Experimental Studies of the Influence of Microencapsulated Phase Change Material on Thermal Parameters of a Flat Liquid Solar Collector
Abstract
:1. Introduction
1.1. Liquids with a Phase Change Material in the Field of Solar Energy
1.2. Letant Functional Thermal Fluid as a Working Fluid in Solar Installations—Previous Research
2. Method and Materials
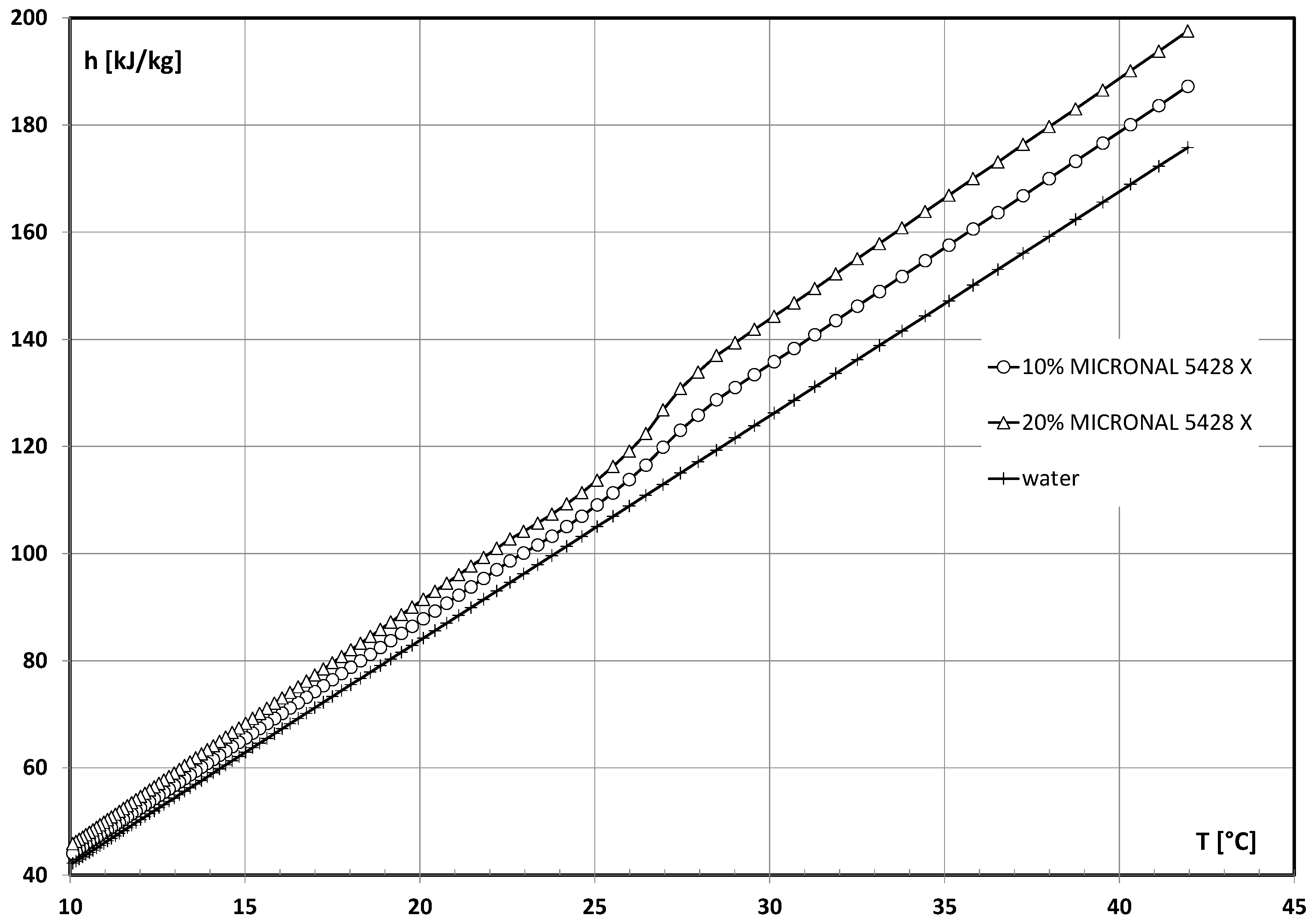
2.1. Working Fluid
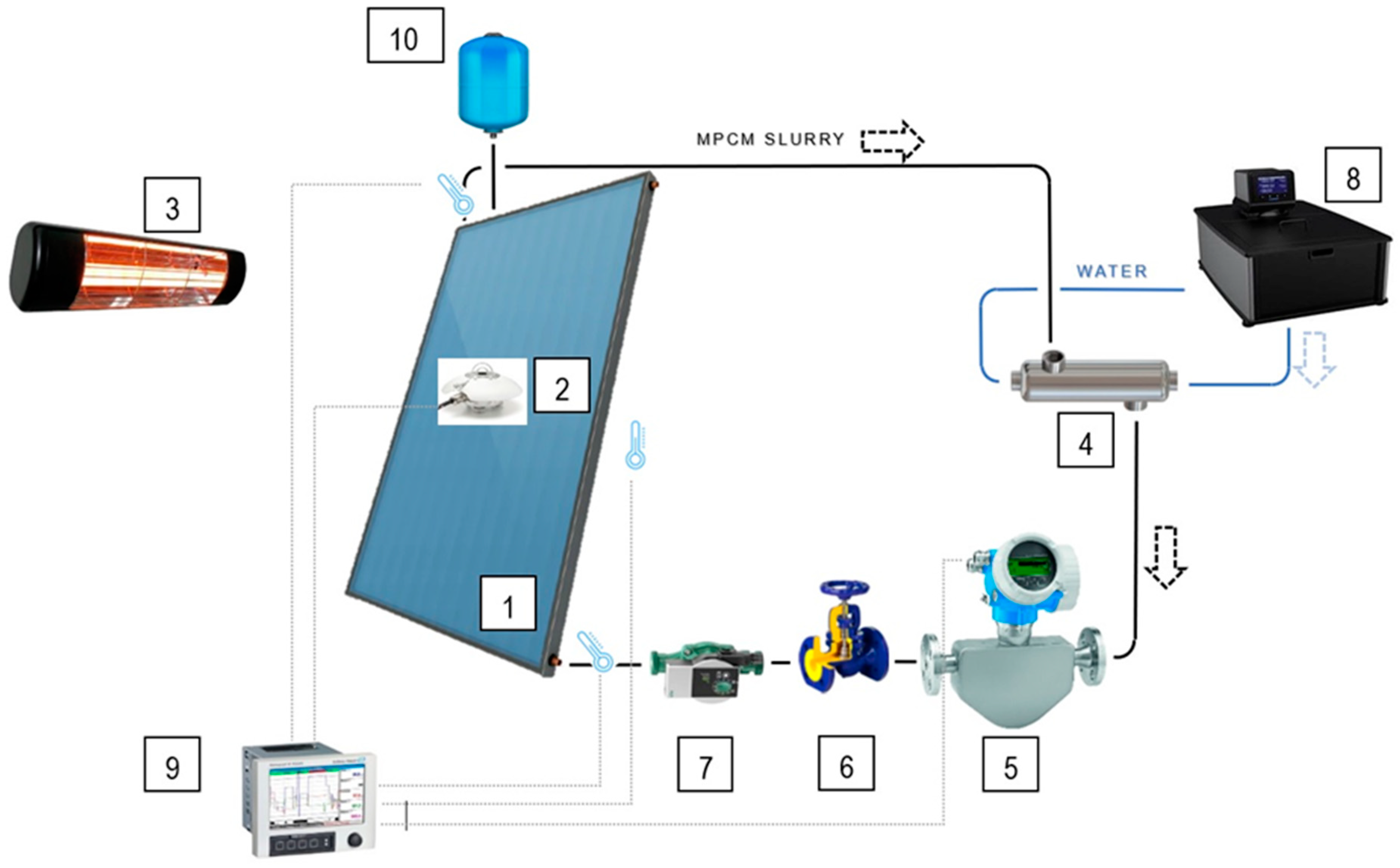
2.2. The Experimental Facility
2.3. Research Procedure and Data Reduction
3. Results and Discussion
3.1. Test Results of a Solar Collector Supplied with the Reference Liquid–Water
3.2. Test Results for a Solar Collector Supplied with mPCM Slurry
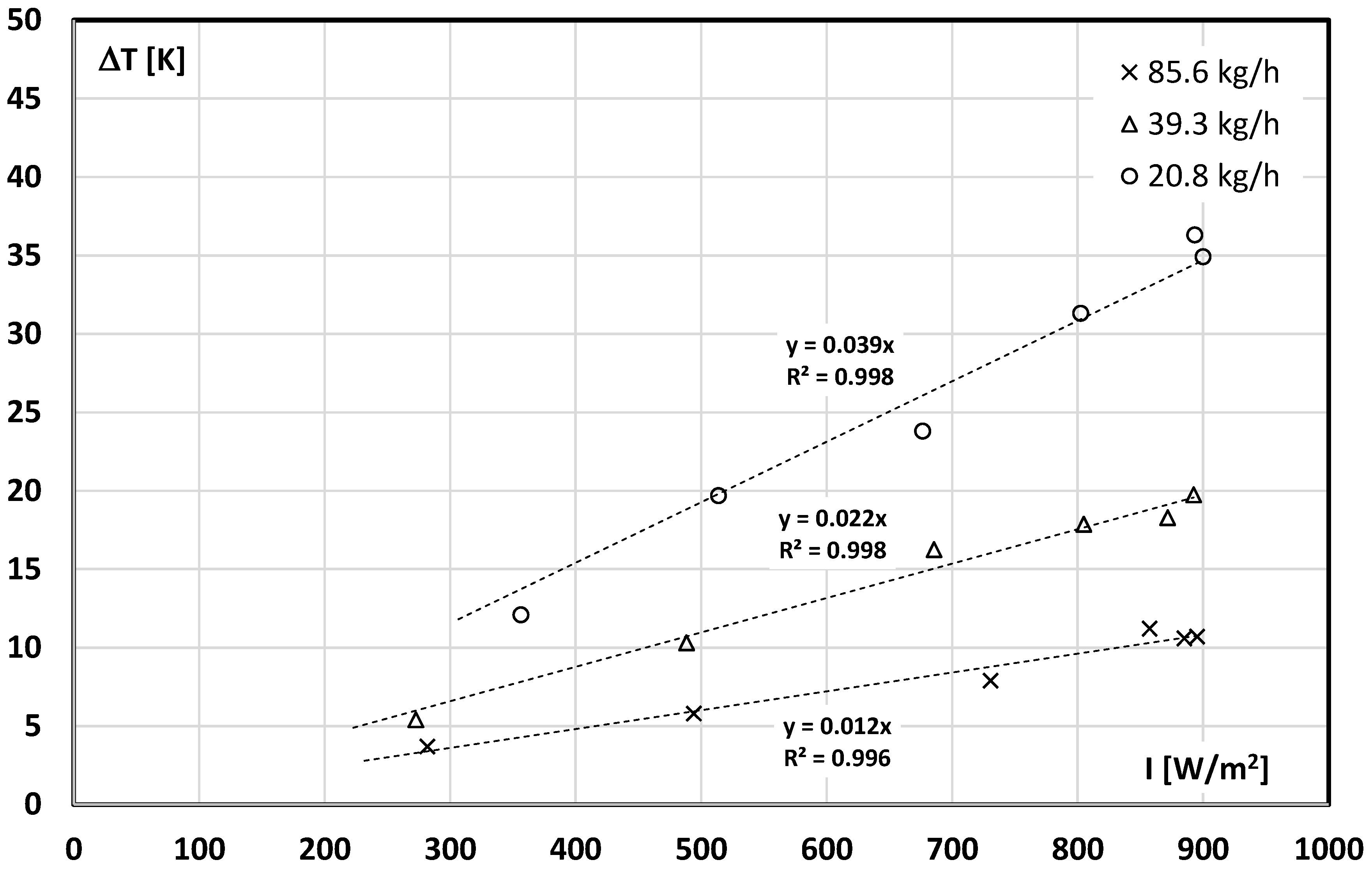
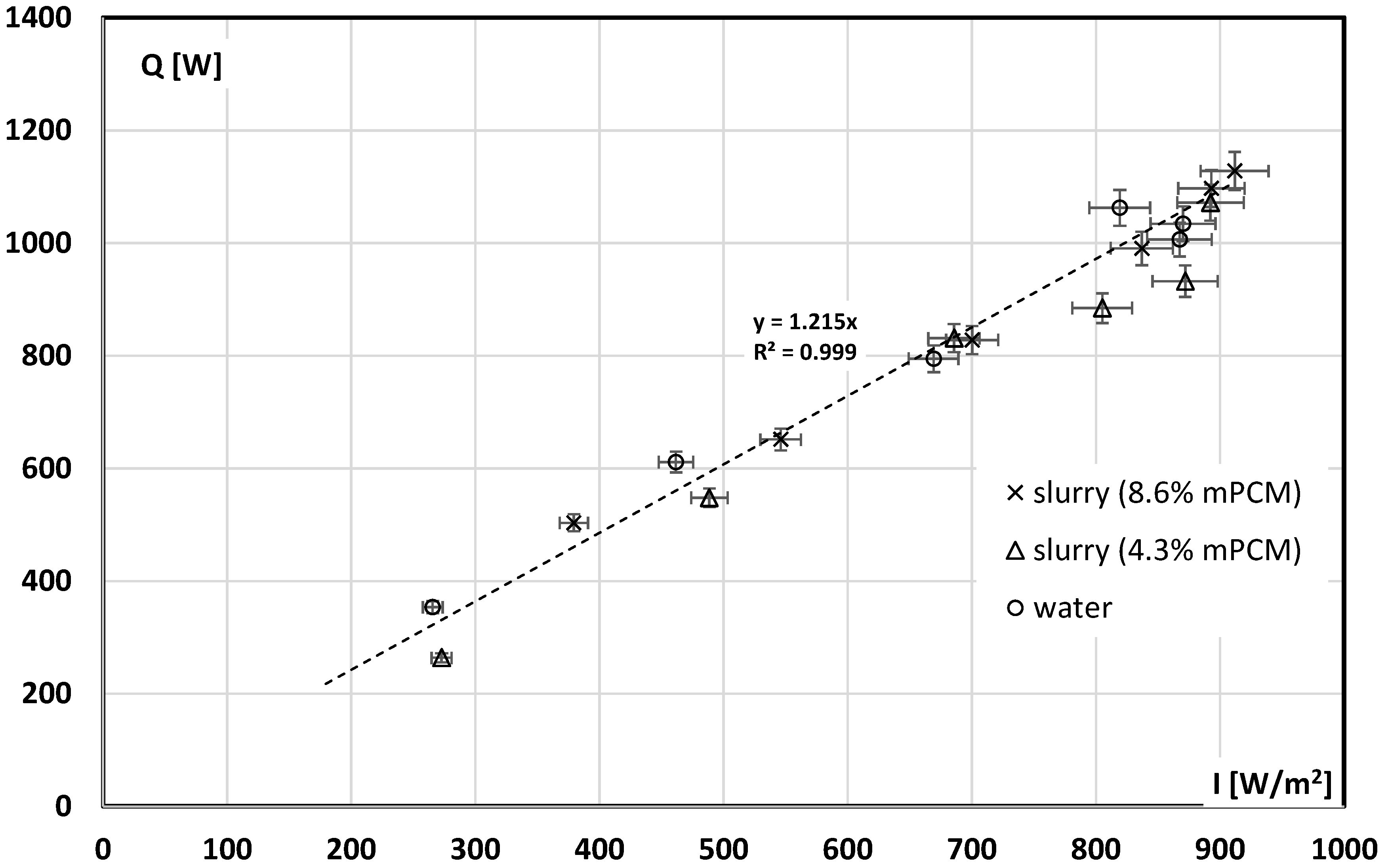
3.3. The Influence of mPCM in the Slurry on the Results of the Solar Collector Tests
- In summer, when hot water consumption is sometimes too low (low demand for hot water, the user has gone on holiday), the water temperature in the tank rises. Then, the temperature of the working liquid flowing through the exchanger also increases. When it reaches the mPCM phase transition temperature, further heat input causes the phase transformation process to start. This is performed at a constant temperature of the working fluid until all PCM in the plant has undergone a phase change. The traditional working fluid may reach boiling point during this time. Then, excessive pressure in the installation causes the safety valve to open and the liquid vapors to be released into the environment. When the working fluid is water, there is no problem, and its deficiency can be easily replenished. However, in temperate regions, due to cold winters, the working fluid is not water but a water–glycol solution. Then, its deficiencies need to be supplemented, and in fact, too high a temperature caused the substance to lose its properties. In this case, all liquid in the system must be replaced;
- In winter, when the temperature of the working liquid is significantly higher than the ambient temperature, the heat losses, including those from the collector, are higher with the higher the temperature of the working liquid in the system. The supply of the same amount of heat to the reservoir with the mPCM slurry at a temperature slightly above the phase transition temperature is more effective than, for example, water, which should have a higher temperature. Reducing heat loss to the environment increases the efficiency of the complete installation.
4. Summary and Conclusions
- The more PCM microcapsules in the working liquid, the lower the temperature of the liquid leaving the collector (for the same irradiance), which results from the use of the latent heat of the PCM material;
- The type of working fluid does not affect the collector’s thermal power obtained for analogous conditions of the experiment (irradiance, mass flow of the working fluid);
- It is possible to use mPCM water slurry as a working fluid in solar collector installations without adversely affecting its performance;
- The use of the phase change heat of the PCM material contained in the microcapsules circulating in the working fluid caused its temperature to be lower than that of water without the addition of mPCM when the same amount of thermal energy was transported;
- The reduced temperature of the working liquid may have a positive effect on the operation of the system:In summer—protection of the working fluid against overheating that results in a phase change and pressure increase in the system, or an irreversible change in the physical properties of the working fluid (e.g., based on propylene glycol);In winter—lowering the temperature of the working liquid circulating in the installation reduces heat loss to the environment (especially through the collector), increasing the overall efficiency of the system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, K.M.; Chaurasiya, R. A review on analysis and development of solar flat plate collector. Renew. Sustain. Energy Rev. 2017, 67, 641–650. [Google Scholar] [CrossRef]
- Raymond, E.; Telkes, M. Dover Sun House, Dover Massachusetts. 1948. Available online: https://energyhistory.yale.edu/library-item/eleanor-raymond-and-maria-telkes-dover-sun-house-dover-massachusetts-1948 (accessed on 2 March 2021).
- Pandey, A.K.; Hossain, M.; Tyagi, V.; Rahim, N.A.; Selvaraj, J.A.; Sari, A. Novel approaches and recent developments on potential applications of phase change materials in solar energy. Renew. Sustain. Energy Rev. 2018, 82, 281–323. [Google Scholar] [CrossRef]
- Javadi, F.; Metselaar, H.; Ganesan, P. Performance improvement of solar thermal systems integrated with phase change materials (PCM), a review. Sol. Energy 2020, 206, 330–352. [Google Scholar] [CrossRef]
- Zayed, M.E.; Zhao, J.; Elsheikh, A.H.; Du, Y.; Hammad, F.A.; Ma, L.; Kabeel, A.; Sadek, S. Performance augmentation of flat plate solar water collector using phase change materials and nanocomposite phase change materials: A review. Process Saf. Environ. Prot. 2019, 128, 135–157. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Ibrahim, N.I.; Mahbubul, I.; Ali, H.M.; Saidur, R.; Al-Sulaiman, F.A. Evaluation of solar collector designs with integrated latent heat thermal energy storage: A review. Sol. Energy 2018, 166, 334–350. [Google Scholar] [CrossRef]
- Bohdal, T.; Kruzel, M. Refrigerant condensation in vertical pipe minichannels under various heat flux density level. Int. J. Heat Mass Transf. 2019, 146, 118849. [Google Scholar] [CrossRef]
- Bohdal, T.; Charun, H.; Kruzel, M.; Sikora, M. An investigation of heat transfer coefficient during refrigerants condensation in vertical pipe minichannels. E3S Web Conf. 2018, 70, 02001. [Google Scholar] [CrossRef]
- Kruzel, M.; Bohdal, T.; Sikora, M. Heat transfer and pressure drop during refrigerants condensation in compact heat exchangers. Int. J. Heat Mass Transf. 2020, 161, 120283. [Google Scholar] [CrossRef]
- Serale, G.; Fabrizio, E.; Perino, M. Design of a low-temperature solar heating system based on a slurry Phase Change Material (PCS). Energy Build. 2015, 106, 44–58. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Qiu, F.; Yang, W.; Zhao, X. Applications of solar water heating system with phase change material. Renew. Sustain. Energy Rev. 2015, 52, 645–652. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, P.; Wang, Z.; Zhao, H.; Zhao, X. PCM and PCM slurries and their application in solar systems. In Advanced Energy Efficiency Technologies for Solar Heating, Cooling and Power Generation; Springer: Berlin, Germany, 2019. [Google Scholar]
- Koohi-Fayegh, S.; Rosen, M. A review of energy storage types, applications and recent developments. J. Energy Storage 2019, 27, 101047. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent thermal energy storage technologies and applications: A review. Int. J. Thermofluids 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Elarem, R.; Alqahtani, T.; Mellouli, S.; Askri, F.; Edacherian, A.; Vineet, T.; Badruddin, I.A.; Abdelmajid, J. A comprehensive review of heat transfer intensification methods for latent heat storage units. Energy Storage 2019, 3, e127. [Google Scholar] [CrossRef] [Green Version]
- Dutkowski, K.; Kruzel, M. Microencapsulated PCM slurries’ dynamic viscosity experimental investigation and temperature-dependent prediction model. Int. J. Heat Mass Transf. 2019, 145, 118741. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M. Experimental investigation of the apparent thermal conductivity of microencapsulated phase-change-material slurry at the phase-transition temperature. Materials 2021, 14, 4124. [Google Scholar] [CrossRef] [PubMed]
- Ikutegbe, C.A.; Farid, M.M. Application of phase change material foam composites in the built environment: A critical review. Renew. Sustain. Energy Rev. 2020, 131, 110008. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. A review on phase change materials for thermal energy storage in buildings: Heating and hybrid applications. J. Energy Storage 2020, 33, 101913. [Google Scholar] [CrossRef]
- Magendran, S.S.; Khan, F.S.A.; Mubarak, N.; Vaka, M.; Walvekar, R.; Khalid, M.; Abdullah, E.; Nizamuddin, S.; Karri, R.R. Synthesis of organic phase change materials (PCM) for energy storage applications: A review. Nano Struct. Nano Objects 2019, 20, 100399. [Google Scholar] [CrossRef]
- Ahmadi, R.; Monadinia, F.; Maleki, M. Passive/active photovoltaic-thermal (PVT) system implementing infiltrated phase change material (PCM) in PS-CNT foam. Sol. Energy Mater. Sol. Cells 2021, 222, 110942. [Google Scholar] [CrossRef]
- Fu, Z.; Liang, X.; Li, Y.; Li, L.; Zhu, Q. Performance improvement of a PVT system using a multilayer structural heat exchanger with PCMs. Renew. Energy 2020, 169, 308–317. [Google Scholar] [CrossRef]
- Kılkış, B. Development of a composite PVT panel with PCM embodiment, TEG modules, flat-plate solar collector, and thermally pulsing heat pipes. Sol. Energy 2019, 200, 89–107. [Google Scholar] [CrossRef]
- Chavan, S.V.; Devaprakasam, D. Improving the performance of solar photovoltaic thermal system using phase change material. Mater. Today Proc. 2020, 46, 5036–5041. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B.; Białko, B. The experimental investigation of mPCM slurries density at phase change temperature. Int. J. Heat Mass Transf. 2020, 159, 120083. [Google Scholar] [CrossRef]
- Akshayveer, K.A.; Singh, A.P.; Singh, O. Effect of novel PCM encapsulation designs on electrical and thermal performance of a hybrid photovoltaic solar panel. Sol. Energy 2020, 205, 320–333. [Google Scholar] [CrossRef]
- Alwaeli, A.H.A.; Kazem, H.A.; Chaichan, M.T.; Sopian, K. Experimental investigation of using nano-PCM/nanofluid on a photovoltaic thermal system (PVT): Technical and economic study. Therm. Sci. Eng. Prog. 2019, 11, 213–230. [Google Scholar] [CrossRef]
- Maatallah, T.; Zachariah, R.; Al-Amri, F.G. Exergo-economic analysis of a serpentine flow type water based photovoltaic thermal system with phase change material (PVT-PCM/water). Sol. Energy 2019, 193, 195–204. [Google Scholar] [CrossRef]
- Alwaeli, A.H.A.; Chaichan, M.T.; Sopian, K.; Kazem, H.A.; Mahood, H.B.; Khadom, A.A. Modeling and experimental validation of a PVT system using nanofluid coolant and nano-PCM. Sol. Energy 2018, 177, 178–191. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Z.; Wang, J.; Li, Y.; Rao, Z. Experimental research on flow and heat transfer characteristics of latent functional thermal fluid with microencapsulated phase change materials. Int. J. Heat Mass Transf. 2017, 115, 737–742. [Google Scholar] [CrossRef]
- Karaipekli, A.; Erdoğan, T.; Barlak, S. The stability and thermophysical properties of a thermal fluid containing surface-functionalized nanoencapsulated PCM. Thermochim. Acta 2019, 682, 178406. [Google Scholar] [CrossRef]
- Hawlader, M.; Uddin, M.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202. [Google Scholar] [CrossRef]
- Chen, L.; Wang, T.; Zhao, Y.; Zhang, X.-R. Characterization of thermal and hydrodynamic properties for microencapsulated phase change slurry (MPCS). Energy Convers. Manag. 2014, 79, 317–333. [Google Scholar] [CrossRef]
- Chai, L.; Shaukat, R.; Wang, L.; Wang, H.S. A review on heat transfer and hydrodynamic characteristics of nano/microencapsulated phase change slurry (N/MPCS) in mini/microchannel heat sinks. Appl. Therm. Eng. 2018, 135, 334–349. [Google Scholar] [CrossRef] [Green Version]
- Alehosseini, E.; Jafari, S.M. Nanoencapsulation of phase change materials (PCMs) and their applications in various fields for energy storage and management. Adv. Colloid Interface Sci. 2020, 283, 102226. [Google Scholar] [CrossRef]
- Usman, M.; Siddiqui, F.; Ehsan, A.; Sadaqat, R.A.; Hussain, A. Improvement of thermal conductivity of paraffin wax, a phase change material with graphite powder. In Proceedings of the 17th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 14–18 January 2020. [Google Scholar]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2019, 25, 251–295. [Google Scholar] [CrossRef]
- Lan, W.; Shang, B.; Wu, R.; Yu, X.; Hu, R.; Luo, X. Thermally-enhanced nanoencapsulated phase change materials for latent functionally thermal fluid. Int. J. Therm. Sci. 2020, 159, 106619. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Zheng, H. A comprehensive review of hydrodynamic mechanisms and heat transfer characteristics for microencapsulated phase change slurry (MPCS) in circular tube. Renew. Sustain. Energy Rev. 2019, 114, 109312. [Google Scholar] [CrossRef]
- Ghoghaei, M.S.; Mahmoudian, A.; Mohammadi, O.; Shafii, M.B.; Mosleh, H.J.; Zandieh, M.; Ahmadi, M.H. A review on the applications of micro-/nano-encapsulated phase change material slurry in heat transfer and thermal storage systems. J. Therm. Anal. Calorim. 2020, 145, 245–268. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, C.; Fang, G. Performance optimization of a photovoltaic/thermal collector using microencapsulated phase change slurry. Int. J. Energy Res. 2019, 44, 1812–1827. [Google Scholar] [CrossRef]
- Ran, F.; Chen, Y.; Cong, R.; Fang, G. Flow and heat transfer characteristics of microencapsulated phase change slurry in thermal energy systems: A review. Renew. Sustain. Energy Rev. 2020, 134, 110101. [Google Scholar] [CrossRef]
- Moshtagh, M.; Jamekhorshid, A.; Azari, A.; Farid, M.M. Experimental and numerical investigation of microencapsulated phase change material slurry heat transfer inside a tube with butterfly tube inserts. Appl. Therm. Eng. 2020, 174, 115270. [Google Scholar] [CrossRef]
- Serale, G.; Cascone, Y.; Capozzoli, A.; Fabrizio, E.; Perino, M. Potentialities of a low temperature solar heating system based on slurry phase change materials (PCS). Energy Procedia 2014, 62, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Baronetto, S.; Serale, G.; Goia, F.; Perino, M. Numerical model of a slurry PCM-based solar thermal collector. In Proceedings of the 8th International Symposium on Heating, Ventilation and Air Conditioning, Xi’an, China, 19–21 October 2013. [Google Scholar]
- Serale, G.; Baronetto, S.; Goia, F.; Perino, M. Characterization and Energy Performance of a Slurry PCM-based Solar Thermal Collector: A Numerical Analysis. Energy Procedia 2014, 48, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Serale, G.; Goia, F.; Perino, M. Numerical model and simulation of a solar thermal collector with slurry Phase Change Material (PCM) as the heat transfer fluid. Sol. Energy 2016, 134, 429–444. [Google Scholar] [CrossRef]
- Prearo, G.; Serale, G.; Perino, M. Numerical model for solar thermal collectors and thermal energy storages based on phase change slurry. In Proceedings of the ISES Solar World Congress 2017 with IEA SHC Solar Heating and Cooling Conference 2017, Abu Dhabi, United Arab Emirates, 29 October–2 November 2017. [Google Scholar]
- Fang, W.; Riffat, S.; Wu, Y. Experimental investigation of evacuated heat pipe solar collector efficiency using phase-change fluid. Int. J. Low-Carbon Technol. 2017, 12, 392–399. [Google Scholar] [CrossRef]
- Sun, L.; Xiang, N.; Yuan, Y.; Cao, X. Experimental investigation on performance comparison of solar water heating-phase change Material system and solar water heating system. Energies 2019, 12, 2347. [Google Scholar] [CrossRef] [Green Version]
- Microtek Labs. Micronal ® 5428 X; Microtek Labs: Moraine, OH, USA, 2018. [Google Scholar]
- KOSPEL. Solar Collector—Technical Brochure; KOSPEL: Koszalin, Poland, 2013. (In Polish) [Google Scholar]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B. Determining the heat of fusion and specific heat of microencapsulated phase change material slurry by thermal delay method. Energies 2020, 14, 179. [Google Scholar] [CrossRef]
- Rajput, N.S.; Shukla, D.D.; Rajput, D.; Sharm, S.K. Performance analysis of flat plate solar collector using Al2O3/Distilled water nanofluid: An experimental investigation. Mater. Today Proc. 2019, 10, 52–59. [Google Scholar] [CrossRef]
- Moravej, M.; Bozorg, M.V.; Guan, Y.; Li, L.K.; Doranehgard, M.H.; Hong, K.; Xiong, Q. Enhancing the efficiency of a symmetric flat-plate solar collector via the use of rutile TiO2-water nanofluids. Sustain. Energy Technol. Assess. 2020, 40, 100783. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Gróf, G. Experimental investigation of flat plate solar collector using CeO 2 -water nanofluid. Energy Convers. Manag. 2018, 155, 32–41. [Google Scholar] [CrossRef]
- Sundar, L.S.; Kirubeil, A.; Punnaiah, V.; Singh, M.K.; Sousa, A. Effectiveness analysis of solar flat plate collector with Al2O3 water nanofluids and with longitudinal strip inserts. Int. J. Heat Mass Transf. 2018, 127, 422–435. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutkowski, K.; Kruzel, M.; Bohdal, T. Experimental Studies of the Influence of Microencapsulated Phase Change Material on Thermal Parameters of a Flat Liquid Solar Collector. Energies 2021, 14, 5135. https://doi.org/10.3390/en14165135
Dutkowski K, Kruzel M, Bohdal T. Experimental Studies of the Influence of Microencapsulated Phase Change Material on Thermal Parameters of a Flat Liquid Solar Collector. Energies. 2021; 14(16):5135. https://doi.org/10.3390/en14165135
Chicago/Turabian StyleDutkowski, Krzysztof, Marcin Kruzel, and Tadeusz Bohdal. 2021. "Experimental Studies of the Influence of Microencapsulated Phase Change Material on Thermal Parameters of a Flat Liquid Solar Collector" Energies 14, no. 16: 5135. https://doi.org/10.3390/en14165135






