Fuel Properties of Torrefied Biomass from Sapindus Pericarp Extraction Residue under a Wide Range of Pyrolysis Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermochemical Properties of Soapberry Pericarp Extraction Residue
2.3. Torrefaction Experiments
2.4. Analysis of Fuel Properties
3. Results
3.1. Thermochemical Characteristics of Soapberry Pericarp (SP) Extraction Residue
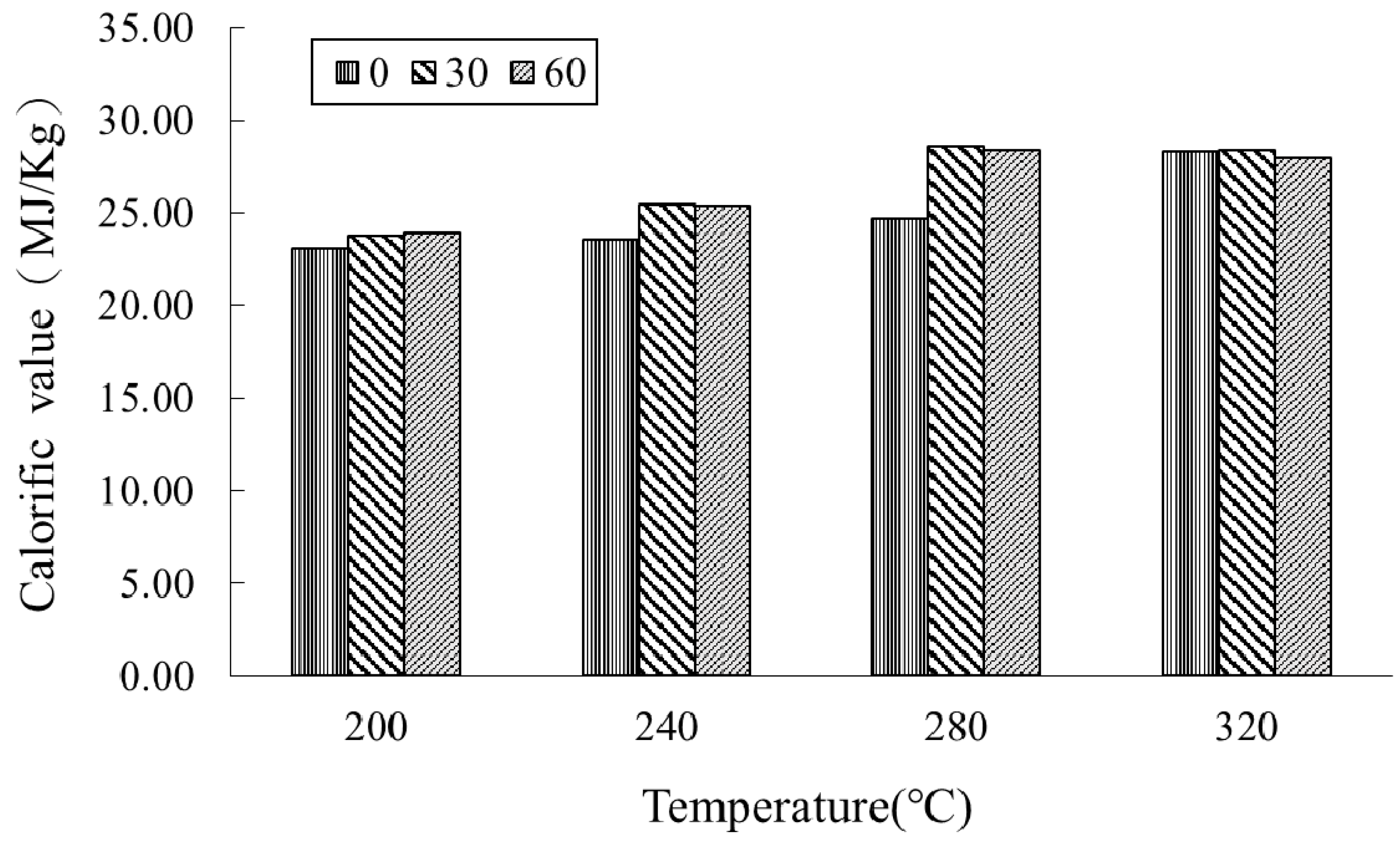
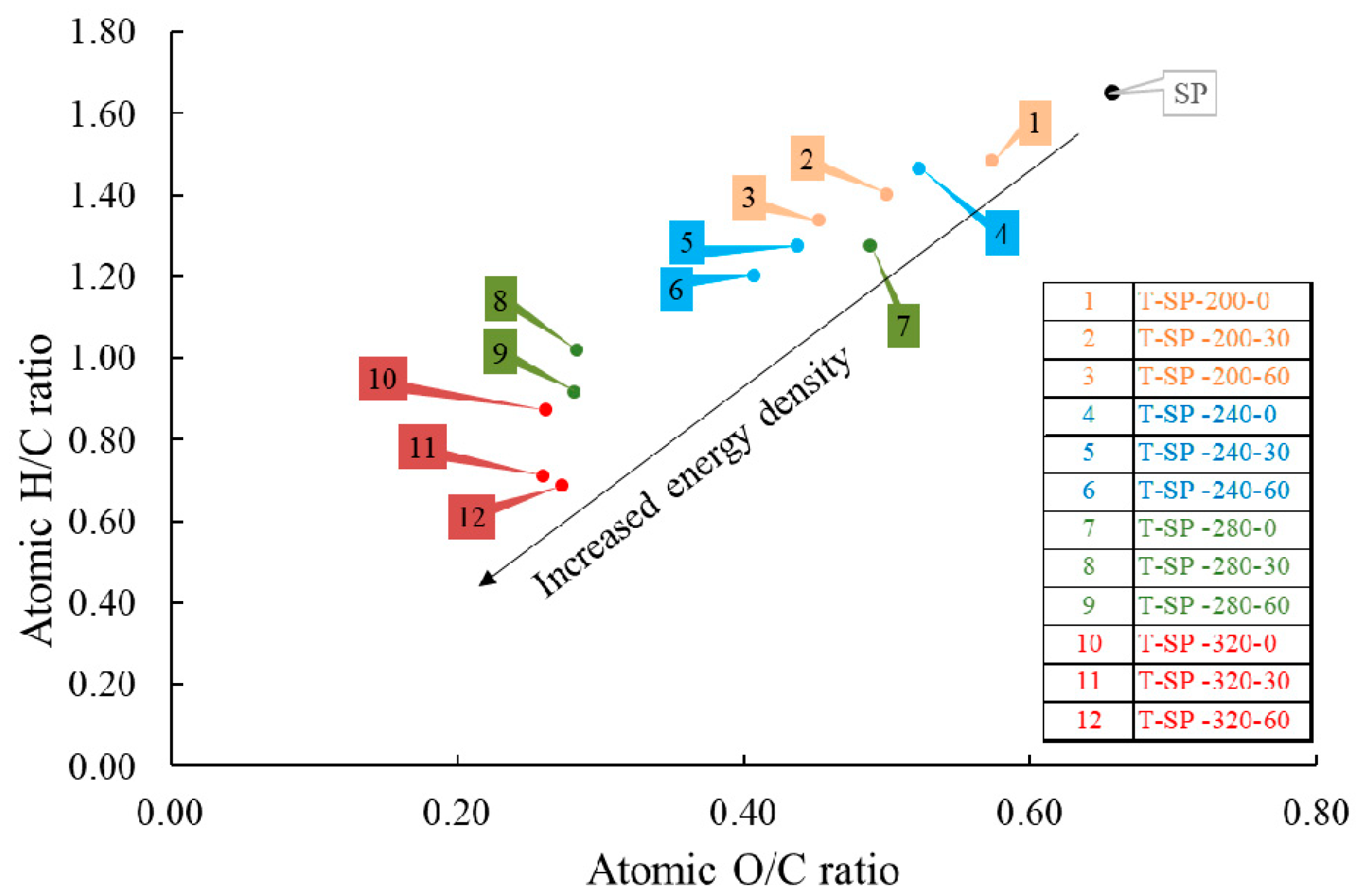
3.2. Fuel Properties of SP-Torrefied Products (T-SP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Academic Press: London, UK, 2013; pp. 87–145. [Google Scholar]
- Li, H.; Liu, X.; Legros, R.; Bi, X.T.; Lim, C.J.; Sokhansan, S. Torrefaction of sawdust in a fluidized bed reactor. Bioresour. Technol. 2012, 103, 453–458. [Google Scholar] [CrossRef]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Negi, S.; Jaswal, G.; Dass, K.; Mazumder, K.; Elumalai, S.; Roy, J.K. Torrefaction: A sustainable method for transforming of agri-wastes to high energy density solids (biocoal). Rev. Environ. Sci. Biotechnol. 2020, 19, 463–488. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Ojo, O.T. Biomass torrefaction for the production of high-grade solid biofuels: A review. Bioenergy Res. 2020, 13, 999–1015. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Knapczyk, A.; Francik, S.; Jewiarz, M.; Zawi´slak, A.; Francik, R. Thermal treatment of biomass: A bibliometric analysis—The torrefaction case. Energies 2021, 14, 162. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Dalai, A.K.; Meda, V. A review of torrefaction technology for upgrading lignocellulosic biomass to solid biofuels. Bioenergy Res. 2021, 14, 645–669. [Google Scholar] [CrossRef]
- Dobhal, U.; Bisht, N.S.; Bhandari, S.L. Traditional values of Sapindus mukorossi gaertn. vern. Ritha: A review. Plant. Arch. 2007, 7, 485–486. [Google Scholar]
- Liu, M.; Chen, Y.L.; Kuo, Y.H.; Lu, M.K.; Liao, C.H. Aqueous extract of Sapindus mukorossi induced cell death of A549 cells and exhibited antitumor property in vivo. Sci. Rep. 2018, 8, 4831. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Gao, Y.; Chen, Z.; Zhao, G.C.; Liu, J.M.; Wang, X.; Gao, S.L.; Zhang, D.G.; Jia, L.M. Metabolomics analysis of the soapberry (Sapindus mukorossi Gaertn.) pericarp during fruit development and ripening based on UHPLC-HRMS. Sci. Rep. 2021, 11, 11657. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Fu, L.L.; He, S.Q.; Lu, X.P.; Wu, Y.Y.; Ma, Z.Q.; Zhang, X. Potent herbicidal activity of Sapindus mukorossi Gaertn. against Avena fatua L. and Amaranthus retroflexus L. Ind. Crops Prod. 2018, 122, 1–6. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Wang, J.; Ma, X.J.; Sun, J.; Tang, F. Laboratory and field evaluation of the phytotoxic activity of Sapindus mukorossi Gaertn pulp extract and identification of a phytotoxic substance. Molecules 2021, 26, 1318. [Google Scholar] [CrossRef]
- Gonzalez, P.J.; Sorensen, P.M. Characterization of saponin foam from Saponaria officinalis for food applications. Food Hydrocoll. 2020, 101, 105541. [Google Scholar] [CrossRef]
- Rai, S.; Acharya, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-derived saponins: A review of their surfactant properties and applications. Preprints 2021, 2021080152. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.H.; Chen, W.H.; Fu, Y.; Chang, J.S.; Bi, X. Oxidative torrefaction of biomass nutshells: Evaluations of energy efficiency as well as biochar transportation and storage. Appl. Energy 2019, 235, 428–441. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Jayaraj, T.; Krishnasamy, R.; Sindhu, J.; Sneka, D.; Subhashini, B.; Vo, D.V.N. Analysis on the removal of emerging contaminant from aqueous solution using biochar derived from soap nut seeds. Environ. Pollut. 2021, 287, 117632. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Huang, P.C. Characterization of acid-leaching cocoa pod husk (CPH) and its resulting activated carbon. Biomass Convers. Biorefin. 2018, 8, 521–528. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q.; Tsai, C.H.; Chung, M.H.; Chu, M.H.; Huang, H.J.; Jao, Y.H.; Yeh, S.I. Conversion of water caltrop husk into biochar by torrefaction. Energy 2020, 195, 116967. [Google Scholar] [CrossRef]
- Tsai, W.T.; Jiang, T.J.; Tang, M.S.; Chang, C.H.; Kuo, T.H. Enhancement of thermochemical properties on rice husk under a wide range of torrefaction conditions. Biomass Convers. Biorefin. 2021, in press. [Google Scholar] [CrossRef]
- Tiwari, D.P.; Sharma, D.N.; Raunija, T.S.K. Sapindus based activated carbon by chemical activation. Res. J. Mater. Sci. 2013, 1, 9–15. [Google Scholar]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Mosek, O.; Johnston, C.T. Thermal Analysis for Biochar Characterisation. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 283–293. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Werner, K.; Pommer, L.; Brostrom, M. Thermal decomposition of hemicelluloses. J. Anal. Appl. Pyrolysis 2014, 110, 130–137. [Google Scholar] [CrossRef]
- Hosokai, S.; Matsuoka, K.; Kuramoto, K.; Suzuki, Y. Modification of Dulong’s formula to estimate heating value of gas, liquid and solid fuels. Fuel Process. Technol. 2016, 152, 399–405. [Google Scholar] [CrossRef]
- Tsai, C.H.; Tsai, W.T.; Liu, S.C.; Lin, Y.Q. Thermochemical characterization of biochar from cocoa pod husk prepared at low pyrolysis temperature. Biomass Conver. Biorefin. 2018, 8, 237–243. [Google Scholar] [CrossRef]
- Lachman, J.; Balas, M.; Lisy, M.; Lisa, H.; Milcak, P.; Elbl, P. An overview of slagging and fouling indicators and their applicability to biomass fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]

| Properties a,b | Value |
|---|---|
| Proximate analysis | |
| Ash (wt%) | 1.62 ± 0.02 |
| Volatile matter (wt%) | 80.82 ± 1.90 |
| Fixed carbon c (wt%) | 17.56 |
| Ultimate analysis | |
| Carbon (wt%) | 42.59 ± 8.60 |
| Hydrogen (wt%) | 5.86 ± 1.17 |
| Oxygen (wt%) | 37.30 ± 0.15 |
| Nitrogen (wt%) | 0.36 ± 0.09 |
| Sulfur (wt%) | 0.11 ± 0.02 |
| Calorific value (MJ/kg) | 20.97 ± 1.89 |
| SP-Torrefied Product a | Mass Yield b (wt%) | Calorific Value b (MJ/kg) | Enhancement Factor c (-) | Energy Yield d (%) |
|---|---|---|---|---|
| T-SP-200-0 e | 91.09 ± 0.08 | 23.07 ± 0.56 | 1.10 | 100.18 |
| T-SP-200-30 | 83.79 ± 1.00 | 23.78 ± 0.76 | 1.13 | 95.00 |
| T-SP-200-60 | 83.00 ± 1.65 | 23.90 ± 0.74 | 1.14 | 94.59 |
| T-SP-240-0 | 84.79 ± 2.29 | 23.54 ± 0.08 | 1.12 | 95.17 |
| T-SP-240-30 | 75.57 ± 2.83 | 25.46 ± 0.73 | 1.21 | 91.72 |
| T-SP-240-60 | 73.34 ± 2.35 | 25.37 ± 0.54 | 1.21 | 88.70 |
| T-SP-280-0 | 74.31 ± 2.95 | 24.66 ± 0.14 | 1.18 | 87.37 |
| T-SP-280-30 | 60.16 ± 3.15 | 28.60 ± 0.60 | 1.36 | 82.04 |
| T-SP-280-60 | 55.60 ± 3.27 | 28.39 ± 0.33 | 1.35 | 75.27 |
| T-SP-320-0 | 48.85 ± 0.49 | 28.32 ± 0.23 | 1.35 | 65.98 |
| T-SP-320-30 | 45.06 ± 2.27 | 28.41 ± 0.79 | 1.35 | 61.03 |
| T-SP-320-60 | 43.52 ± 2.90 | 27.97 ± 0.34 | 1.33 | 58.05 |
| Sapindus Pericarp (SP) and Its Torrefied Products (SP-T) | Organic Elements (wt%) | H/C a | O/C a | ||||
|---|---|---|---|---|---|---|---|
| C | H | N | O | S | |||
| SP | 42.59 ± 8.60 | 5.86 ± 1.17 | 0.36 ± 0.09 | 37.30 ± 0.15 | 0.11 ± 0.02 | 1.65 | 0.66 |
| T-SP-200-0 e | 54.39 ± 0.54 | 6.72 ± 0.12 | 1.68 ± 0.13 | 41.62 ± 0.78 | 0.38 ± 0.13 | 1.48 | 0.57 |
| T-SP-200-30 | 57.84 ± 1.99 | 6.76 ± 0.05 | 1.53 ± 0.23 | 38.55 ± 0.12 | 0.24 ± 0.03 | 1.40 | 0.50 |
| T-SP-200-60 | 58.62 ± 3.01 | 6.53 ± 0.25 | 1.63 ± 0.01 | 35.36 ± 0.85 | 0.23 ± 0.05 | 1.34 | 0.45 |
| T-SP-240-0 | 54.92 ± 1.97 | 6.70 ± 0.22 | 1.29 ± 0.41 | 38.30 ± 3.90 | 0.15 ± 0.01 | 1.46 | 0.52 |
| T-SP-240-30 | 58.66 ± 0.18 | 6.24 ± 0.05 | 1.36 ± 0.08 | 34.20 ± 1.27 | 0.15 ± 0.01 | 1.28 | 0.44 |
| T-SP-240-60 | 61.76 ± 1.20 | 6.19 ± 0.10 | 1.50 ± 0.06 | 33.57 ± 0.70 | 0.14 ± 0.00 | 1.20 | 0.41 |
| T-SP-280-0 | 57.50 ± 1.85 | 6.11 ± 0.16 | 0.78 ± 0.01 | 37.47 ± 3.06 | 0.12 ± 0.00 | 1.28 | 0.49 |
| T-SP-280-30 | 67.05 ± 0.71 | 5.69 ± 0.14 | 1.76 ± 0.16 | 25.41 ± 0.43 | 0.14 ± 0.01 | 1.02 | 0.28 |
| T-SP-280-60 | 68.59 ± 1.28 | 5.24 ± 00.34 | 1.86 ± 0.01 | 25.78 ± 1.22 | 0.14 ± 0.00 | 0.92 | 0.28 |
| T-SP-320-0 | 69.72 ± 0.94 | 5.08 ± 0.22 | 1.26 ± 0.10 | 24.32 ± 0.66 | 0.12 ± 0.01 | 0.87 | 0.26 |
| T-SP-320-30 | 70.33 ± 0.39 | 4.17 ± 0.02 | 1.19 ± 0.11 | 24.37 ± 0.03 | 0.11 ± 0.01 | 0.71 | 0.26 |
| T-SP-320-60 | 68.77 ± 3.44 | 3.94 ± 0.57 | 1.59 ± 0.25 | 25.05 ± 0.19 | 0.14 ± 0.01 | 0.69 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-T.; Jiang, T.-J.; Lin, Y.-Q.; Zhang, X.; Yeh, K.-S.; Tsai, C.-H. Fuel Properties of Torrefied Biomass from Sapindus Pericarp Extraction Residue under a Wide Range of Pyrolysis Conditions. Energies 2021, 14, 7122. https://doi.org/10.3390/en14217122
Tsai W-T, Jiang T-J, Lin Y-Q, Zhang X, Yeh K-S, Tsai C-H. Fuel Properties of Torrefied Biomass from Sapindus Pericarp Extraction Residue under a Wide Range of Pyrolysis Conditions. Energies. 2021; 14(21):7122. https://doi.org/10.3390/en14217122
Chicago/Turabian StyleTsai, Wen-Tien, Tasi-Jung Jiang, Yu-Quan Lin, Xiang Zhang, Kung-Sheng Yeh, and Chi-Hung Tsai. 2021. "Fuel Properties of Torrefied Biomass from Sapindus Pericarp Extraction Residue under a Wide Range of Pyrolysis Conditions" Energies 14, no. 21: 7122. https://doi.org/10.3390/en14217122







