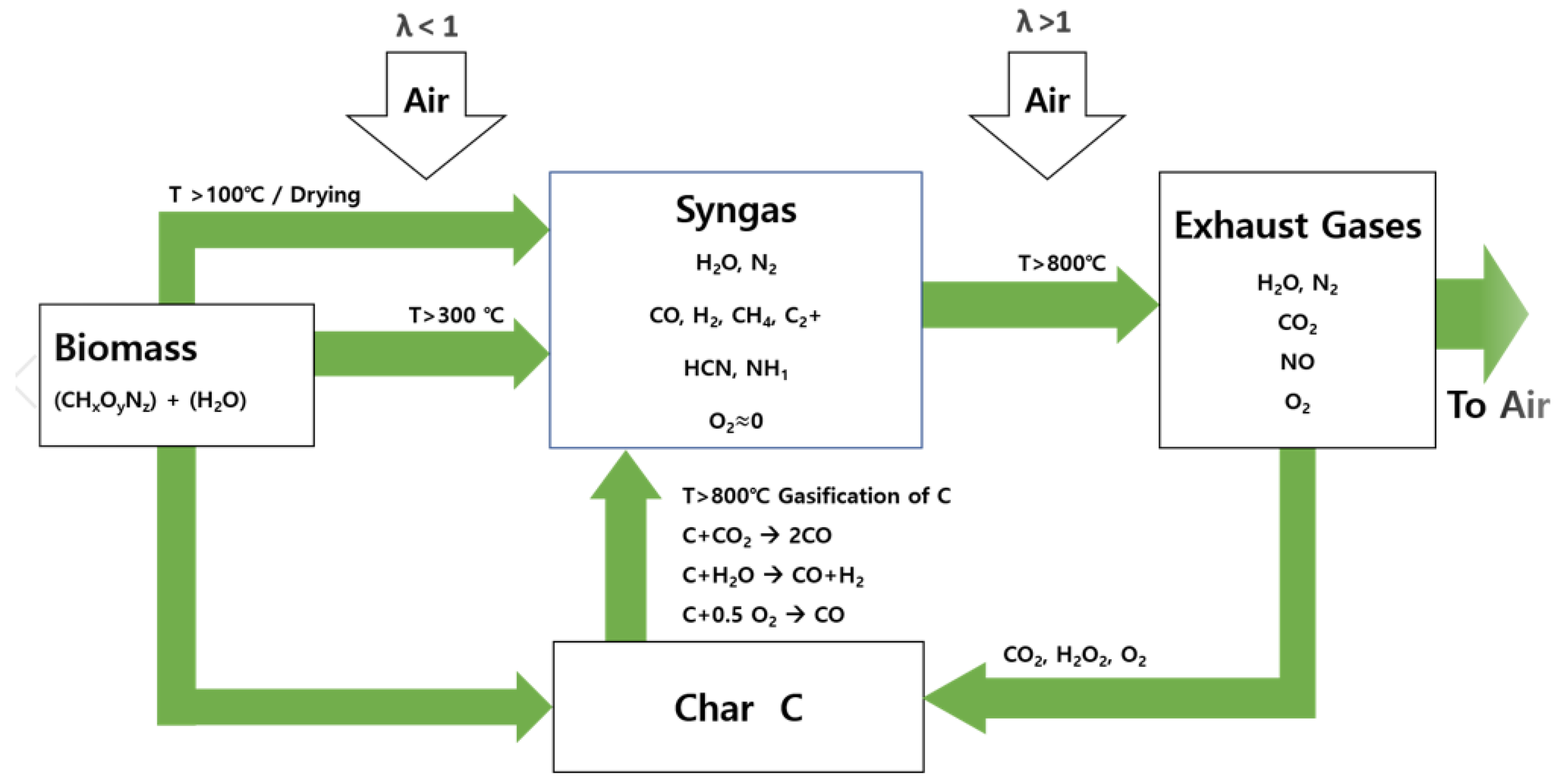
3. Biomass Combustion with Cyclone Burner
→ Intermediates (C, CO, H2, CO2, CmHn etc.)
→ CO2 + 0.72 H2O + (λ − 1) O2 + λ 3.87 N2 − 439 KJ/kmol
4. NOx Emission and Ash Deposit
5. Co-Firing and Gasification of Cyclone Burner
6. Conclusions
- Design factors for the type 2 cyclone burner can be derived from the following three dimensionless numbers: swirl intensity (S), Strouhal number (St), and Reynolds number (Re). Critical design factors in the geometry of the combustor include the diameter of the chamber, longitudinal length or height of the chamber, and cross sectional area of the tangential inlet [25].
- At the excess air ratio (λ) = 1.2, the cyclone combustor acquired the minimum CO and NOx. With micron-sized pulverized wood (less than 250 μm), Luo et al. [5] obtained CO = 0.015 ppm and NOx = 182 ppm, while Choe [25] acquired 39 ppm and 49 ppm, respectively, for non-pulverized wood chips (about 5–10 cm size).
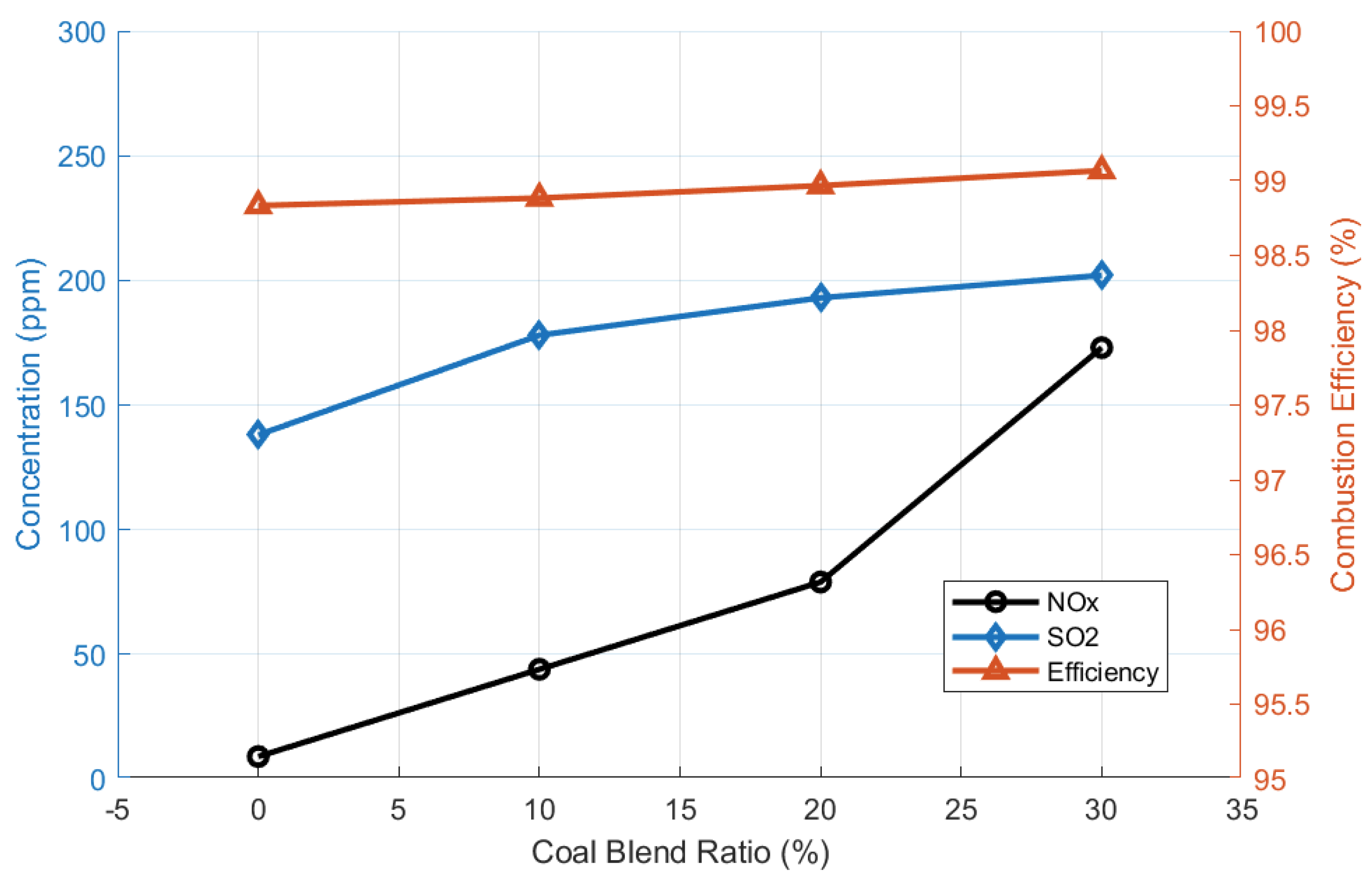
- The co-firing of wood biomass with coal (up to 30%) resulted in an increase in combustion efficiency, from 95% to 98%, and lowered the base-to-acid ratio (Rb/a), from 2.3 to 0.9 [58]. However, NOx and SOx moderately increased.
- Through the cyclone gasifier, the fine wood powder and BMF produced, on average, 44% syngas (H2, CO, CO2, CH4). Alkali retention in the collected ash was at a maximum of 70% by Syred et al. [71]. The amount of K and Na, the cause of fouling/slagging, decreased by 50%, from 300 mg/kg at 800 °C, to 150 mg/kg at 920 °C [35].
Funding
Conflicts of Interest
References
- The Handbook of Biomass Combustion & Co-Firing; Van Loo, S.; Koppejan, J. (Eds.) Earthscan: New York, NY, USA, 2008; Chapter 1. [Google Scholar]
- Klason, T.; Bai, X.-S. Computational study of the combustion process and NO formation in a small-scale wood pellet furnace. Fuel 2007, 86, 1465–1474. [Google Scholar] [CrossRef]
- Demirbas, A. Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S.; He, M. Experimental study on combustion of biomass micron fuel (BMF) in cyclone furnace. Energy Convers. Manag. 2010, 51, 2098–2102. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory; Sandia National Laboratory; University of California; Foster Wheeler Development Corporation; US Bureau of Mines; Miles, T.R. Alkali Deposits Found in Biomass Power Plants; Research Report NREL/TP-433-8142 SAND96-8225 Vols I and II.; National Renewable Energy Laboratory: Oakridge, TN, USA, 1996. [Google Scholar]
- Gaur, S.; Reed, T.B. An Atlas of Thermal Data for Biomass and Other Fuels; (No. NREL/TP-433-7965); National Renewable Energy Laboratory: Golden, CO, USA, 1995. [Google Scholar]
- Sander, B. Properties of Danish biofuels and the requirements for power production. Biomass Bioenergy 1997, 12, 177–183. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Skrifvars, B.-J.; Lauren, T.; Hupa, M.; Korbee, R.; Ljung, P. Ash behaviour in a pulverized wood fired boiler—A case study. Fuel 2004, 83, 371–1379. [Google Scholar] [CrossRef]
- Smoot, L.D.; Smith, P.J. Coal Combustion and Gasification; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Xilin, W.; Zhanhua, M.; Dexin, W.; Lixing, Z. Experimental Study of Coal Combustion in an Improved Spouting-cyclone Combustor. Tsinghua Sci. Technol. 1998, 3, 959–962. [Google Scholar]
- Joo, N.R.; Kim, H.Y.; Chung, J.T.; Choi, S.I. The characteristics of pulverized coal combustion in the two stage cyclone combustor. KSME Int. J. 2002, 16, 1112–1120. [Google Scholar] [CrossRef]
- Osborne, D. The Coal Handbook: Towards Cleaner Production; Coal Utilisation; Woodhead Publishing Series in Energy: Oxford, UK, 2013; Volume 2. [Google Scholar]
- Zarzycki, R.; Bis, Z. Analysis of Coal Dust Combustion and Gasification in the Cyclone Furnace. In Proceedings of the E3S Web of Conferences 13, Cracow, Poland, 12–14 October 2016; p. 05003. [Google Scholar]
- Zarzycki, R. Pulverized Coal Gasification with Steam and Flue Gas. In Proceedings of the MATEC Web of Conferences 240, Cracow, Poland, 21–24 May 2018; p. 05036. [Google Scholar]
- Zhou, L.X.; Chen, T.; Xu, Y.; Ma, Z.H.; Guo, Y.C. Strongly swirling gas-particle flows and coal combustion in a cyclone combustor. Symp. Int. Combust. 1998, 27, 3119–3126. [Google Scholar] [CrossRef]
- Zarzycki, R.; Bis, Z. Modelling of Coal Dust Gasification in a Cyclone Furnace under Oxy-fuel Combustion Conditions. Procedia Eng. 2016, 157, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Zarzycki, R.; Bis, Z.; Kobyłecki, R. The Concept of Coal Burning in a Cyclone Furnace. Procedia Eng. 2016, 157, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Zarzycki, R.; Bis, Z. Modelling of the process of coal dust combustion in a cyclone furnace. J. Therm. Sci. 2017, 26, 192–198. [Google Scholar] [CrossRef]
- Zarzycki, R.; Jedras, J.; Kobyłecki, R. Fuel Processing in a Swirl Flow: Numerical Modelling of Combustion and Gasification. In Proceedings of the E3S Web of Conferences, Warsaw, Poland, 3–6 December 2019; Volume 137, p. 01052. [Google Scholar]
- Zarzycki, R. Swirl Chamber for Vitrification of Fly Ashes. In Proceedings of the E3S Web of Conferences 13, Cracow, Poland, 12–14 October 2016; p. 05004. [Google Scholar]
- Zarzycki, R.; Jędras, J.; Kobyłecki, R. Gasification of Coal Dust in a Cyclone Furnace in an O2/H2O Atmosphere. Energies 2020, 13, 2253. [Google Scholar] [CrossRef]
- Gupta, A.K.; Lilley, D.G.; Syred, N. Swirl Flows; Abacus Press: Kent, WA, USA, 1995; Chapter 5. [Google Scholar]
- Choe, K.; Lee, Y.; Lee, S.; Weedon, M. Experimental Study on Non-Pulverized Wood Biomass Combustion with a New Three-Way Swirling Combustion Cyclone Combustor. J. Therm. Sci. Eng. Appl. 2019, 12, 1–32. [Google Scholar] [CrossRef]
- Ustimenko, B.P.; Bukham, M.A. Turbulent flow structure in a cyclone chamber. Therm. Eng. 1968, 21, 64. [Google Scholar]
- Deissler, R.; Perlmutter, M. Analysis of the flow and energy separation in a turbulent vortex. Int. J. Heat Mass Transf. 1960, 1, 173–191. [Google Scholar] [CrossRef]
- Sibulkin, M. Unsteady, viscous, circular flow, part III. Application to the Ranque–Hilsch vortex tube. J. Fluid Mech. 1962, 12, 269–293. [Google Scholar] [CrossRef]
- Syred, N.; Dahmen, K.R.; Styles, A.C.; Najim, S.A. Review of combustion problems associated with low calorific value gases. J. Inst. Energy 1997, 50, 195–207. [Google Scholar]
- Crowe, C.T. Conservation equations for vapor-droplet flows. In Proceedings of the Heat Transfer and Fluid Mechanics Institute Meeting 25th, Davis, CA, USA, 21–23 June 1976; (A76-35401 17-34). Stanford University Press: Stanford, CA, USA, 1976; pp. 214–228. [Google Scholar]
- Weller, A.E.; Dennis, W.R.; Putnam, A.A. The Development of a Surface Hardening Burner with a High Rate of Heat Transfer Resulting from a Self Generating Oscillation; Final Battelle Report; Cincinnati Milling Machine Company: Cincinnati, OH, USA, 1955. [Google Scholar]
- Choe, K.; Chae, J.; Cheah, W.; Na, S. A New Three-Way Swirling Combustion Technology with Air Curtain Insulation Effect for Solid Waste. J. Therm. Sci. Eng. Appl. 2018, 10, 041021. [Google Scholar] [CrossRef]
- Smith, J.L. An Experimental Study of the Vortex in the Cyclone Separator. J. Basic Eng. 1962, 84, 602–608. [Google Scholar] [CrossRef]
- Syred, N.; Beér, J. Combustion in swirling flows: A review. Combust. Flame 1974, 23, 143–201. [Google Scholar] [CrossRef]
- Fredriksson, C. Exploratory Experimental and Theoretical Studies of Cyclone Gasification of Wood Powder. Ph.D. Thesis, Lulea University of Technology, Luleå, Sweden, 2003. [Google Scholar]
- Bourgouin, J.F.; Moeck, J.; Durox, D.; Schuller, T.; Candel, S. Sensitivity of swirling flows to small changes in the swirler geometry. Comptes Rendus Mec. 2013, 341, 211–219. [Google Scholar] [CrossRef]
- Sosa-Arnao, J.H.; Ferreira, D.J.O.; Santos, C.G.; Justo, E.; Alvarez, J.E.; Rangel, L.P.; Park, S.W. The Influence of Swirl Burner Geometry on the Sugar-Cane Bagasse Injection and Burning. Technol. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2015, 9, 798–801. [Google Scholar]
- Ziqiang, L.; Guangqiang, L.; Yingjie, L. Optimization Study on Bias Angle of a Swirl Burner with Tangential Inlet Air. Int. J. Smart Home 2016, 10, 171–180. [Google Scholar] [CrossRef]
- Pasymi, P.; Budhi, Y.W.; Bindar, Y. Experimental and Numerical Investigations of Fluid Flow Behaviors in a Biomass Cyclone Burner. ASEAN J. Chem. Eng. 2020, 20, 88–98. [Google Scholar] [CrossRef]
- Nemoda, S.; Bakić, V.; Oka, S.; Zivković, G.; Crnomarkovic, N. Experimental and numerical investigation of gaseous fuel combustion in swirl chamber. Int. J. Heat Mass Transf. 2005, 48, 4623–4632. [Google Scholar] [CrossRef]
- Pasymi, P.; Budhi, Y.W.; Bindar, Y. Effect of initial tangential intensity on the fluid dynamic characteristics in tangential burner. MATEC Web. Conf. 2017, 101, 03001. [Google Scholar] [CrossRef] [Green Version]
- Pasymi, P.; Budhi, Y.W.; Bindar, Y. Three dimensional cyclonic turbulent flow structures at various geometries, inlet-outlet orientations, and operating conditions. J. Mech. Eng. Sci. 2018, 12, 4300–4328. [Google Scholar] [CrossRef]
- Aydin, O.; Avci, M.; Markal, B.; Yazici, M.Y. An experimental study on the decaying swirl flow in a tube. Int. Commun. Heat Mass Transf. 2014, 55, 22–28. [Google Scholar] [CrossRef]
- Gawali, S.S.; Bhambere, M.B. Computational fluid dynamics approach for predictions of cyclone separator pressure drop. Int. J. Mech. Eng. Robot. Res. 2015, 4, 374–377. [Google Scholar]
- Syred, N.; Fick, W.; O’Doherty, T.; Griffiths, A.J. The Effect of the Processing Vortex Core on Combustion in a Swirl Number. Combust. Sci. Technol. 1997, 125, 139–157. [Google Scholar] [CrossRef]
- Chen, J.; Haynes, B.S.; Fletcher, D.F.A. Numerical and experimental study of tangentially injected swirling pipe flows. In Proceedings of the 2nd International Conference on CFD in the Minerals and Process Industries CSIRO, Melbourne, Australia, 6–8 December 1999; pp. 485–490. [Google Scholar]
- De Bruyn Kops, S.M.; Malte, P.C. Simulation and Modeling of Wood Dust Combustion in Cyclone Burners. Available online: http://faculty.washington.edu/malte/OldSite/pubs/final_report_wood_dust_combustion_in_cyclone_burners.pdf (accessed on 1 April 2021).
- Al-Abdeli, Y.M.; Masri, A.R. Review of laboratory swirl burners and experiments for model validation. Exp. Therm. Fluid Sci. 2015, 69, 178–196. [Google Scholar] [CrossRef]
- Styles, A.C.; Syred, N.; Najim, S.E. Stabilization of Low Calorific Value Gases in a Cyclone Combustor. J. Energy 1981, 5, 159. [Google Scholar]
- Nussbaumer, T. Biomass Combustion in Europe Overview on Technologies and Regulations; Final Report 08–03; New York State Energy Research and Development Authority: Albany, NY, USA, 2008. [Google Scholar]
- Nussbaumer, T. Furnace Design and Combustion Control to Reduce Emissions and Avoid Ash Slagging; R&D Report. 1997. Available online: https://silo.tips/download/furnace-design-and-combustion-control-to-reduce-emissions-and-avoid-ash-siagging (accessed on 1 April 2021).
- Yorulmaz, S.Y.; Atimtay, A.T. Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process. Technol. 2009, 90, 939–946. [Google Scholar] [CrossRef]
- Baxter, L. Ash deposition during biomass and coal combustion: A mechanistic approach. Biomass Bioenergy 1993, 4, 85–102. [Google Scholar] [CrossRef]
- Fungtammasan, B.; Jittrepit, P. An Experimental Study on the Combustion Characteristics of Sawdust in a Cyclone Combustor. In The Journal of KMUTNB; King Mongkut’s University of Technology: North Bangkok, Thailand, 2008. [Google Scholar]
- Nussbaumer, T. Combustion and Co-combustion of Biomass: Fundamentals, Technologies, and Primary Measures for Emission Reduction. Energy Fuels 2003, 17, 1510–1521. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Baeyens, J.; Seville, J.P. NOx formation and selective non-catalytic reduction (SNCR) in a fluidized bed combustor of biomass. Biomass Bioenergy 2010, 34, 1393–1409. [Google Scholar] [CrossRef]
- Seo, Y. Study of Characteristics of Emission Gases from Waste Wood; R&D Report; National Institute of Environmental Research: Incheon, Korean, 2008. [Google Scholar]
- Yi, Q.; Qi, F.; Xioa, B.; Hu, Z.; Liu, S. Co-firing ramie residue with supplementary coal in a cy-clone furnace. Bio Resour. 2013, 8, 844–854. [Google Scholar]
- Vamvuka, D.; Kakaras, E. Ash properties and environmental impact of various biomass and coal fuels and their blends. Fuel Process. Technol. 2011, 92, 570–581. [Google Scholar] [CrossRef]
- Dayton, D.; Jenkins, B.; Turn, S.Q.; Bakker, R.R.; Williams, R.B.; Belle-Oudry, A.D.; Hill†, L.M. Release of Inorganic Constituents from Leached Biomass during Thermal Conversion. Energy Fuels 1999, 13, 860–870. [Google Scholar] [CrossRef]
- Miles, T.R.; Miles, T.R., Jr.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Oden, L.L. Boiler deposits from firing biomass fuels. Biomass Bioenergy 1996, 10, 125–138. [Google Scholar] [CrossRef]
- Tillman, D.A. Biomass cofiring: The technology, the experience, the combustion consequences. Biomass Bioenergy 2000, 19, 365–384. [Google Scholar] [CrossRef]
- Paulrud, S.; Nilsson, C. The effects of particle characteristics on emissions from burning wood fuel powder. Fuel 2004, 83, 813–821. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Mukunda, H.; Dasappa, S.; Paul, P.; Rajan, N.; Shrinivasa, U. Gasifiers and combustors for biomass—Technology and field studies. Energy Sustain. Dev. 1994, 1, 27–38. [Google Scholar] [CrossRef]
- Demirbaş, A. Sustainable cofiring of biomass with coal. Energy Convers. Manag. 2003, 44, 1465–1479. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Hughes, E. Biomass cofiring: Economics, policy and opportunities. Biomass Bioenergy 2000, 19, 457–465. [Google Scholar] [CrossRef]
- Banhart, J.S.; Laurendeau, N.M. Pulverised coal combustion and gasification in a cyclone reactor. Ind. Eng. Chem. Process. Des. Dev. 1982, 21, 671–680. [Google Scholar] [CrossRef]
- Cousins, J.W.; Robinson, W.H. Gasification of sawdust in an air-blown cyclone gasifier. Ind. Eng. Chem. Process. Des. Dev. 1985, 24, 1281–1287. [Google Scholar] [CrossRef]
- Syred, C.; Fick, W.; Griffiths, A.; Syred, N. Cyclone gasifier and cyclone combustor for the use of biomass derived gas in the operation of a small gas turbine in cogeneration plants. Fuel 2004, 83, 2381–2392. [Google Scholar] [CrossRef]
- Gabra, M.; Nordin, A.; Ohman, M.; Kjellstrom, B. Alkali retention/separation during bagasse gasification: A comparison between a fluidized bed and a cyclone gasifier. Biomass Bioenergy 2001, 21, 461–476. [Google Scholar] [CrossRef]
- Demirbaş, A. Yields of hydrogen-rich gaseous products via pyrolysis from selected biomass samples. Fuel 2001, 80, 1885–1891. [Google Scholar] [CrossRef]
- Morf, P.; Hasler, P.; Nussbaumer, T. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips. Fuel 2002, 81, 843–853. [Google Scholar] [CrossRef]
- Guoa, X.; Xiaoa, B.; Liua, S.; Hu, Z.; Luo, S.; He, M. An experimental study on air gasification of biomass micron fuel (BMF) in a cyclone gasifier. Int. J. Hydrog. Energy 2009, 34, 1265–1269. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Y.; Tian, H.; Ling, F.; Su, F. Experimental study on cyclone air gasification of wood powder. Bioresour. Technol. 2009, 100, 4047–4049. [Google Scholar] [CrossRef]
- Al-Attab, K.; Zainal, Z.; Alattab, K. Syngas production and combustion characteristics in a biomass fixed bed gasifier with cyclone combustor. Appl. Therm. Eng. 2017, 113, 714–721. [Google Scholar] [CrossRef]
- Cheng, G.; He, P.-W.; Xiao, B.; Hu, Z.-Q.; Liu, S.-M.; Zhang, L.-G.; Cai, L. Gasification of biomass micron fuel with oxygen-enriched air: Thermogravimetric analysis and gasification in a cyclone furnace. Energy 2012, 43, 329–333. [Google Scholar] [CrossRef]

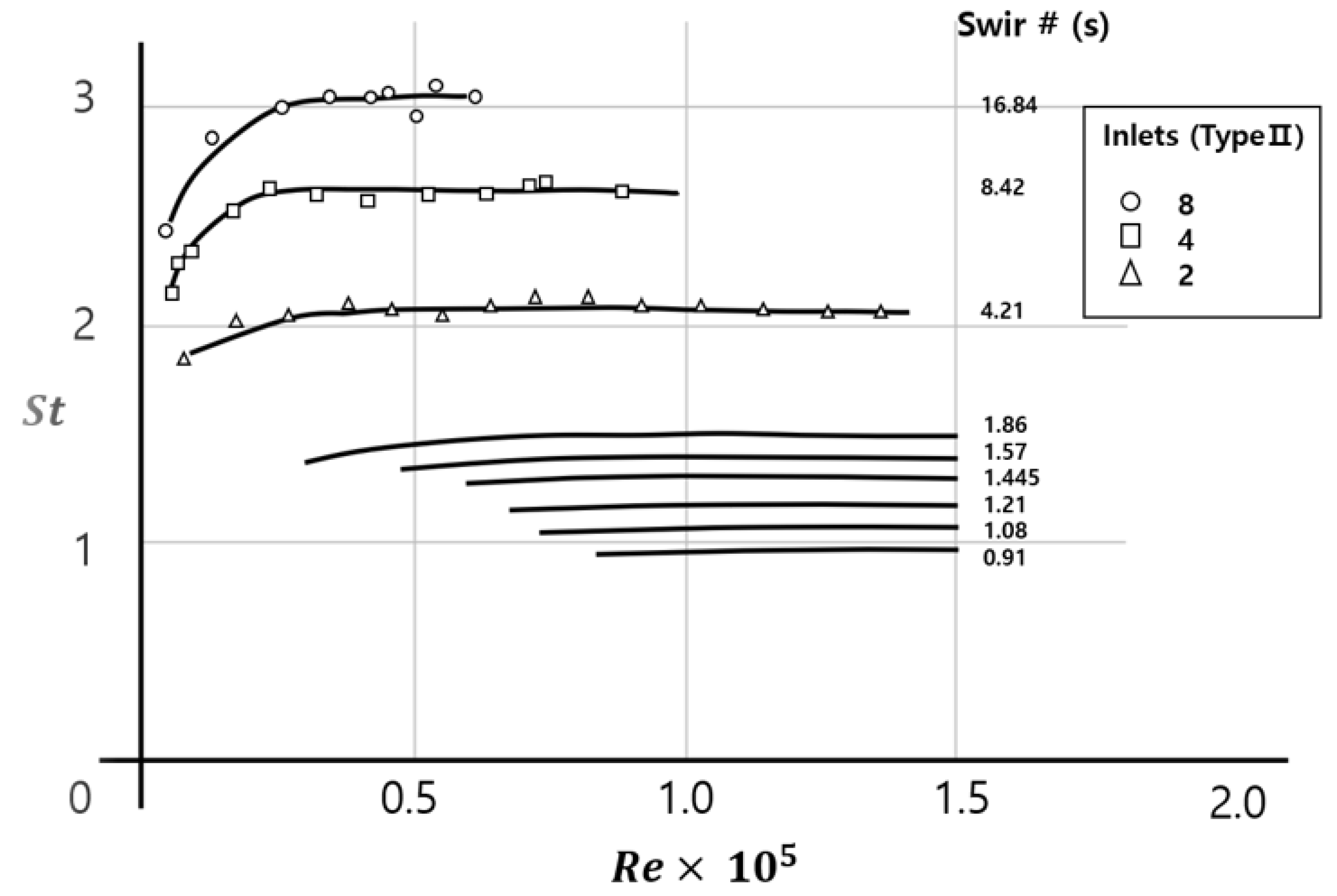
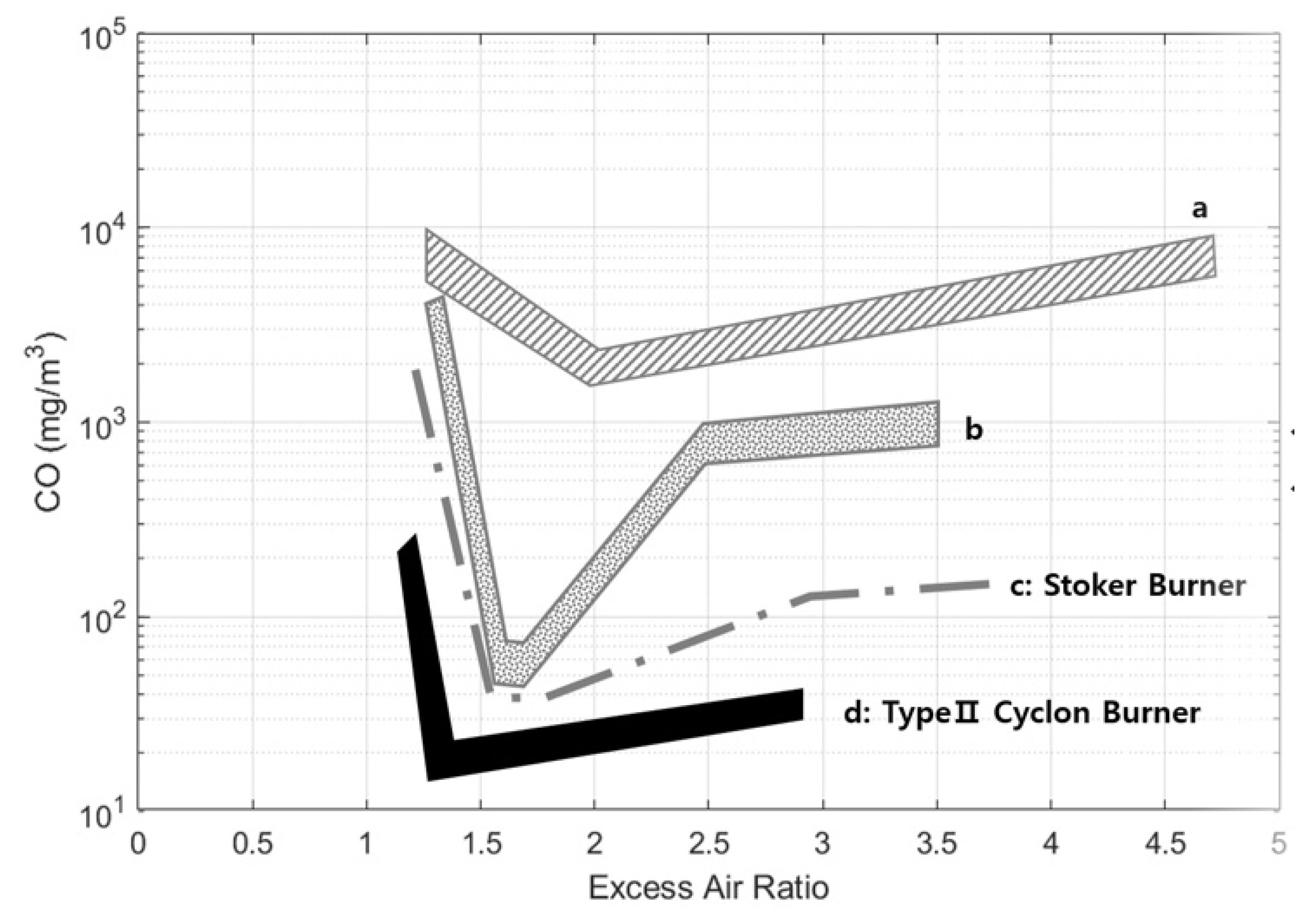
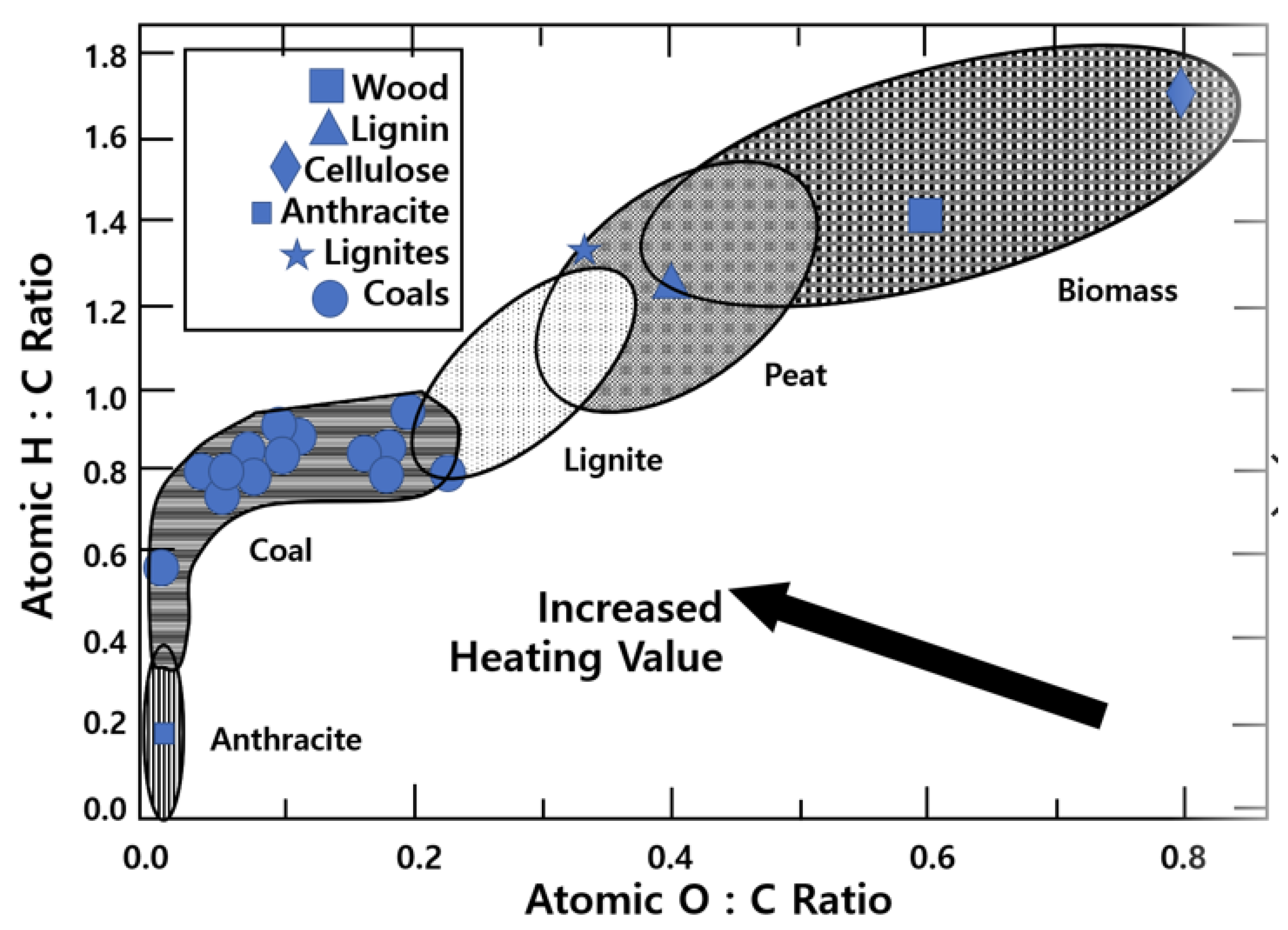
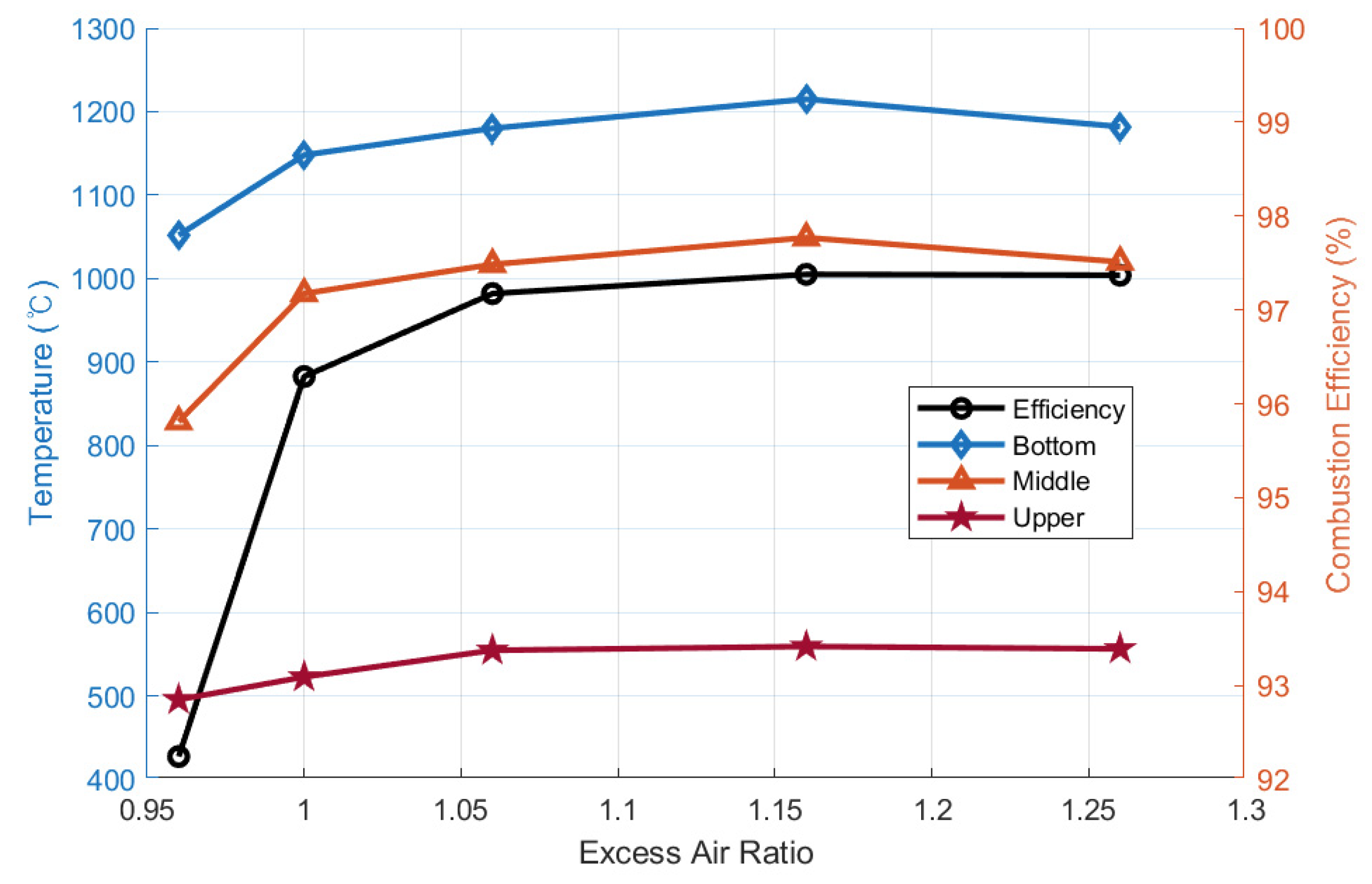
| Cyclone Type | S (Swirl #) | De/Do | L/De | Fuel | Configuration |
|---|---|---|---|---|---|
| Type1 [26] | 2–11 | 0.4–0.7 | 1–3 | High calorific value and high volatile | Bottom exit |
| Type2 [27,28] | 8–20 | 0.4–0.5 | 1–1.25 | High ash | Top exit/ash through bottom exit |
| Type3 [29] | 3 or higher | 0.3 or less | 5 or higher | Low calorific and high volatile | Horizontal multi-inlets |
| Type4 [30] | 3 or higher | About 0.4 | About 3 | Oil (sulfuric) | Top inlet/bottom exit |
| Type5 [31] | 3 or less | 0.4–0.6 | 2.2–2.9 | Metal | Top exit |
| S | L/Do | De/Do | St [41] | Re @Max. St [41] | |
|---|---|---|---|---|---|
| TSC [25] | 14.53 | 1.9 | 0.54 | 2.93 | 0.347 × 105 |
| Mildsteel Combustor [46] | 6.8 | 2.0 | 0.44 | - | - |
| Perpex Model [46] | 8.8 | 2.06 | 0.51 | - | |
| Type 2 Cyclone [41] | ≤20 | ≤4 | ≥0.4, ≤0.5 | ≤3 | - |
| Sample | Volatile Matter | Ash | Fixed Carbon | Calorific Value (MJ/kg) | C | H | N | O | S |
|---|---|---|---|---|---|---|---|---|---|
| Pine | 88.02 | 0.62 | 11.29 | 19.72 | 53.28 | 6.35 | 0.16 | 40.21 | - |
| MDF | 86.68 | 2.29 | 11.06 | 19.31 | 49.57 | 6.33 | 4.44 | 39.66 | - |
| Paticleboard | 83.82 | 1.22 | 14.38 | 17.51 | 46.26 | 5.83 | 2.36 | 45.51 | 0.04 |
| Plywood | 85.79 | 0.80 | 13.40 | 18.64 | 47.12 | 5.92 | 1.19 | 45.72 | 0.05 |
| Fuel Type | Max. Temp (°C) | O2 (%) | CO (ppm) | NOx (ppm) | SOx (ppm) | Excess Air Ratio (λ) | |
|---|---|---|---|---|---|---|---|
| Fungtammasan et al. [54] | Sawdust | 1275 | - | 3196 | - | - | 1.50 |
| Luo et al. [5] | Micron-sized wood (less than 250 μm) | 1270 | 0.6 | 0.015 | 182.0 | 96.0 | 1.20 |
| Choe et al. [25] | Non-pulverized wood chips (5–10 cm) | 1058 | 3.53 | 39.46 | 48.67 | 0.3 | 1.21 |
| Construction Waste [1] | Non-MDF [57] | MDF [57] | Antisepsis Wood [57] | Wood [1] | Luo et al. [5] | Choe et al. [25] | |
|---|---|---|---|---|---|---|---|
| NOx (ppm) | 176 | 480 | 681 | 603 | 125–261 | 182 | 49 |
| CO (ppm) | 4243 | 2673 | 5480 | 12,628 | 96–1547 | 0.015 | 39 |
| Components (%) | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | Na2O | CaO | P2O5 | SO3 | TiO2 | Rb/a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMF | 14.28 | 3.16 | 2.38 | 7.14 | 8.10 | 6.40 | 42.82 | 4.80 | 2.91 | 2.91 | 3.28 |
| Coal | 45.58 | 30.02 | 9.69 | - | 2.02 | - | 5.48 | 0.85 | 4.34 | 1.64 | 0.223 |
| Process | Excess Air Ratio (λ) | Temperature (°C) | Main Product |
|---|---|---|---|
| Combustion | >1 | 800–1300 | Hot exhaust gas |
| Gasification | 0.2 < λ < 0.5 | 700–900 | Producer gas of high thermal value |
| Pyrolysis | 0 < λ < 0.2 | 400–700 | Liquid of high thermal value (pyrolysis oil) |
| Wood Powder [71] | Sawdust [71] | Powdery Biomass [65] | BMF (2) [75] | Wood Powder [76] | Furniture Wood [77] | BMF (2) [78] | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Operating Conditions | Feed Rate (kg/h) | Sampling Time (Min) | Particle Size (mesh) | Secondary Air Ratio (%) (1) | Syngas Flow Rate (m3/h) | Oxygen Concentration (vol %) | ||||||||||||||||
| 26 | 40 | 28 | 40 | 59 | 200 | 404 | 60–100 | 100–180 | <180 | 19 | 23 | 31 | 50 | 100 | 150 | 200 | 21 | 23 | 25 | 28 | 31 | |
| Temperature (°C) | 818 | 842 | 819 | 915 | 650–700 | 755 | 790 | 814 | 850–1000 | 310 | 450 | 550 | 620 | 798 | 803 | 820 | 838 | 845 | ||||
| H2 (%, v/v) | 9.06 | 8.56 | 3.71 | 7.14 | 16.51 | 16.84 | 18.72 | 4.77 | 6.92 | 8.26 | 5.03 | 7.10 | 6.00 | 12.30 | 11.10 | 11.50 | 11.20 | 8.83 | 9.84 | 11.27 | 11.95 | 12.01 |
| O2 (%, v/v) | 1.17 | 1.48 | 1.97 | 1.69 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1.80 | 1.20 | 2.30 | 3.00 | NA | NA | NA | NA | NA |
| N2 (%, v/v) | 56.87 | 52.09 | 68.34 | 60.33 | 49.23 | 60.55 | 48.62 | NA | NA | NA | NA | NA | NA | 52.90 | 54.30 | 52.90 | 58.40 | NA | NA | NA | NA | NA |
| CH4 (%, v/v) | 1.95 | 3.31 | 1.06 | 1.95 | 0.69 | 0.64 | 1.29 | 2.42 | 1.94 | 1.58 | 3.84 | 3.22 | 4.47 | 2.40 | 1.40 | 1.40 | 1.00 | 2.96 | 3.41 | 3.85 | 4.44 | 4.66 |
| CO (%, v/v) | 15.37 | 18.18 | 7.05 | 12.08 | 10.44 | 19.39 | 19.79 | 17.27 | 18.95 | 19.29 | 18.60 | 22.00 | 20.50 | 15.00 | 20.00 | 18.00 | 15.40 | 18.08 | 20.98 | 22.17 | 24.01 | 23.93 |
| CO2 (%, v/v) | 13.39 | 12.63 | 16.47 | 14.55 | 9.95 | 10.97 | 12.07 | 12.14 | 13.57 | 13.82 | 16.01 | 18.01 | 15.81 | 15.60 | 12.00 | 13.90 | 11.00 | 9.41 | 10.94 | 12.51 | 14.54 | 15.09 |
| SO2 (mg/m) | 4.40 | 3.10 | 1.60 | 3.80 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Calorific Value of Exhaust gas (MJ/m3)/(MJ/kg) | 4.26 | 5.91 | 0.78 (3) | 2.99 (4) | 4.16 | 4.25 | 4.50 | 4.08 | 4.22 | 4.25 | 4.57 | 5.67 | 5.22 | 4.40 | 4.50 | 4.30 | 3.75 | 4.38 | 4.93 | 5.39 | 5.91 | 6.20 |
| Syngases (H2, CH4, CO, CO2) Conversion Rate (%, v/v) | 39.77 | 42.68 | 28.29 | 35.72 | 37.59 | 47.84 | 51.87 | 36.60 | 41.38 | 42.95 | 43.48 | 50.33 | 46.78 | 45.30 | 44.50 | 44.80 | 38.60 | 39.28 | 45.17 | 49.80 | 54.94 | 55.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, K. Review of Wood Biomass Cyclone Burner. Energies 2021, 14, 4807. https://doi.org/10.3390/en14164807
Choe K. Review of Wood Biomass Cyclone Burner. Energies. 2021; 14(16):4807. https://doi.org/10.3390/en14164807
Chicago/Turabian StyleChoe, Kangil. 2021. "Review of Wood Biomass Cyclone Burner" Energies 14, no. 16: 4807. https://doi.org/10.3390/en14164807





