Comparative Exergy Analysis of Units for the Porous Ammonium Nitrate Granulation
Abstract
:1. Introduction
2. Description of Schemes
3. Methodology
4. Assessing the Systems Effectiveness
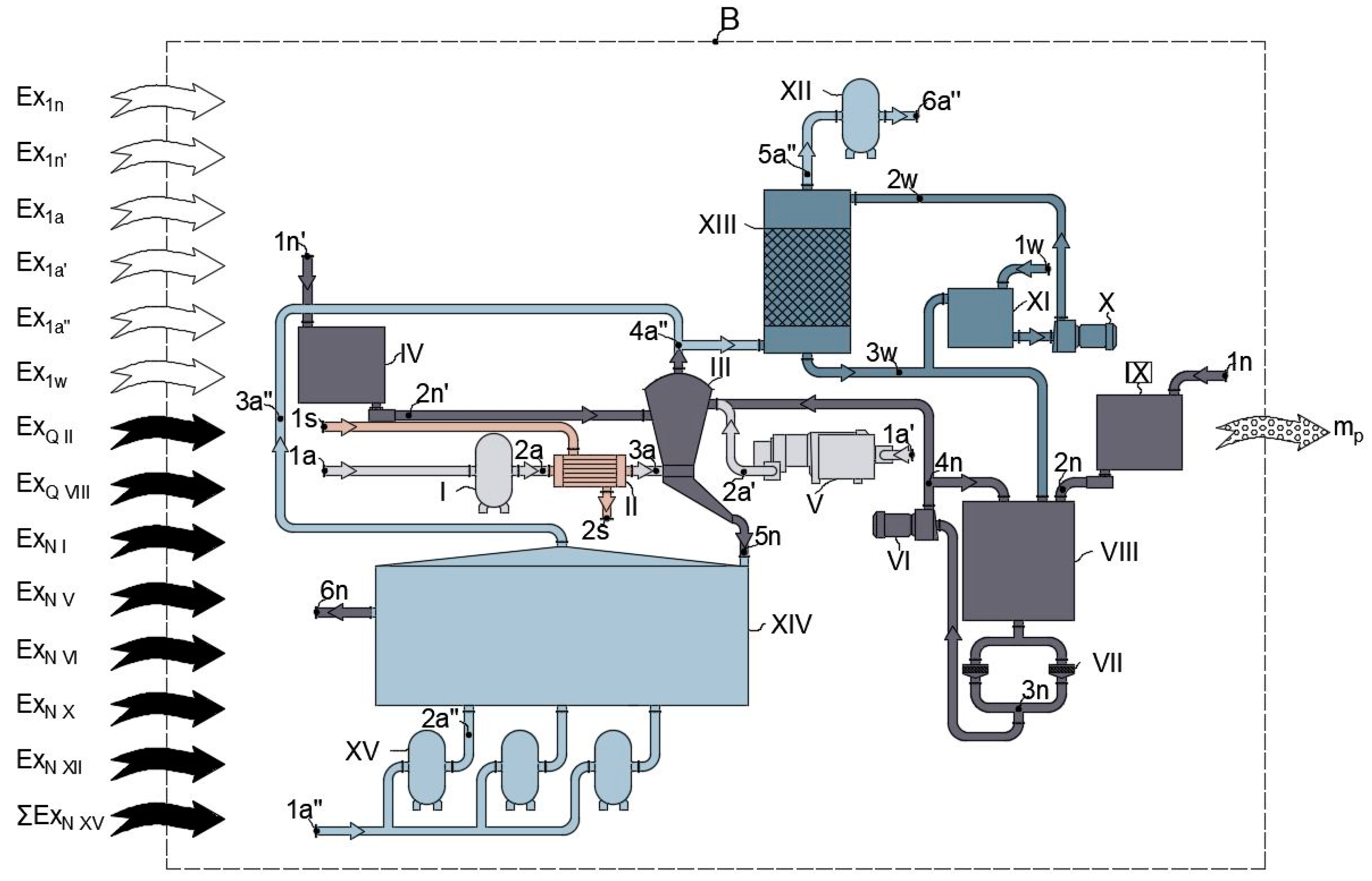
4.1. Basic Scheme
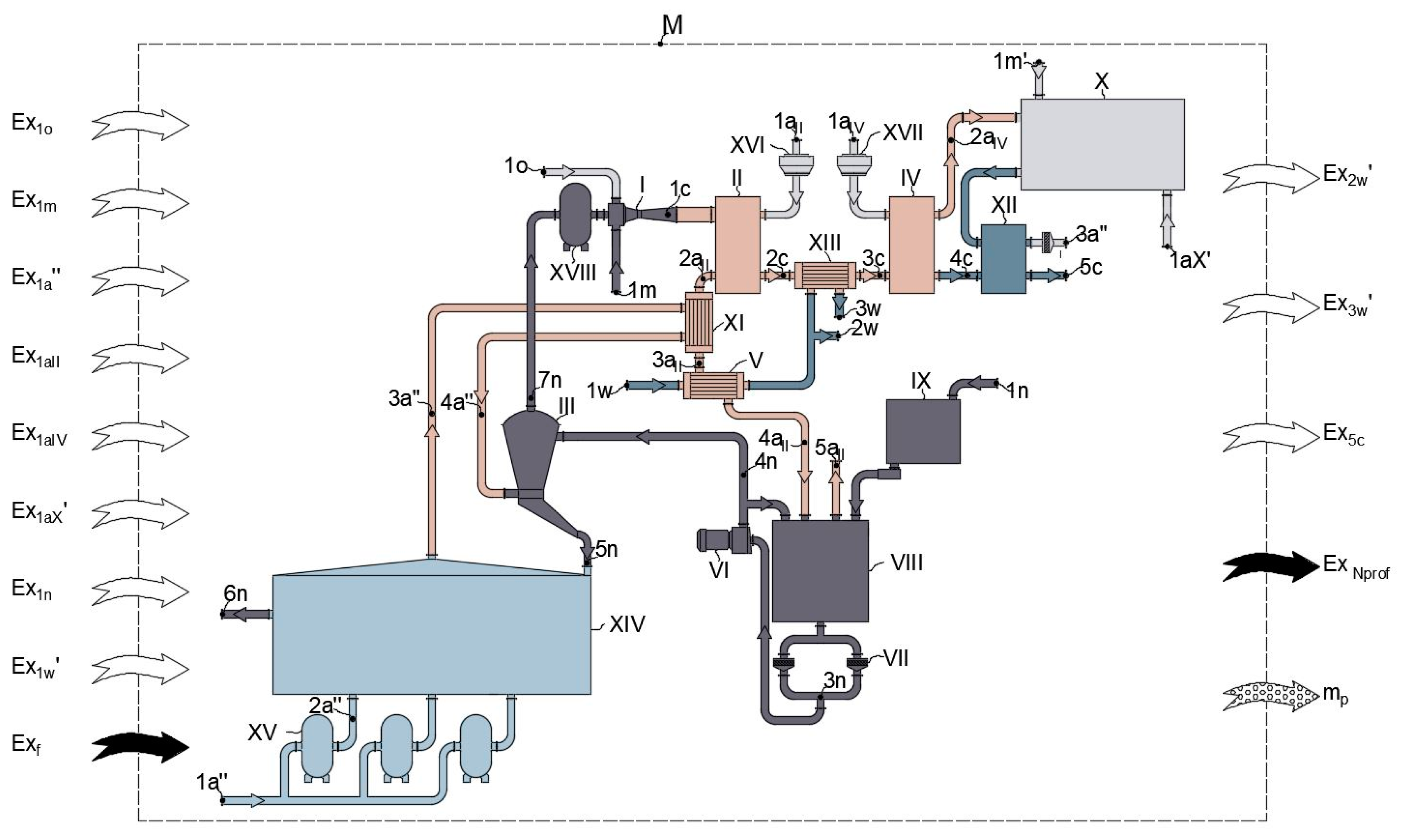
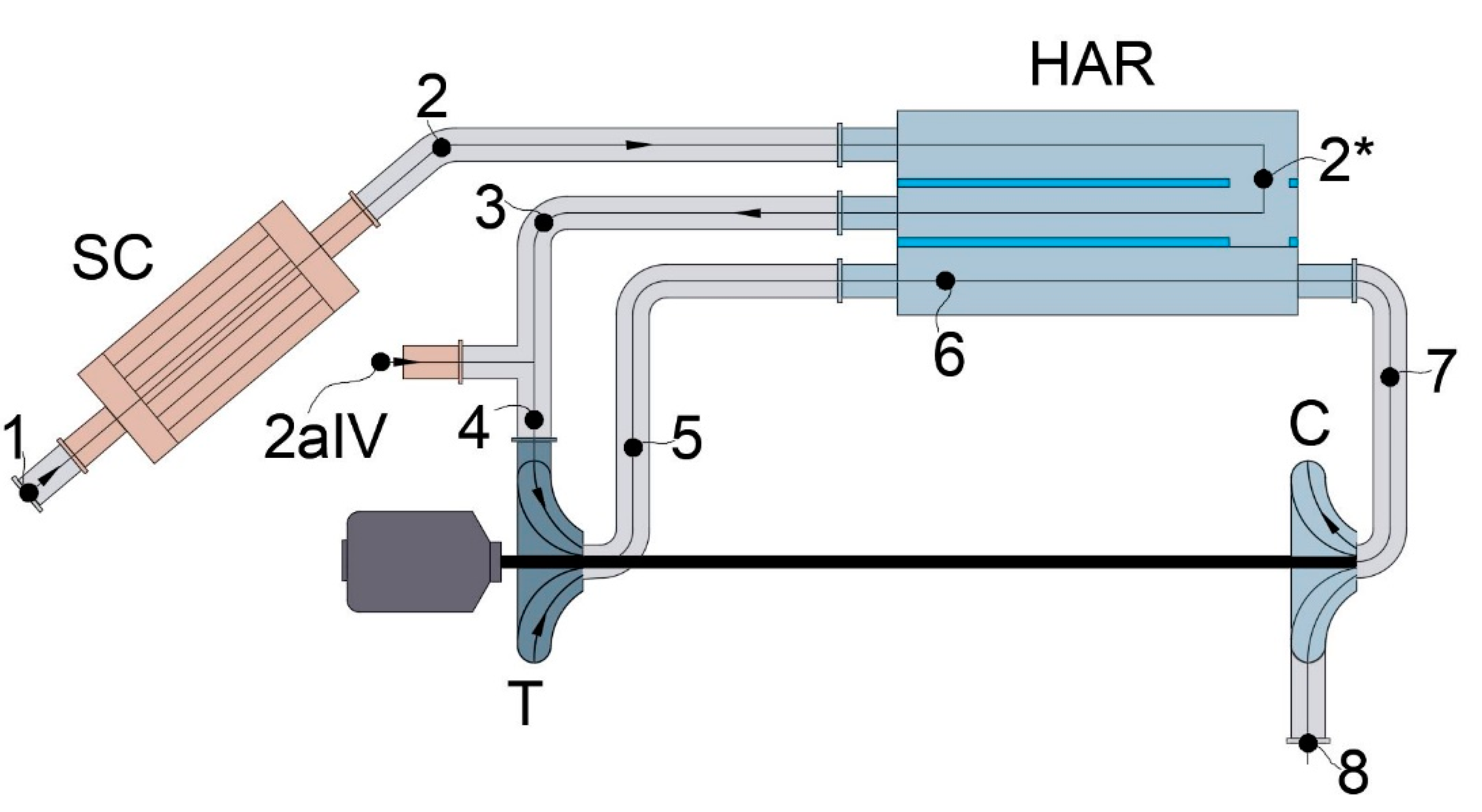
4.2. Modified Scheme
5. Discussion
6. Conclusions
- -
- optimization of flow arrangement in the vortex granulator as a core device in the scheme to provide additional utilization of fine fraction of ammonium nitrate;
- -
- consideration of various combinations of operating modes in the same workspace of the device to meet the drying agent’s optimal heat potential;
- -
- detailed calculations of the proposed scheme and its further study are required, considering the design features, materials and processes inside the equipment;
- -
- experimental investigation of devices for indirect regenerative evaporative cooling;
- -
- detailed analysis of the proposed sub-atmospheric inverse Brayton cycle and other cycles as a core for power generation and heat recovery module.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| specific isobaric heat capacity | |
| humidity ratio in humid air | |
| system efficiency indicator | |
| specific exergy flow of the substance | |
| enthalpy | |
| specific work | |
| mass flow rate | |
| pressure | |
| specific heat flow | |
| entropy | |
| temperature | |
| substance exergy flow | |
| power | |
| heat flow | |
| gas constant | |
| absolute temperature | |
| Greek symbols | |
| efficiency coefficient | |
| Subscripts | |
| dead state | |
| process air | |
| air to spray solution | |
| air for product cooling | |
| ambient air | |
| ambient air | |
| ambient air supplied to Brayton’s sub-atmospheric inverse cycle with HAR | |
| a substandard stream that passed the recycling module in which lower oxides passed into higher ones; it is also a thermal energy source necessary for the unit’s operation | |
| irregular flow | |
| dry air | |
| destruction | |
| exergy | |
| fuel | |
| humid air | |
| input | |
| isentropic | |
| index | |
| loss | |
| ammonium nitrate | |
| seeding agent of the ammonium nitrate | |
| output | |
| product | |
| useful effect | |
| saturation state | |
| water vapor | |
| water | |
| Superscripts | |
| basic | |
| modified | |
| Designations on the diagrams | |
 | exergy of the substance flow |
 | material flow |
 | exergy of energy flow |
References
- Carole, T.; Scheihing, P.; Sousa, L. Energy Efficiency and Use in the Chemical Industry. ACEEE Proc. 2001, 1, 267–275. [Google Scholar]
- Fawkes, S.; Oung, K.; Thorpe, D. Best Practices and Case Studies for Industrial Energy Efficiency Improvement. In An Introduction for Policy Makers; UNEP DTU Partnership: Copenhagen, Denmark, 2016; p. 173. [Google Scholar]
- Patt, J.J.; Banholzer, W.F. Improving Energy Efficiency in the Chemical Industry. Bridg. Energy Effic. 2009, 39, 15–21. [Google Scholar]
- Duroudier, J.-P. Heat Transfer in the Chemical, Food and Pharmaceutical Industries; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-1-78548-188-8. [Google Scholar]
- Hipple, J. Chemical Engineering for Non-Chemical Engineers; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-1-119-30965-9. [Google Scholar]
- Kapustenko, P.O.; Kukulka, D.J.; Arsenyeva, O.P. Intensification of heat transfer processes. Chem. Eng. Trans. 2015, 45, 1729–1734. [Google Scholar] [CrossRef]
- Beller, M.; Böhland, T.; Demtröder, D.; Ebenhoech, J.; Ernst, S.; Ewers, J.; Haber, S.; Hoer, R.; Hirth, T.; Jahn, D.; et al. Change in the Raw Materials Base; GDCh: Frankfurt, Germany, 2010. [Google Scholar]
- Ali, M.F.; Ali, B.M.; Speight, J.G. Handbook of Industrial Chemistry: Organic Chemicals; McGraw-Hill Education: New York, NY, USA, 2005; ISBN 9780071410373. [Google Scholar]
- Stahl, H.; Van Vaerenbergh, G. Single-Pot Processing. In Handbook of Pharmaceutical Granulation Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 311–332. ISBN 9780849354953. [Google Scholar]
- Litster, J.; Ennis, B. The Science and Engineering of Granulation Processes; 31 March 2004; Springer: Berlin/Heidelberg, Germany, 2004; ISBN 978-94-017-0546-2. [Google Scholar]
- Stahl, H. Comparing Granulation Methods; GEA Pharma Systems: Hürth, Germany, 2010. [Google Scholar]
- Muralidhar, P.; Bhargav, E.; Sowmya, C. Novel Techniques of Granulation: A Review. Int. Res. J. Pharm. 2016, 7, 8–13. [Google Scholar] [CrossRef]
- Stahl, H. Comparing Different Granulation Techniques. In Pharmaceutical Technology; PharmTech: Muellheim, Germany, 2004; pp. 22–33. [Google Scholar]
- Sahoo, C.K.; Rao, S.R.M.; Sudhakar, M.; Bhaskar, J. Advances in granulation technology. Res. J. Pharm. Technol. 2016, 9, 571–580. [Google Scholar] [CrossRef]
- Athar, M. A technical note on granulation technology: A way to optimise granules. Int. J. Pharm. Sci. Rev. Res. 2013, 4, 55–67. [Google Scholar]
- Artyukhov, A.Y.; Sklabinskiy, V.I. Experimental and industrial implementation of porous ammonium nitrate producing process in vortex granulators. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2013, 6, 42–48. [Google Scholar]
- Cardarelli, F. Fuels, Propellants, and Explosives. In Materials Handbook; Springer International Publishing: Cham, Switzerland, 2018; pp. 1465–1496. ISBN 3527302107. [Google Scholar]
- Janssen, T.J. Explosive Materials: Classification, Composition and Properties; Nova Science Publishers: New York, NY, USA, 2011; ISBN 1617611883. [Google Scholar]
- Artyukhov, A.E.; Sklabinskiy, V.I. Investigation of the Temperature Field of Coolant in the Installations for Obtaining 3D Nanostructured Porous Surface Layer on the Granules of Ammonium Nitrate. J. Nano-Electron. Phys. 2017, 9, 01015. [Google Scholar] [CrossRef]
- Artyukhova, N.O. Morphological Features of the Nanoporous Structure in the Ammonium Nitrate Granules at the Final Drying Stage in Multistage Devices. J. Nano-Electron. Phys. 2020, 12, 04036. [Google Scholar] [CrossRef]
- Artyukhov, A.; Obodiak, V.; Boiko, P.; Rossi, P. Computer modeling of hydrodynamic and heat-mass transfer processes in the vortex type granulation devices. In CEUR Workshop Proceedings; ICTERI: Kyiv, Ukraine, 2017; pp. 33–47. [Google Scholar]
- Artyukhov, A.E.; Artyukhova, N.O. Technology and the Main Technological Equipment of the Process to Obtain N4HNO3 with Nanoporous Structure. In Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 585–594. [Google Scholar]
- Artyukhov, A.E.; Ivaniia, A.V. Obtaining porous ammonium nitrate in multistage and multifunctional vortex granulators. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2017, 7, 68–75. [Google Scholar]
- Levchenko, D.; Yurko, I.; Artyukhov, A.; Baga, V. Maisotsenko cycle applications for multistage compressors cooling. IOP Conf. Ser. Mater. Sci. Eng. 2017, 233, 012023. [Google Scholar] [CrossRef] [Green Version]
- Levchenko, D.; Pavlenko, I.; Shulumei, A.; Ochowiak, M.; Manzharov, A. Parameter Identification of the Capillary Rising Process in Nanomaterials for Evaporative Cooling Applications. In Design, Simulation, Manufacturing: The Innovation Exchange; Springer: Cham, Switzerland, 2020; pp. 201–215. [Google Scholar]
- Choi, C.-H.; Krishnan, S.; TeGrotenhuis, W.; Chang, C.-H. Capillary Rise of Nanostructured Microwicks. Micromachines 2018, 9, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artyukhova, N.O.; Krmela, J. Nanoporous Structure of the Ammonium Nitrate Granules at the Final Drying: The Effect of the Dryer Operation Mode. J. Nano-Electron. Phys. 2019, 11, 04006. [Google Scholar] [CrossRef]
- Levchenko, D.O.; Artyukhov, A.E.; Yurko, I.V. Maisotsenko cycle applications in multi-stage ejector recycling module for chemical production. IOP Conf. Ser. Mater. Sci. Eng. 2017, 233, 012024. [Google Scholar] [CrossRef] [Green Version]
- Buyadgie, D.; Buyadgie, O.; Drakhnia, O.; Brodetsky, P.; Maisotsenko, V. Solar low-pressure turbo-ejector Maisotsenko cycle-based power system for electricity, heating, cooling and distillation. Int. J. Low-Carbon Technol. 2015, 10, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Khalatov, A.A.; Severin, S.D.; Brodetsky, P.I.; Maisotsenko, V.S. Brayton’s subatmospheric inverse cycle with regeneration of output heat by Maisotsenko’s cicle. Rep. Natl. Acad. Sci. Ukr. 2015, 72–79. [Google Scholar] [CrossRef]
- Mahmood, M.H.; Sultan, M.; Miyazaki, T.; Koyama, S.; Maisotsenko, V.S. Overview of the Maisotsenko cycle—A way towards dew point evaporative cooling. Renew. Sustain. Energy Rev. 2016, 66, 537–555. [Google Scholar] [CrossRef]
- Kozlov, A.; Chudnovsky, Y. Combined Cooling, Heating And Power System. U.S. patent 16/297928, 12 September 2019. [Google Scholar]
- Piacentino, A. Thermal analysis and new insights to support decision making in retrofit and relaxation of heat exchanger networks. Appl. Therm. Eng. 2011, 31, 3479–3499. [Google Scholar] [CrossRef] [Green Version]
- Asante, N.D.K.; Zhu, X.X. An Automated and Interactive Approach for Heat Exchanger Network Retrofit. Chem. Eng. Res. Des. 1997, 75, 349–360. [Google Scholar] [CrossRef]
- Safder, U.; Ifaei, P.; Yoo, C. A novel approach for optimal energy recovery using pressure retarded osmosis technology: Chemical exergy pinch analysis—Case study in a sugar mill plant. Energy Convers. Manag. 2020, 213, 112810. [Google Scholar] [CrossRef]
- Bejan, A. Method of entropy generation minimization, or modeling and optimization based on combined heat transfer and thermodynamics. Rev. Générale Therm. 1996, 35, 637–646. [Google Scholar] [CrossRef]
- Korpyś, M.; Gancarczyk, A.; Iwaniszyn, M.; Sindera, K.; Jodłowski, P.J.; Kołodziej, A. Analysis of entropy production in structured chemical reactors: Optimization for catalytic combustion of air pollutants. Entropy 2020, 22, 1017. [Google Scholar] [CrossRef] [PubMed]
- Besagni, G. Ejectors on the cutting edge: The past, the present and the perspective. Energy 2019, 170, 998–1003. [Google Scholar] [CrossRef]
- Брoдянский, B.; Фратшер, B.; Михалек, K. Эксергетический метoд и егo прилoжения; Энергoатoм: Мoсква, Рoссия, 1988; ISBN 5-283-00152-0. [Google Scholar]
- Morosuk, T.; Tsatsaronis, G. Advanced exergy-based methods used to understand and improve energy-conversion systems. Energy 2019, 169, 238–246. [Google Scholar] [CrossRef]
- Sayadi, S.; Tsatsaronis, G.; Morosuk, T.; Baranski, M.; Sangi, R.; Müller, D. Exergy-based control strategies for the efficient operation of building energy systems. J. Clean. Prod. 2019, 241, 118277. [Google Scholar] [CrossRef]
- Athari, H.; Soltani, S.; Rosen, M.A.; Gavifekr, M.K.; Morosuk, T. Exergoeconomic study of gas turbine steam injection and combined power cycles using fog inlet cooling and biomass fuel. Renew. Energy 2016, 96, 715–726. [Google Scholar] [CrossRef]
- Fazelpour, F.; Morosuk, T. Exergoeconomic analysis of carbon dioxide transcritical refrigeration machines. Int. J. Refrig. 2014, 38, 128–139. [Google Scholar] [CrossRef]
- Jannatkhah, J.; Najafi, B.; Ghaebi, H. Energy and exergy analysis of combined ORC—ERC system for biodiesel-fed diesel engine waste heat recovery. Energy Convers. Manag. 2020, 209, 112658. [Google Scholar] [CrossRef]
- MOROSUK, T.; TSATSARONIS, G. A new approach to the exergy analysis of absorption refrigeration machines. Energy 2008, 33, 890–907. [Google Scholar] [CrossRef]
- Morosuk, T.; Tsatsaronis, G. Advanced exergy analysis for chemically reacting systems—Application to a simple open gas-turbine system. Int. J. Thermodyn. 2009, 12, 105–111. [Google Scholar] [CrossRef]
- Morosuk, T.; Tsatsaronis, G. Splitting physical exergy: Theory and application. Energy 2019, 167, 698–707. [Google Scholar] [CrossRef]
- Shargut, Y. Exergy; Shargut, R.P., Ed.; Energiia: Moscow, Russia, 1968. [Google Scholar]
- Yukhimenko, N.; Vakal, S. The exergy analysis of energy efficiency of the technology of granulated phosphorus-potassium fertilizers. East.-Eur. J. Enterp. Technol. 2016, 5, 4–10. [Google Scholar] [CrossRef]
- Quoilin, S.; Lemort, V. Technological and Economical Survey of Organic Rankine Cycle Systems. In Proceedings of the 5th European Conference Economics and Management of Energy in Industry, Algarve, Portugal, 14–17 April 2009; Volume 278, p. 12. [Google Scholar]
- Madhawa, H.; Golubovic, M.; Worek, W.; Ikegami, Y. Optimum design criteria for an Organic Rankine cycle using low-temperature geothermal heat sources. Energy 2007, 32, 1698–1706. [Google Scholar] [CrossRef]
- Živić, M.; Galović, A.; Avsec, J.; Holik, M. Exergy analysis of a Brayton cycle with variable physical properties and variable composition of working substance. Teh. Vjesn. Tech. Gaz. 2016, 23, 801–808. [Google Scholar] [CrossRef] [Green Version]



| Index | |||||
|---|---|---|---|---|---|
| 1608 | 293 | 101,325 | - | 0 | |
| 835 | 293 | 101,325 | - | 0 | |
| 15,000 | 293 | 101,325 | 0.00877 | 0 | |
| 453.6 | 293 | 101,325 | 0.00877 | 0 | |
| 12,000 | 293 | 101,325 | 0.00877 | 0 | |
| 557 | 293 | 101,325 | - | 0 | |
| - | - | - | - | 120 | |
| - | - | - | - | 32 | |
| - | - | - | - | 75 | |
| - | - | - | - | 2.5 | |
| - | - | - | - | 3 | |
| - | - | - | - | 3 | |
| - | - | - | - | 40 | |
| - | - | - | - | 120 | |
| 1608 | 293 | 101,325 | - | 0 | |
| 395.5 |
| Index | |||||
|---|---|---|---|---|---|
| 33.6 | 293 | 101,325 | - | 0 | |
| 8.4 | 293 | 101,325 | - | 110.8 | |
| 12,000 | 293 | 101,325 | 0.00877 | 0 | |
| 18,097 | 293 | 101,325 | 0.00877 | 0 | |
| 2243 | 293 | 101,325 | 0.00877 | 0 | |
| 1188 | 293 | 101,325 | 0.00877 | 0 | |
| 2442 | 293 | - | - | 0 | |
| 6516 | 291 | 101,325 | - | 0 | |
| - | - | - | - | 3 | |
| - | - | - | - | 120 | |
| - | - | - | - | 7.6 | |
| - | - | - | - | 0.2 | |
| - | - | - | - | 385 | |
| - | 626.5 | ||||
| 415.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levchenko, D.; Manzharov, A.; Artyukhov, A.; Artyukhova, N.; Krmela, J. Comparative Exergy Analysis of Units for the Porous Ammonium Nitrate Granulation. Energies 2021, 14, 280. https://doi.org/10.3390/en14020280
Levchenko D, Manzharov A, Artyukhov A, Artyukhova N, Krmela J. Comparative Exergy Analysis of Units for the Porous Ammonium Nitrate Granulation. Energies. 2021; 14(2):280. https://doi.org/10.3390/en14020280
Chicago/Turabian StyleLevchenko, Dmytro, Andrii Manzharov, Artem Artyukhov, Nadiya Artyukhova, and Jan Krmela. 2021. "Comparative Exergy Analysis of Units for the Porous Ammonium Nitrate Granulation" Energies 14, no. 2: 280. https://doi.org/10.3390/en14020280






