Evaporation of Methanol Solution for a Methanol Steam Reforming System
Abstract
:1. Introduction
2. Experimental Approaches for the Evaporator of the Methanol-Reforming System
2.1. Design of the Methanol/Steam Mixture Evaporator for Reforming System
2.2. Experimental Apparatus for the Methanol-Reforming System with Different Evaporators
3. Result and Discussion
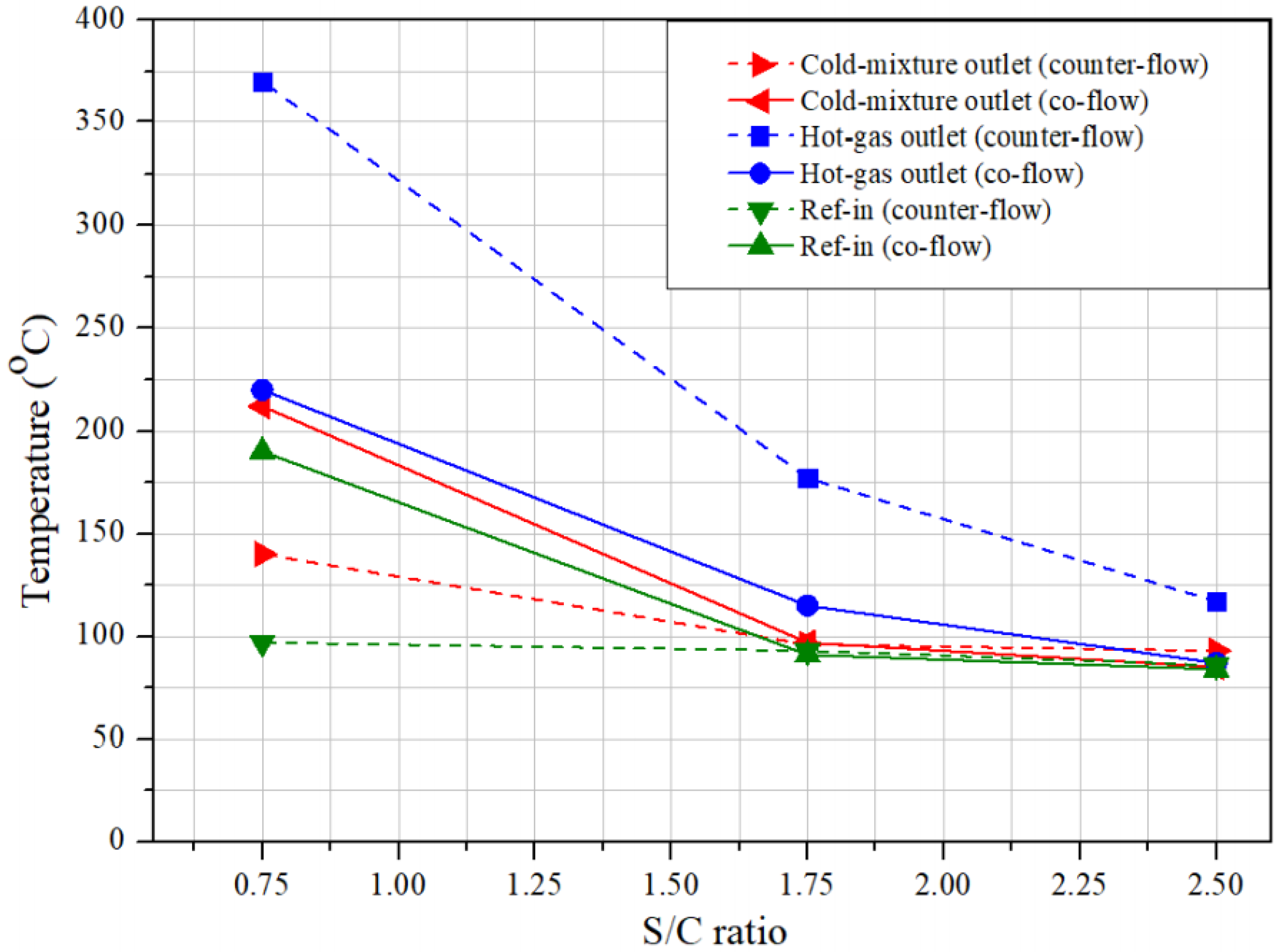
3.1. Evaporation Process Evaluation for the Establishment of the Reference Conditions
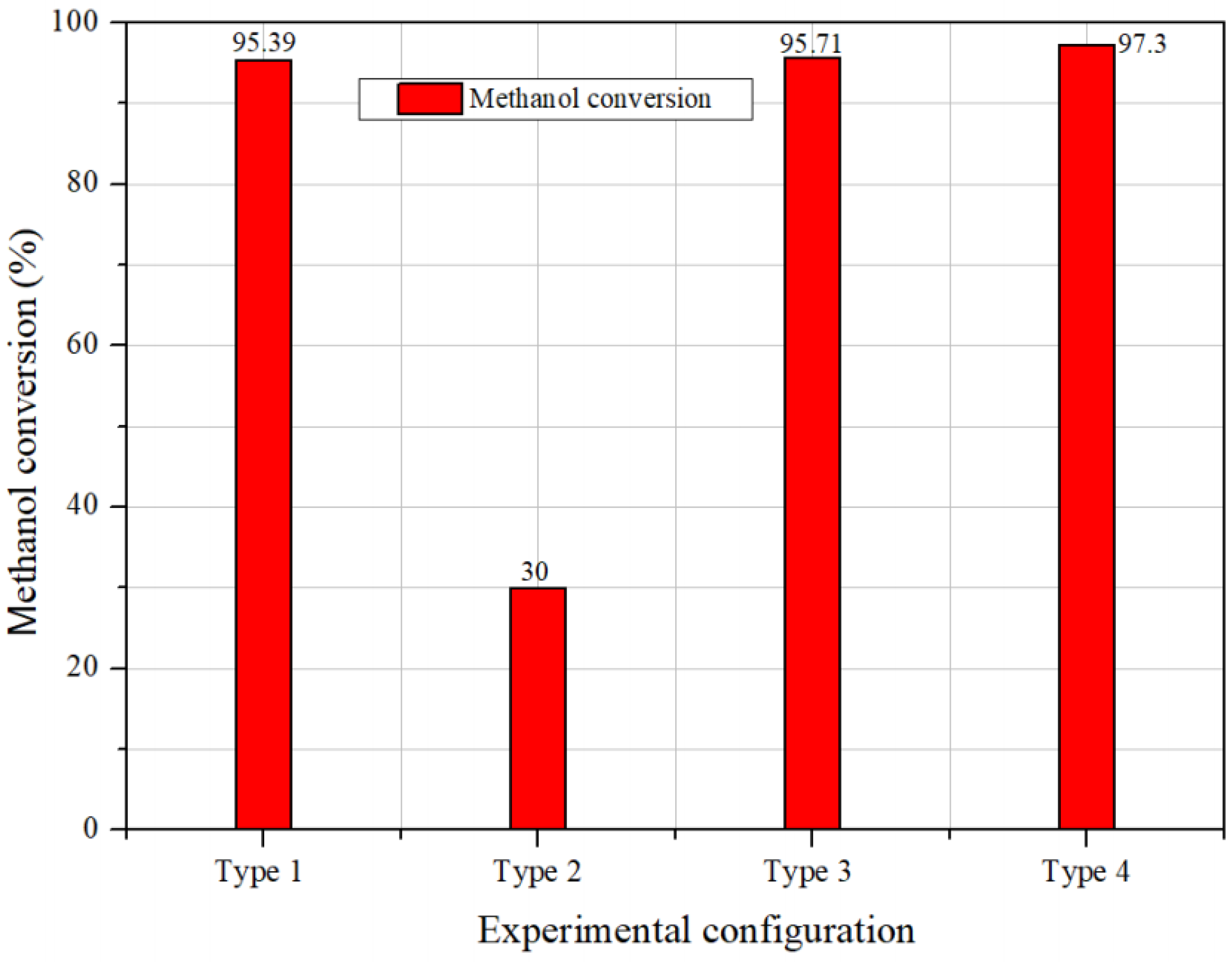
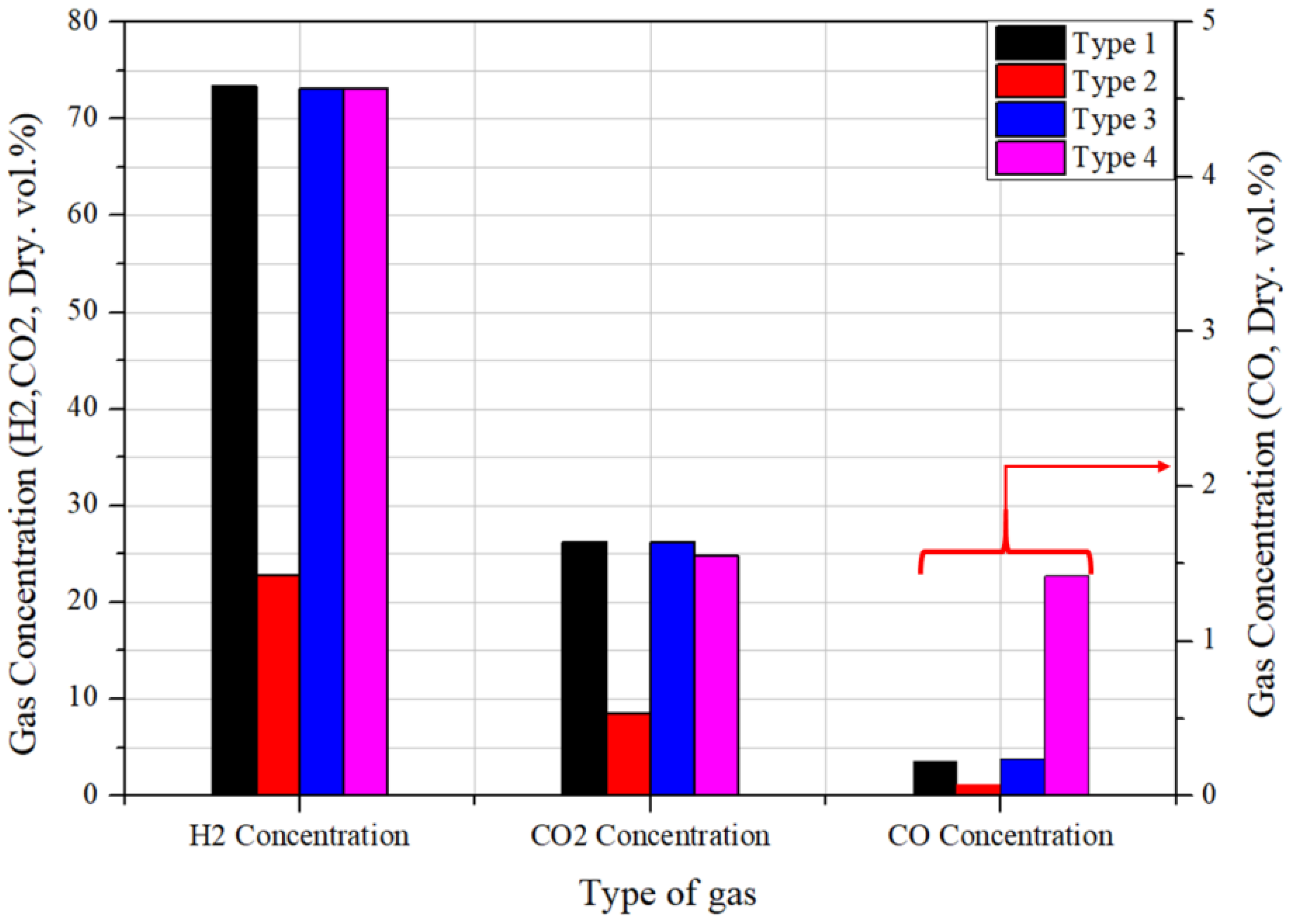
3.2. Performance Comparison of the Four Different Setups at Identical Heat Capacity
4. Conclusions
- The flow-field mode was chosen for the system; the co-flow arrangement was more suitable for the methanol-reforming system than the counter-flow mode because of the insignificant difference in the evaporator outlet temperature.
- Comparison between the four types of evaporators proceeded at the same heat capacity; the type-4 evaporator demonstrated the most effective methanol conversion and temperature development performance. The methanol conversion reached over 97%, with the highest temperature of the evaporator outlet.
- Internal evaporation was feasible for the evaporating process; however, it should be combined with the external evaporation (shell-and-tube) to use the wasted thermal energy to improve the system’s performance.
- While the external evaporation method has received extensive attention from researchers, the system’s performance increases moderately. As a result, the performance of the system rises significantly when two methods of evaporation proceed simultaneously. This work will provide future researchers with many advantages for designing an integrated methanol-reforming system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| S/C ratio | steam to carbon ratio |
| CH3OH | methanol |
| H2O | water |
| CO | carbon monoxide |
| CO2 | carbon monoxide dioxide |
| TC | thermocouple |
| GHSV | Gas hourly space velocity |
| ZnO | zinc oxide |
| CuO | copper (ii) oxide |
References
- Sarkar, J.; Bhattacharyya, S. Application of graphene and graphene-based materials in clean energy-related devices Minghui. Arch. Thermodyn. 2012, 33, 23–40. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef] [Green Version]
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Rathour, R.K.; Ahuja, V.; Bhatia, R.K.; Bhatt, A.K. Biobutanol: New era of biofuels. Int. J. Energy Res. 2018, 42, 4532–4545. [Google Scholar] [CrossRef]
- Palmer, G.; Floyd, J. Hydrogen as an energy carrier. In Energy Storage and Civilization; Springer: Cham, Switzerland, 2020; Volume 40. [Google Scholar]
- Moon, C.S. Estimations of the lethal and exposure doses for representative methanol symptoms in humans. Ann. Occup. Environ. Med. 2017, 29, 44. [Google Scholar] [CrossRef]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A review of the methanol economy: The fuel cell route. Energies 2020, 13, 596. [Google Scholar] [CrossRef] [Green Version]
- Vlachos, D.G.; Caratzoulas, S. The roles of catalysis and reaction engineering in overcoming the energy and the environment crisis. Chem. Eng. Sci. 2010, 65, 18–29. [Google Scholar] [CrossRef]
- Schubert, A.W.K.; Brandner, J.; Fichtner, M.; Linder, G.; Schygulla, U. Microstucture devices for applications in thermal and chemical process engineering. Microscale Thermophys. Eng. 2001, 5, 17–39. [Google Scholar]
- Holladay, J.D.; Jones, E.O.; Dagle, R.A.; Xia, G.G.; Cao, C.; Wang, Y. High efficiency and low carbon monoxide micro-scale methanol processors. J. Power Sources 2004, 131, 69–72. [Google Scholar] [CrossRef]
- Pattekar, A.V.; Kothare, M.V. A microreactor for hydrogen production in micro fuel cell applications. J. Microelectromech. Syst. 2004, 13, 7–18. [Google Scholar] [CrossRef]
- Brooks, K.P.; Davis, J.M.; Fischer, C.M.; King, D.L.; Pederson, L.R.; Rawlings, G.C.; Stenkamp, V.S.; TeGrotenhuis, W.; Wegeng, R.S.; Whyatt, G.A. Fuel Reformation: Microchannel Reactor Design. In Microreactor Technology and Process Intensification: ACS Symposium Series; Wang, Y., Holladay, J.D., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 238–257. [Google Scholar]
- Kolb, G.; Hessel, V. Micro-structured reactors for gas phase reactions. Chem. Eng. J. 2004, 98, 1–38. [Google Scholar] [CrossRef]
- Wegeng, R.S.; Pederson, L.R.; TeGrotenhuis, W.E.; Whyatt, G.A.; Saffell, B.J. Compact fuel processors for fuel cell powered automobiles based on microchannel technology. Fuel Cells Bull. 2001, 3, 8–13. [Google Scholar] [CrossRef]
- Tezcan, I.; Avci, A.K. Parametric investigation of oxidative coupling of methane in a heat-exchange integrated microchannel reactor. J. Chem. Technol. Biotechnol. 2015, 90, 1827–1838. [Google Scholar] [CrossRef]
- Cuta, J.M.; Bennett, W.D.; McDonald, C.E.; Ravigururajan, T.S. Fabrication and testing of microchannel heat exchangers. In Proceedings of the Microlithography and Metrology in Micromachining, International Society for Optics and Photonics, Austin, TX, USA, 26 September 1995; Volume 2640. [Google Scholar] [CrossRef]
- Yoshida, K.; Tanaka, S.; Hiraki, H.; Esashi, M. A micro fuel reformer integrated with a combustor and a microchannel evaporator. J. Micromech. Microeng. 2006, 16, S191. [Google Scholar] [CrossRef]
- Kolios, G.; Frauhammer, J.; Eigenberger, G. Efficient reactor concepts for coupling of endothermic and exothermic reactions. Chem. Eng. Sci. 2002, 57, 1505–1510. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Castaldo, F.; Ricca, A.; Boettge, D. Ethanol steam reforming over bimetallic coated ceramic foams: Effect of reactor configuration and catalytic support. Int. J. Hydrog. Energy 2015, 40, 12650–12662. [Google Scholar] [CrossRef]
- Cui, X.; Kær, S.K. Two-dimensional thermal analysis of radial heat transfer of monoliths in small-scale steam methane reforming. Int. J. Hydrog. Energy 2018, 43, 11952–11968. [Google Scholar] [CrossRef]
- Yun, J.; Kim, Y.; Yu, S. Interactive heat transfer characteristics of 5 kW class shell-and-tube methane steam reformer with intermediate temperature heat source. Int. J. Hydrog. Energy 2020, 45, 21767–21778. [Google Scholar] [CrossRef]
- Yun, J.; Yu, S. Transient behavior of 5 kW class shell-and-tube methane steam reformer with intermediate temperature heat source. Int. J. Heat Mass Transf. 2019, 134, 600–609. [Google Scholar] [CrossRef]
- Shin, G.; Yun, J.; Yu, S. Thermal design of methane steam reformer with low-temperature non-reactive heat source for high efficiency engine-hybrid stationary fuel cell system. Int. J. Hydrog. Energy 2017, 42, 14697–14707. [Google Scholar] [CrossRef]
- Yun, J.; Cho, K.; Lee, Y.D.; Yu, S. Four different configurations of a 5 kW class shell-and-tube methane steam reformer with a low-temperature heat source. Int. J. Hydrog. Energy 2018, 43, 4546–4562. [Google Scholar] [CrossRef]

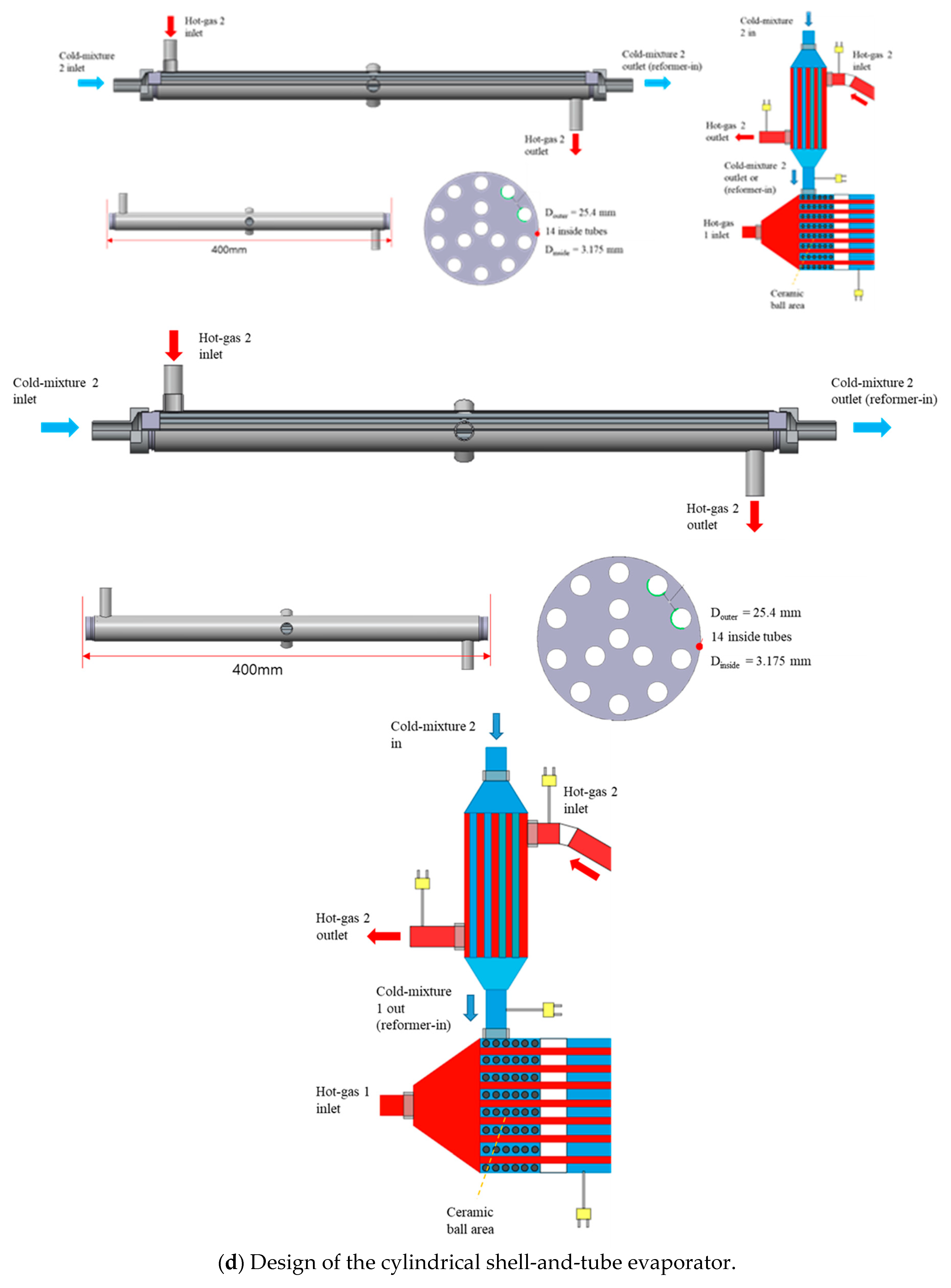
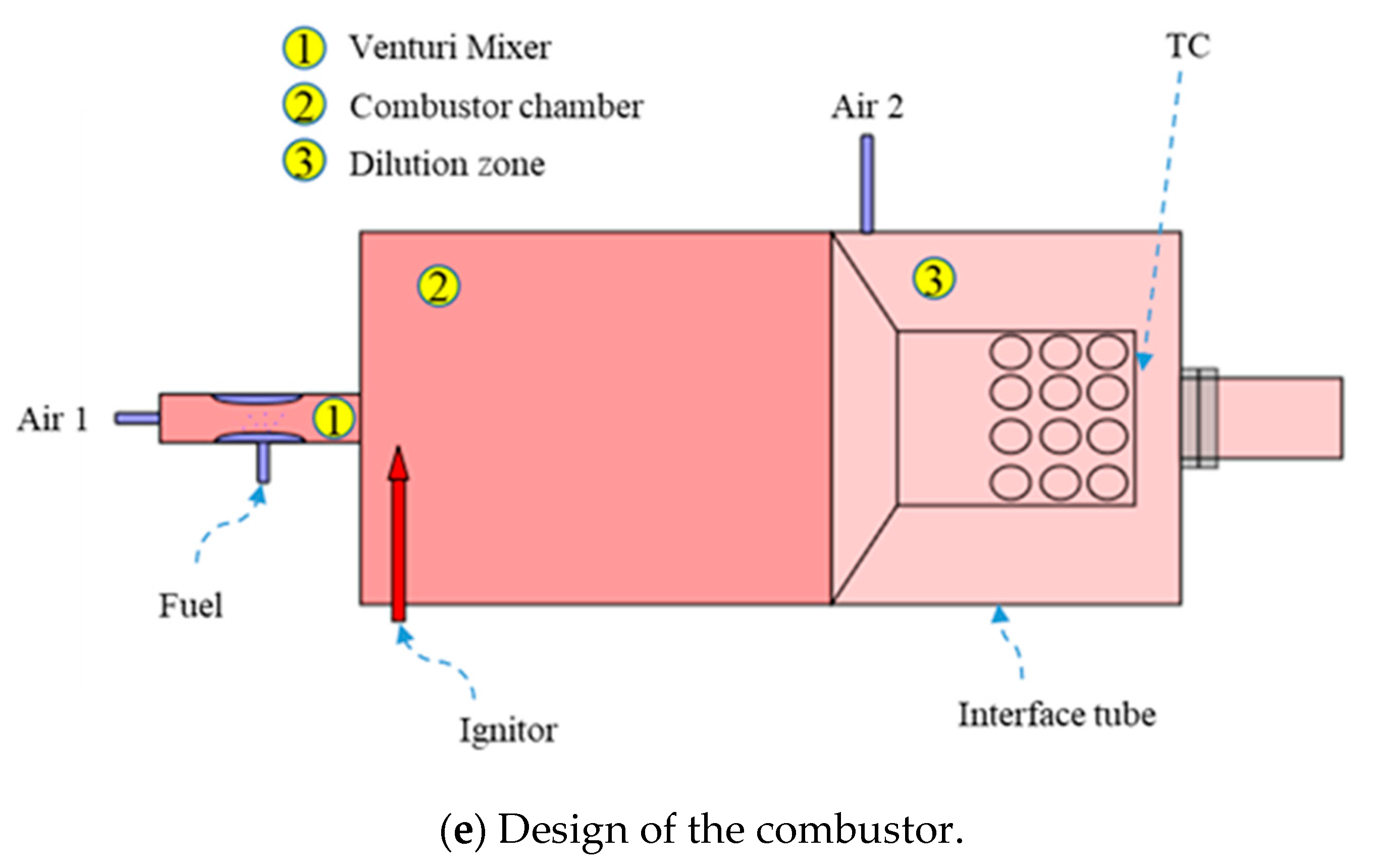
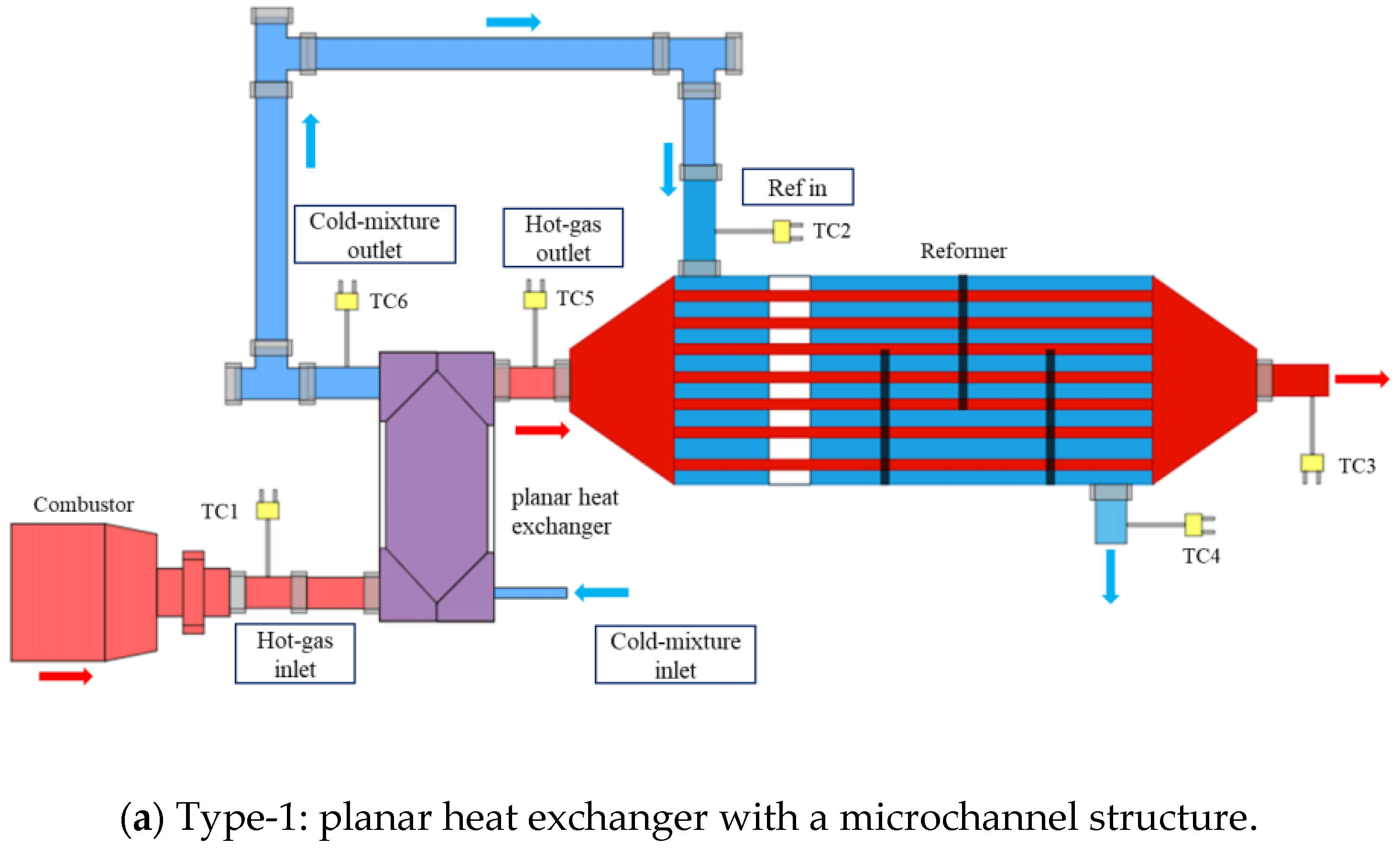
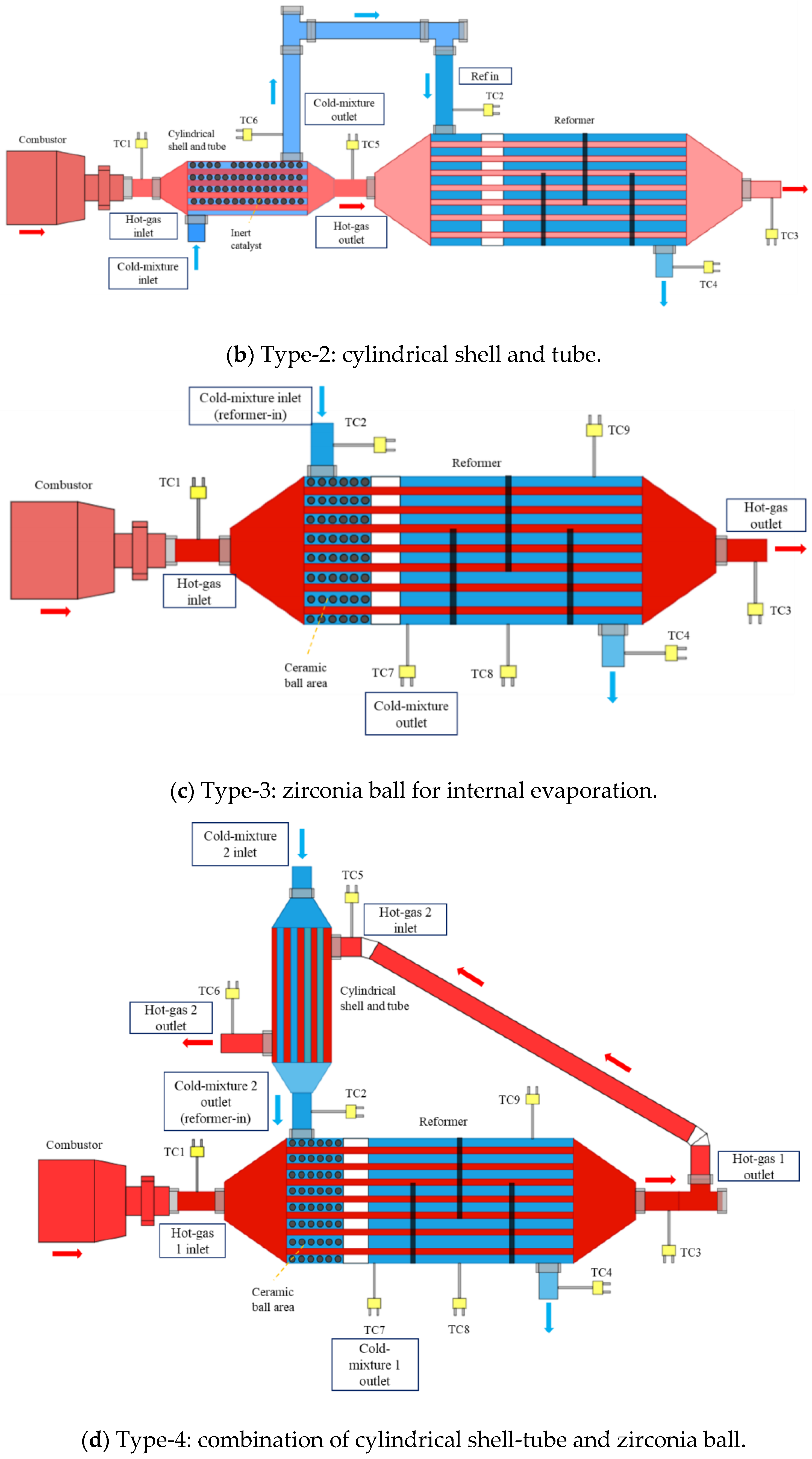




| Parameters | Values | Units |
|---|---|---|
| Reference conditions | ||
| Temperature of reactants for reforming | 25 | °C |
| Flow rate of methanol for reforming | 0.003 | mol/s |
| Flow rate of methanol for burner | 0.0015 | mol/s |
| S/C ratio | 2.5 | - |
| GHSV | 2400 | 1/h |
| Standard operating temperature | 300 | °C |
| Experiment parameters | ||
| S/C ratio | 0.75, 1.75, 2, 2.25, 2.5, 2.75, 3 | - |
| Flow rate of methanol for burner | 60, 70, 80, 90, 100 | % |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, N.V.; Kim, Y.; Kim, H.; Yu, S. Evaporation of Methanol Solution for a Methanol Steam Reforming System. Energies 2021, 14, 4862. https://doi.org/10.3390/en14164862
Trinh NV, Kim Y, Kim H, Yu S. Evaporation of Methanol Solution for a Methanol Steam Reforming System. Energies. 2021; 14(16):4862. https://doi.org/10.3390/en14164862
Chicago/Turabian StyleTrinh, Ngoc Van, Younghyeon Kim, Hongjip Kim, and Sangseok Yu. 2021. "Evaporation of Methanol Solution for a Methanol Steam Reforming System" Energies 14, no. 16: 4862. https://doi.org/10.3390/en14164862







